Abstract
Thioredoxin (TRX) is a redox regulating protein which has protective effects against oxidative stress-induced damage to cells and tissues. In this study, we investigated the effects of orally administered TRX derived from edible yeast, Saccharomyces cerevisiae, on gastric mucosa. First, we examined the digestibility of orally administered yeast TRX in mice, and detected yeast TRX in the stomach for 4 h after administration. Next, we investigated the mitigation of gastric mucosal injury after the oral administration of yeast TRX in water-immersion restraint stress and HCl/ethanol-induced gastric ulcer models. Furthermore, we conducted DNA microarray analysis, using the HCl/ethanol-induced model, which revealed that several groups of genes related to tissue repair were upregulated in ulcer regions in the stomachs of rats administered with yeast TRX. These results demonstrated the viability of the use of oral administrations of yeast TRX to protect the gastric mucosa.
Graphical Abstract
Oral administration of yeast thioredoxin (TRX) mitigates gastric mucosal injuries in water-immersion restraint stress (WRS) and HCl/ethanol-induced gastric ulcer models.
Thioredoxin (TRX) is a small ubiquitous thiol protein (12 kDa) that has a catalytically active site (Cys-Gly-Pro-Cys) which is conserved in animals, plants, and bacteria.Citation1–3) Cysteines in the active site undergo a reversible redox change between an oxidized disulfide (–S–S–) and a reduced dithiol (–SH, –SH). TRX is oxidized when it cleaves disulfide bonds in the target proteins and oxidized TRX is regenerated to the reduced state by NADPH-dependent thioredoxin reductase (NTR). It has been reported that, via the active site, TRX has multiple regulatory functions that maintain cellular redox homeostasis. By itself, TRX scavenges reactive oxygen species (ROS), such as singlet oxygen and hydroxyl radicals.Citation4) TRX coordinately reduces H2O2 with peroxiredoxin, a member of the thioredoxin-dependent peroxidase family.Citation5) Moreover, TRX plays an important role in cellular signaling through its redox regulative activity. Transcription factors containing sulfhydryl residues, such as AP-1 and NF-κB, increase DNA-binding activity through change in the redox state of the residues induced by TRX directly or indirectly.Citation3,6,7) Apoptosis signal-regulating kinase-1 (ASK1), a MAP kinase kinase kinase, is inhibited by being bound to reduced TRX.Citation8,9) It has been suggested that TRX plays a defensive role against several diseases, including gastrointestinal disease, through these functions. Previous studies have shown attenuation of dextran sulfate sodium-induced colitis,Citation10) Helicobacter felis-induced gastritisCitation11) and also indomethacin-induced gastric mucosal injuryCitation12) in TRX-overexpressing transgenic mice or mice after the systemic administration of recombinant human TRX. Recently, Nakajima et al. reported that oral administration of sake yeast extracts with a high TRX content reduced indomethacin-induced gastric injury,Citation13) suggesting that orally administered TRX, and not merely endogenous TRX or injected TRX, can protect the gastric mucosa. Until the present study, no investigations have been conducted on how long orally administered TRX remains in the stomach.
In this study, we first examined the digestibility of orally administered TRX derived from yeast, which is commonly used in fermented foods, and analyzed the protective effect of yeast TRX on the gastric mucosa both in in vitro and in vivo models (water-immersion restraint stress (WRS) and HCl/ethanol-induced gastric ulcer models). Next, we conducted DNA microarray analysis of ulcerated gastric mucosa in HCl/ethanol-induced gastric ulcer models.
Materials and methods
Materials
Recombinant yeast TRX2 (Saccharomyces cerevisiae has three isoforms of thioredoxin; cytosolic TRX1 and TRX2 and mitochondrial TRX3.) and yeast NTR produced and purified from Escherichia coli were obtained from Oriental Yeast Co., Ltd. (Tokyo, Japan). The other biochemicals, reagents, and enzymes employed in the study were all analytical grade.
Cell culture
Rat gastric mucosal cell line cells (RGM-1), kindly provided by Dr Matsui (University of Tsukuba), were maintained in DMEM/Ham’s F-12 medium (50%/50%, Nacalai Tesque, Inc., Kyoto, Japan) containing 20% fetal calf serum, 100 μg/mL streptomycin and 100 U/mL penicillin. The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Animals
Male Spraque-Dawley rats were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). Male C57BL/6 mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). Rabbits were obtained from Kitayama Labes Co., Ltd. (Nagano, Japan). These animals were housed at 23 ± 3 °C and 40–60% humidity under a 12 h light cycle, with access to solid laboratory feed (Oriental Yeast Co.) and tap water ad libitum.
All of the animal experiments conducted in this study were approved by the Animal Care and Use Committee at Oriental Yeast Co., and the animals were treated according to the guidelines issued by the Committee.
Production of polyclonal antibody against yeast TRX
One milligram of recombinant yeast TRX2 was injected subcutaneously into two rabbits with Freund’s complete adjuvant, and booster injections of 0.5 mg of yeast TRX2 with Freund’s incomplete adjuvant were repeated thrice at 14-day intervals. Fourteen days after the final immunization, the antisera were collected. The IgG fractions in the rabbit sera were collected using protein A affinity chromatography (GE Healthcare UK Ltd., Buckinghamshire, England).
Oral administration study of yeast TRX
Male C57BL/6 mice were used for the experiment at eight weeks of age after a 1-week acclimatization period. The animals were fasted for 12 h before the oral administration study. Five groups of three animals were orally administered with 100 mg/kg body weight of recombinant yeast TRX2 in a 0.85% NaCl solution through a stomach tube. At 1, 2, 4, 6, and 24 h after administration, the mice were anesthetized and the stomachs were excised. In three animals employed as a negative control group, stomachs were excised immediately after the administration of a 0.85% NaCl solution alone. Stomach contents were collected from the excised stomachs, and the stomachs were washed thrice with ice-cold 1.15% KCl solution. The 1.15% KCl solutions used for washing were also collected as stomach contents. After removal of the stomach contents, the stomachs were used as gastric walls in the following experiments.
Detection of yeast TRX in the stomachs by the Western blot method
The gastric walls were homogenized in 1.15% KCl solution and lysis buffer was added at the following final concentrations (50 mm Tris–HCl, pH 8.0, 150 mm NaCl, 1% (w/v) Nonidet P-40®, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v)SDS, 0.5 mm DTT, 1 mm PMSF, 5 μg/mL leupeptin, 5 μg/mL aprotinin, 1 mm Na2VO4, and 10 mm NaF), and then incubated on ice for 2 h. The yeast TRX remaining in the gastric walls was extracted by immunoprecipitation. The homogenates, containing 1.5 mg proteins with the addition of 26 μg of anti-yeast TRX polyclonal antibodies, were incubated for 1 h at 4 °C, and then mixed gently with 20 μL of protein A/G plus-Agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4 °C. The beads were washed thrice with TBS containing 0.05% Tween 20 (20 mm Tris–HCl, pH 7.6, 137 mm NaCl, TBS-T), and centrifuged at 7500 × g for 3 min.
The stomach contents were diluted 100-fold with lysis buffer and 10 μL of the lysates were analyzed by the western blot method as follows.
The precipitated beads from the gastric walls and the lysates from the stomach contents were separated on 20% SDS-PAGE gels and transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking in 5% skim milk in TBS-T, the membrane was incubated with anti-yeast TRX polyclonal antibodies (1:200) for 1 h, followed by incubation with HRP-conjugated anti-rabbit IgG antibody (1:15,000) for 1 h. Chemiluminescence signals were detected with an ECL plus western blot detection kit (GE Healthcare UK).
Assay for ethanol-induced cytotoxicity
RGM-1 cells were seeded onto 96-well plates at 8 × 104 cells/mL and cultured for 48 h. Then, the cells were incubated with ethanol and recombinant yeast TRX2 in Dulbecco’s phosphate-buffered saline containing 0.9 mm CaCl2 and 0.49 mm MgCl2 (PBS (+)) for the time period required. Cell viability was assessed by LDH leakage. LDH released from damaged cells was determined in aliquots of the culture medium using a LDH assay kit (Kyokuto Pharmaceutical Industrial, Co., Ltd., Tokyo, Japan).
Assay of TRX reducing activity
Reaction buffer (100 mm potassium phosphate buffer, pH 7.0, 2 mm EDTA, 1.125 mg/mL insulin, and 200 μm NADPH) was mixed with each sample (or a dilution series of recombinant yeast TRX2 as a standard) to a final volume of 980 μL. Twenty microliter of 1 mg/mL NTR was added to the mixture and the change in absorbance at 340 nm due to oxidation of NADPH at 25 °C was monitored.
WRS-induced gastric ulcers
Male Spraque-Dawley rats were used for the experiment at six weeks of age after a 1-week acclimatization period. The animals were fasted for 12–14 h before the induction of the ulcers. Three groups of 5 animals each were orally administered with 10 mg/kg body weight of recombinant yeast TRX2 in a 0.85% NaCl solution, ovalbumin (OVA) in a 0.85% NaCl solution or a 0.85% NaCl solution alone through a stomach tube 15 min before water-immersion (pre-treatment groups). The other three groups of 5 animals each were orally administered with samples 15 min after water-immersion (post-treatment groups). A control rat was administered with a 0.85% NaCl solution alone and not exposed to WRS. Gastric mucosal injury was induced by immersing the rats in the water for 6 h, and then 5 h later the rats were anesthetized and the stomachs were excised. After fixation in 1% formalin solution for 24 h, the stomachs were incised, the numbers of the gastric mucosal lesions were counted, and the lengths of the lesions were measured. The sum of the lesion lengths in the gastric mucosa was used as a lesion index.
HCl/ethanol-induced gastric ulcers
Male Spraque-Dawley rats were used for the experiment at six weeks of age after a 1-week acclimatization period. The animals were fasted for 12–14 h and then administered orally with 10 mg/kg body weight of recombinant yeast TRX2 in a 0.85% NaCl solution, OVA in a 0.85% NaCl solution or a 0.85% NaCl solution alone through a stomach tube daily for 3 days. At 1 h after the final administration, gastric mucosal injury was induced by oral administration of HCl/ethanol (150 mm HCl in 80% ethanol), and then 1 h later the rats were anesthetized and the stomachs were excised. The other experimental procedures employed were the same as for the WRS-induced gastric ulcers described above.
Microarray analysis
Two groups of 3 male Spraque-Dawley rats each were treated with recombinant yeast TRX2 in a 0.85% NaCl solution or a 0.85% NaCl solution alone daily for 3 days. The gastric mucosal injuries were induced by HCl/ethanol, using the same procedures as described above. One hour later, the rats were anesthetized and the stomachs were excised. The collected stomachs were separated into ulcerated and non-ulcerated regions (Fig. (A)). These samples were frozen by liquid nitrogen and stored at −70 °C until analysis.
Total RNA was extracted from 10 mg of the tissues with a RNeasy Mini Kit (Qiagen, Inc., Hilden, Germany), and treated with DNase (RNase-Free DNase Set, Qiagen). The quality of the RNA samples was evaluated using a 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA).
An Agilent Rat V2 Oligo Microarray (Agilent Technologies, G4130B) containing more than 20,000 features was used for the one-color microarray analysis in this study. Cy3-labeled complementary RNA (cRNA) was synthesized from 0.5 μg of the total RNA samples using an Agilent Low RNA Input Fluorescent Linear Amplification Kit (Agilent Technologies). Microarray hybridization was carried out for 17 h at 65 °C with 1.5 μg of cRNA in a Hybridization Chamber (Agilent Technologies, G2534A) according to procedures provided by the company. After washing, the microarrays were scanned with an Agilent Technologies Microarray Scanner with the scanning resolution set to 10 μm and analyzed with Agilent Feature Extraction Software 8.5. Subsequent analyses were performed using GeneSpring GX software (Agilent Technologies). Raw signal values were thresholded to 1, log2 transformed, normalized using a 50th percentile shift, and baseline transformed to the median of each non-ulcerated region sample. After transformation, data were filtered by flags and by expression (20–100th percentile). The three independent biological replicates data-sets for rats that ingested yeast TRX2 or rats that ingested a 0.85% NaCl alone were initially filtered using a one-sample Student’s t test (p < 0.05) to identify statistically significant differences in genes expressed within each treatment group. The resulting genes of the two groups were subsequently analyzed using a moderated t test with Benjamini-Hochberg multiple-testing correction (p < 0.05). Selected genes were annotated and the biological processes were analyzed using the web-based tool DAVID (Database for Annotation, Visualization, and Integrated Discovery) V6.7.Citation14,15)
Statistical analysis
Values were given as means ± SD. When two groups were compared, the data were analyzed using Student’s t test. When multiple groups were compared, the data were analyzed using Tukey’s test. A p value < 0.05 was considered significant.
The statistical analysis of the microarray experiment was performed as described above.
Results
The digestibility of yeast TRX in the stomach
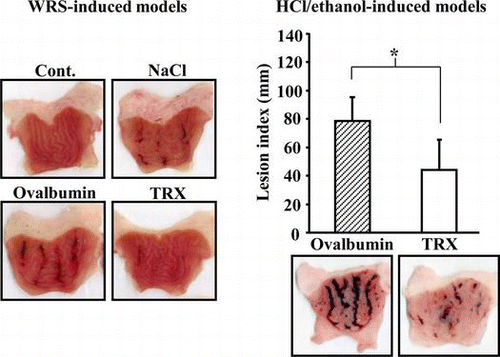
First, we conducted tests to determine how long orally administered TRX proteins remain in the stomach. We conducted assays to detect yeast TRX in the gastric walls and the stomach contents from mice which were administered with 100 mg/kg body weight of yeast TRX orally. As shown in Fig. , yeast TRX in the stomach contents was detected for 2 h after administration. In the gastric walls, the TRX was detected for 4 h, which was 2 h longer than the stomach contents, and the yeast TRX was detected as a double-banded pattern at 4 h. These results suggest that a part of yeast TRX remains in the stomach for several hours.
Fig. 1. The digestibility of yeast TRX in the stomach of the mouse.
Notes: Recombinant yeast TRX was administered orally to mice at a dosage of 100 mg/kg body weight in a 0.85% NaCl solution. At 1, 2, 4, 6, and 24 h after administration, the stomachs were excised from the mice. Yeast TRX in the stomach contents were detected by the western blot method (A). Yeast TRX in the gastric walls was detected by immunoprecipitation and the western blot method (B). Ten nanograms of recombinant yeast TRX was loaded as a control for detection.
The effects of yeast TRX on ethanol-induced cytotoxicity in RGM-1 cells
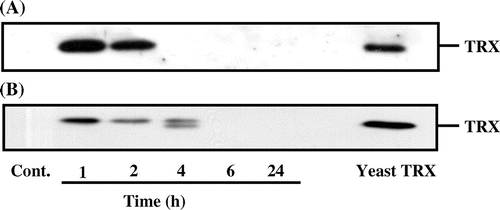
It has been reported that human TRX showed a protective effect against ethanol-induced cytotoxicity in an experimental gastric mucosal injury model.Citation16) To reconfirm this finding, we examined the effect of yeast TRX on RGM-1 cells treated with 10% ethanol. The cells treated with 10−7 m yeast TRX showed delayed progression of cell injury, compared with untreated cells. Furthermore, in a group treated with yeast TRX, the rate of injured cells reached a plateau at 80% after 30–35 min of ethanol treatment, while almost 100% of the untreated cells were injured (Fig. (A)). Cells treated with 10−7 m yeast TRX showed significant reduction in cell injury compared with untreated cells, while cells treated with 10−7 m thermal yeast TRX did not (Fig. (B)). These results suggest that the reducing activity of TRX is required for a reduction in cell injuries. We have ascertained that the TRX retains its reducing activity in 10% ethanol for 60 min (Fig. (C)) and that thermally denatured TRX lost its reducing activity (Fig. (D)).
Fig. 2. The effects of recombinant yeast TRX on ethanol-induced cytotoxicity in RGM-1 cells.
Notes: (A) Time course of the LDH release. RGM-1 cells were incubated with 10% ethanol and 10−7 m recombinant yeast TRX for the time period required. (B) Relative cytotoxicity. The cells were incubated with 10% ethanol and 10−8 or 10−7 m recombinant yeast TRX, or 10−7 m thermal denatured recombinant yeast TRX for 35 min. Panel (A) shows the result of a representative experiment. In panel (B), the relative cytotoxicity (%) is shown relative to the cytotoxicity of the control cells (set as 100%) as an average value from 3 independent experiments. The values are given as mean ± SD. The data were analyzed using two-sided one-sample Student’s t tests. The * symbol indicates values significantly different from those for cells incubated without yeast TRX at p < 0.05. (C) Time course of the remaining reducing activity. Yeast TRX was incubated with 10% ethanol for the time period required. (D) Remaining reducing activity of native and thermally denatured TRX. Yeast TRX was denatured at 100 °C for 20 min. The remaining activity (%) was measured by an assay of the TRX reducing activity.
The effects of yeast TRX on WRS-induced gastric ulcers
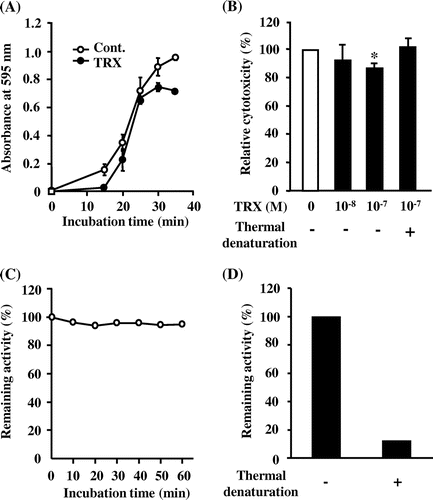
The results mentioned above suggest that, in part, orally ingested yeast TRX remains in the stomach, and has a protective effect against gastric mucosal cell injury in vitro. Next, we examined the effect of orally administered yeast TRX in WRS-induced gastric ulcer model rats. In the post-treatment groups, the lesion index was 39.4 ± 31.5 mm in rats administered with a 0.85% NaCl solution alone, 109.3 ± 53.3 mm in rats administered with protein OVA, and 20.4 ± 22.7 mm in rats administered with yeast TRX (p < 0.01 vs. rats administered with OVA). In the pre-treatment groups, lesion index was 65.4 ± 79.4 mm in rats administered with a 0.85% NaCl solution alone, 44.3 ± 44.0 mm in rats administered with OVA, and 9.0 ± 10.0 mm in rats administered with yeast TRX. Rats administered with yeast TRX in the pre-treatment group showed a tendency for a reduction of the lesion index, although it was not a statistically significant difference, compared with rats administered with other reagents (Fig. (A)). The number of lesions in both the pre-treatment and post-treatment groups showed results similar to the lesion index (Fig. (B)). Macroscopically, the gastric mucosal injuries were observed as black hemorrhagic lesion sites (Fig. (C)).
Fig. 3. The effects of yeast TRX on WRS-induced gastric ulcers.
Notes: Lesion indexes (A) and the number of gastric ulcer lesions (B) in rats pre-treatment or post-treatment with the administration of recombinant yeast TRX in a 0.85% NaCl solution, OVA in a 0.85% NaCl solution, or a 0.85% NaCl solution alone. The animals employed as a negative control group were administered with a 0.85% NaCl solution alone and not exposed to WRS. The values are given as mean ± SD (n = 5). The *symbols indicate values significantly different at p < 0.05. The **symbol indicates values significantly different at p < 0.01. ND: not detected. (C) Pictures of excised stomachs from rats in the post-treatment groups.
The effects of yeast TRX on HCl/ethanol-induced gastric ulcers
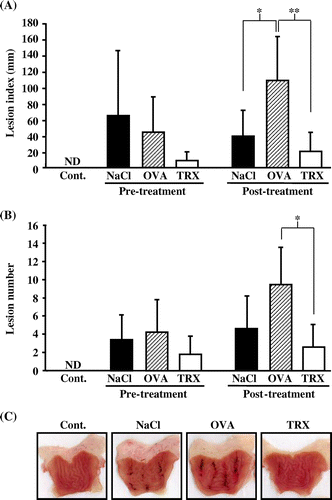
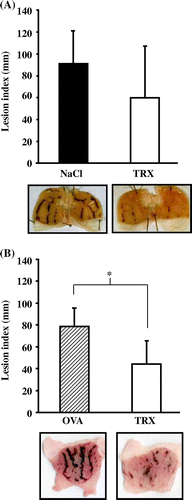
Furthermore, we examined the effect of orally administered yeast TRX for 3 days in another model, where the gastric ulcers were induced by HCl/ethanol. Compared with the group where the rats were administered with a 0.85% NaCl solution alone (91.6 ± 29.2 mm), rats administered with yeast TRX (60.3 ± 46.8 mm) showed a tendency for a reduction of the lesion index, but the difference was not statistically significant (Fig. (A)). Compared with rats administered with OVA, there was a significant difference in the lesion index for the rats administered with yeast TRX (respectively 78.4 ± 16.4 mm vs. 44.1 ± 20.9 mm, p < 0.05) (Fig. (B)). Macroscopically, extensive hemorrhagic lesions were shown in both rats administered with a 0.85% NaCl alone and OVA, and the extent of the lesions was much smaller in rats administered with yeast TRX. We have ascertained that rats administered with yeast TRX orally for 3 days showed no significant changes in body weight, compared with untreated rats (data not shown).
Fig. 4. The effects of yeast TRX on HCl/ethanol-induced gastric ulcers.
Notes: (A) Lesion indexes of gastric ulcer (upper figure) and pictures of excised stomachs (lower figure) in recombinant yeast TRX in a 0.85% NaCl solution or a 0.85% NaCl solution alone administered rats (n = 10 in each group). (B) Lesion indexes of gastric ulcer (upper figure) and pictures of excised stomachs (lower figure) in recombinant yeast TRX in a 0.85% NaCl solution (n = 6) or OVA in a 0.85% NaCl solution (n = 5) administered rats. The values are given as mean ± SD. The *symbol indicates values significantly different at p < 0.05.
In order to evaluate the genetic changes induced by orally administered yeast TRX on gastric mucosal cell injuries, we performed microarray analysis to compare gene expression patterns in ulcerated and non-ulcerated regions of the stomachs of rats that ingested yeast TRX or a 0.85% NaCl solution alone (Fig. (A)). There were 385 genes that showed a significantly higher expression ratio (ulcerated vs. non-ulcerated regions) in rats that ingested yeast TRX, compared to rats that ingested a 0.85% NaCl solution alone (TRX-up) (Fig. (B), the genes identified were shown in Supplemental Table 1; see Biosci. Biotechnol. Biochem Website). On the other hand, there were 65 genes that showed a significantly lower ratio in rats that ingested yeast TRX (TRX-down) (Fig. (B), the genes identified were shown in Supplemental Table 2). We performed Gene Ontology (GO) analysis at the biological process level for the selected genes mentioned above. The enriched GO terms were divided into broad categories such as “localization,” “developmental process,” “biological regulation,” “metabolic process,” “cellular process,” and “response to stimulus.” The lower classes in the GO hierarchy are listed in Table . In the TRX-up genes, the terms related to repair of injuries, such as “mammary gland development,” “myoblast differentiation,” “blood vessel development,” and “regulation of blood coagulation,” were demonstrated as significant GO terms with p < 0.05.
Fig. 5. DNA microarray analysis of HCl/ethanol-induced gastric ulcers in rats.
Notes: (A) The method employed for the DNA microarray experiment. Two groups of rats were administered with recombinant yeast TRX in a 0.85% NaCl solution or a 0.85% NaCl solution alone and gastric mucosal injuries were induced by HCl/ethanol. Stomachs were excised from the rats, and separated into ulcerated and non-ulcerated regions. RNA extraction and DNA Microarray procedures were performed for each separate sample. (B) Scatter plot of gene expression values (log2 ratios of ulcerated vs. non-ulcerated regions) in two groups. The x-axis: rats that ingested a 0.85% NaCl solution alone, the y-axis: rats that ingested yeast TRX. The red and green spots respectively depict significantly higher and lower expressed genes in rats that ingested yeast TRX, compared to rats that ingested a 0.85% NaCl solution alone.
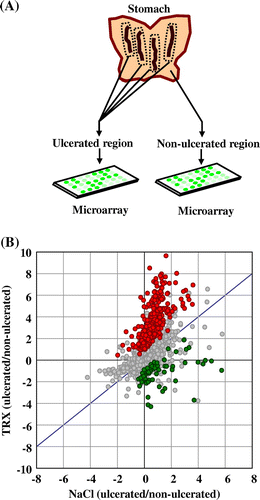
Table 1. Gene-annotation enrichment analysis based on the GO databases using differentially expressed genes between rats that ingested yeast TRX and rats that ingested a 0.85% NaCl solution alone.
Discussion
In this study, we demonstrated that orally administered yeast TRX protects the gastric mucosal surface before being digested by gastric juice. Orally administered proteins are exposed to acid and enzymatically degraded to peptides. It is possible that the yeast TRX protein is also denatured and degraded before acting on the gastric wall. Therefore, we examined the digestibility of yeast TRX in the stomach by detecting yeast TRX in the gastric walls and the stomach contents collected from mice which were orally administered with yeast TRX. The TRX in the gastric walls was detected for 4 h after administration, 2 h longer than the stomach contents (Fig. ). We previously confirmed that yeast TRX maintained its reducing activity after treatment at pH 3.0 for 24 h (data not shown). In addition, it has been reported that the structure of E. coli TRX is stable from pH 3.0 to 7.0.Citation17) It has been reported that the reducing activity of Dromedary TRX remained after it was purified through acidic treatment (pH 2.5).Citation18) Considering the interspecies-conserved structure of TRX, these studies support the acid stability of yeast TRX, and therefore, it is reasonable to assume that undegraded yeast TRX has reducing activity in acidic gastric juice. To support this assumption, further studies of yeast TRX activity in the gastric environment are required.
In the present study, we showed that the oral administration of yeast TRX mitigated gastric ulcers in both a WRS-induced gastric ulcer model and an HCl/ethanol-induced gastric ulcer model (Figs. and ). Gastric injury induced by HCl/ethanol is accompanied by the production of ROS, which is caused by the depletion of endogenous antioxidantsCitation19) or direct oxidative damage due to ethanol.Citation20) Since TRX scavenges ROS directlyCitation4) and reduces H2O2 via peroxiredoxin, a member of the thioredoxin-dependent peroxidase family,Citation5) the TRX-mediated mitigation of the gastric injuries seems to be partly due to the decreases in ROS induced by HCl/ethanol. Moreover, previous report showed that pretreatment with recombinant human TRX suppressed indomethacin-induced ROS generation in rat gastric mucosal cells,Citation12) which supports our hypothesis. In the WRS-induced gastric ulcer model, yeast TRX may exert its protective effect through an antioxidative mechanism, since several antioxidants have been reported to attenuate gastric ulcer in the same model.Citation21,22) The WRS-induced gastric ulcer model is a typical stress ulcer model based on the stimulation of gastric secretion via the nervous system due to extreme mental and psychological stress on rodents.Citation23,24) The oral administration of yeast TRX might also have effects on gastric secretion. In this study, we showed that the degree of the gastric mucosal lesions in rats administered with yeast TRX was significantly less than that of rats administered with OVA, while a comparison of the rats administered with TRX and rats administered with a 0.85% NaCl solution alone showed that while there was a tendency for a reduction in the degree of the lesions, the difference was not statistically significant (the post-treatment groups in Figs. and ). We consider that the ulcerations might be increased in the groups of rats that ingested yeast TRX or OVA compared with the group of rats that ingested a 0.85% NaCl solution alone resulting from gastric acid secretion stimulated by ingestion of proteins such as yeast TRX or OVA. Thus, compared to rats administered with OVA, it can be inferred that the mucosal damage caused by the secreted gastric acid is decreased, or that the gastric acid secretion is suppressed, when yeast TRX is administered. Yeast TRX2, which was used in this study, is a stress-inducible cytosolic isoform in S. cerevisiae,Citation25,26) and its structure is similar to those of the homologs from other organisms.Citation27) Considering the high structural conservation and interspecies cross-reactivity of TRX,Citation1,28,29) yeast TRX might interact with gastric mucosal cells and affect the secretion of gastric mucus or gastric acid. However, it has not been previously reported about direct interaction of orally administered TRX and gastrointestinal cells. We infer that yeast TRX affects the gastric cells mainly from the extracellular side in this study. In the previous report, recombinant human TRX was observed to enter into ATL2 cells gradually over 24 h.Citation30–32) We show that yeast TRX is digested within only several hours (Fig. ), and thus it is unlikely that large fraction of active yeast TRX without denaturation enters the gastric cells. Further studies are required to clarify these issues.
In order to examine the gene changes due to the oral administration of yeast TRX in the stomach, we conducted microarray analyses employing an HCl/ethanol-induced gastric ulcer rat model. As a result, the expression of comparatively large amounts of genes (385 genes) were shown to be significantly higher in ulcerated regions in the group of rats that ingested yeast TRX, compared with the group of rats that ingested a 0.85% NaCl solution alone (TRX-up), while the expression of 65 genes were shown to be significantly lower (TRX-down) (Fig. (B)). These results suggested that there were some vigorous responses on the ulcerated region in the group of rats that ingested yeast TRX. In the GO analyses, terms related to the repair of injuries, such as “mammary gland development” (e.g. Hk2 and Hoxa9), “myoblast differentiation” (e.g. Myod1 and Pitx1), “blood vessel development” (e.g. Sox18, Egf, Epgn and Gja4) and “regulation of blood coagulation” (e.g. Hrg, Plg and Proc), were detected in TRX-up genes (Table ). These results provide clues to the mechanism of the TRX action in the model. The GO terms “calcium ion transport” (e.g. Cyp27B1, Trpm7 and Tmem37) (TRX-up) and “calcium ion homeostasis” (e.g. Casr, Lpar1, and Trim24) (TRX-down) were also detected. Calcium ion regulates several processes through wound repairs, such as inflammation, cell proliferation, and cell migration, and it has been reported that calcium ion transport is essential during early wound healing.Citation33) The changes shown in the genes due to the administration of yeast TRX may be associated with wound healing. Healing processes are shown in the gastric ulcer margin, and various signaling factors, including redox-sensitive proteins are involved in the induction of healing.Citation34,35) In the group of rats that ingested yeast TRX, signal transduction might be enhanced, resulting from the suppression of ROS by TRX, and consequently, genes related to tissue repair might be up-regulated. On the other hand, the GO term “regulation of Ras protein signal transduction” (e.g. Acap2 and Shoc2) were detected in TRX-down genes with comparatively low p value, although the effects of orally administered yeast TRX on signal transduction is not yet clear in this experiment. We consider that a major cause is one-point and short-term analysis from ulcer induction, and continuous analysis from ulcer induction is required. Additionally, to ascertain whether the oral administration of yeast TRX has adverse effects such as carcinogenicity, further studies should be conducted.
The content of protein and non-protein sulfhydryls and disulfides in the gastric mucosa is closely related to gastric mucosal damage, and a rapid decrease or oxidation of sulfhydryl groups is observed after ulceration.Citation36) Sulfhydryl groups contribute to the maintenance of gastric function, and accordingly, an exogenous supply of SH compounds (GSH, N-acetylcysteine) has been reported to protect gastric mucosa.Citation36–38) It is probable that the protective effects exerted by the exogenous supplement of TRX share similar mechanisms, at least in part, with other SH compounds. In this study, we demonstrated that the direct ingestion of TRX significantly suppressed gastric mucosal injuries and had an effect on gene expression in the ulcerated regions. TRX seems to provide more active protection of gastric mucosa than non-protein SH groups through its characteristic bioactivity.Citation5–9) In addition, the gene expression results shown in this study raise the interesting possibility of promoting wound-healing responses by the administration of TRX, although the data are still preliminary and further studies are required. Collectively, we hereby propose that yeast TRX is available for the development of gastroprotective functional foods, although further evaluation of the safety of yeast TRX ingestion is necessary.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.915733.
Supplemental Tables 1 and 2
Download PDF (126.2 KB)Acknowledgment
We would like to express our thanks to Dr Yoshiyuki Matsuo (Kyoto University, Kyoto, Japan) for his helpful discussions and suggestions. This study was supported by a grant-aid from the Research and Development Program for New Bio-Industry Initiatives.
Notes
Abbreviations: TRX, thioredoxin; NTR, NADPH-dependent thioredoxin reductase; ROS, reactive oxygen species; WRS, water-immersion restraint stress; OVA, ovalbumin; GO, Gene Ontology.
References
- Arnér ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109.
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annu. Rev. Plant Biol. 2005;56:187–220.
- Kondo N, Nakamura H, Masutani H, Yodoi J. Redox regulation of human thioredoxin network. Antioxid. Redox Signaling. 2006;8:1881–1890.
- Das KC, Das CK. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: redox independent functions. Biochem. Biophys. Res. Commun. 2000;277:443–447.
- Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods Enzymol. 1999;300:219–226.
- Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Nat. Acad. Sci. USA. 1997;94:3633–3638.
- Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J. Biol. Chem. 1999;274:27891–27897.
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606.
- Liu H, Zhang H, Iles KE, Rinna A, Merrill G, Yodoi J, Torres M, Forman HJ. The ADP-stimulated NADPH oxidase activates the ASK-1/MKK4/JNK pathway in alveolar macrophages. Free Radical Res. 2006;40:865–874.
- Tamaki H, Nakamura H, Nishio A, Nakase H, Ueno S, Uza N, Kido M, Inoue S, Mikami S, Asada M, Kiriya K, Kitamura H, Ohashi S, Fukui T, Kawasaki K, Matsuura M, Ishii Y, Okazaki K, Yodoi J, Chiba T. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131:1110–1121.
- Kawasaki K, Nishio A, Nakamura H, Uchida K, Fukui T, Ohana M, Yoshizawa H, Ohashi S, Tamaki H, Matsuura M, Asada M, Nishi T, Nakase H, Toyokuni S, Liu W, Yodoi J, Okazaki K, Chiba T. Helicobacter felis-induced gastritis was suppressed in mice overexpressing thioredoxin-1. Lab. Invest. 2005;85:1104–1117.
- Tan A, Nakamura H, Kondo N, Tanito M, Kwon YW, Ahsan MK, Matsui H, Narita M, Yodoi J. Thioredoxin-1 attenuates indomethacin-induced gastric mucosal injury in mice. Free Radical Res. 2007;41:861–869.
- Nakajima A, Fukui T, Takahashi Y, Kishimoto M, Yamashina M, Nakayama S, Sakaguchi Y, Yoshida K, Uchida K, Nishio A, Yodoi J, Okazaki K. Attenuation of indomethacin-induced gastric mucosal injury by prophylactic administration of sake yeast-derived thioredoxin. J. Gastroenterol. 2012;47:978–987.
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57.
- Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13.
- Dekigai H, Nakamura H, Bai J, Tanito M, Masutani H, Hirota K, Matsui H, Murakami M, Yodoi J. Geranylgeranylacetone promotes induction and secretion of thioredoxin in gastric mucosal cells and peripheral blood lymphocytes. Free Radical Res. 2001;35:23–30.
- Hamid Wani A, Udgaonkar JB. HX-ESI-MS and optical studies of the unfolding of thioredoxin indicate stabilization of a partially unfolded, aggregation-competent intermediate at low pH. Biochemistry. 2006;45:11226–11238.
- Ben Bacha AG, Mejdoub H. Purification and biochemical characterization of an organic-solvent-tolerant thioredoxin from dromedary pancreas. Protein J. 2012;31:1–7.
- Olaleye SB, Farombi EO. Attenuation of indomethacin- and HCl/ethanol-induced oxidative gastric mucosa damage in rats by kolaviron, a natural biflavonoid of Garcinia kola seed. Phytother. Res. 2006;20:14–20.
- Natale G, Lazzeri G, Lubrano V, Colucci R, Vassalle C, Fornai M, Blandizzi C, Del Tacca M. Mechanisms of gastroprotection by lansoprazole pretreatment against experimentally induced injury in rats: role of mucosal oxidative damage and sulfhydryl compounds. Toxicol. Appl. Pharmacol. 2004;195:62–72.
- Ohta Y, Nishida K. Protective effect of coadministered superoxide dismutase and catalase against stress-induced gastric mucosal lesions. Clin. Exp. Pharmacol. Physiol. 2003;30:545–550.
- Hamaishi K, Kojima R, Ito M. Anti-ulcer effect of tea catechin in rats. Biol. Pharm. Bull. 2006;29:2206–2213.
- Xie YF, Jiao Q, Guo S, Wang FZ, Cao JM, Zhang ZG. Role of parasympathetic overactivity in water immersion stress-induced gastric mucosal lesion in rat. J. Appl. Physiol. 2005;99:2416–2422.
- Kitagawa H, Fujiwara M, Osumi Y. Effects of water-immersion stress on gastric secretion and mucosal blood flow in rats. Gastroenterology. 1979;77:298–302.
- Morgan BA, Banks GR, Toone WM, Raitt D, Kuge S, Johnston LH. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 1997;16:1035–1044.
- Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano MB. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 1999;274:16040–16046.
- Bao R, Chen Y, Tang YJ, Janin J, Zhou CZ. Crystal structure of the yeast cytoplasmic thioredoxin Trx2. Proteins. 2007;66:246–249.
- Holmgren A. Thioredoxin. Annu. Rev. Biochem. 1985;54:237–271.
- Taketani Y, Kinugasa K, Furukawa S, Nakamura H, Otsuki R, Yasuda H, Fujita T, Kanzaki K, Masutani H, Yodoi J. Yeast thioredoxin-enriched extracts for mitigating the allergenicity of foods. Biosci. Biotechnol. Biochem. 2011;75:1872–1879.
- Kondo N, Ishii Y, Kwon YW, Tanito M, Horita H, Nishinaka Y, Nakamura H, Yodoi J. Redox-sensing release of human thioredoxin from T lymphocytes with negative feedback loops. J. Immunol. 2004;172:442–448.
- Hara T, Kondo N, Nakamura H, Okuyama H, Mitsui A, Hoshino Y, Yodoi J. Cell surface thioredoxin-1; possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts. Antioxid. Redox Signaling. 2007;9:1427–1437.
- Kondo N, Ishii Y, Kwon YW, Tanito M, Sakakura-Nishiyama J, Mochizuki M, Maeda M, Suzuki S, Kojima M, Kim YC, Son A, Nakamura H, Yodoi J. Lipid raft-mediated uptake of cysteine-modified thioredoxin-1: apoptosis enhancement by inhibiting the endogenous thioredoxin-1. Antioxid. Redox Signaling. 2007;9:1439–1448.
- Cordeiro JV, Jacinto A. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat. Rev. Mol. Cell Biol. 2013;14:249–262.
- Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. 2005;50:S24–S33.
- Sen CK, Roy S. Redox signals in wound healing. Biochim. Biophys. Acta. 2008;1780:1348–1361.
- Nagy L, Nagata M, Szabo S. Protein and non-protein sulfhydryls and disulfides in gastric mucosa and liver after gastrotoxic chemicals and sucralfate: possible new targets of pharmacologic agents. World J. Gastroenterol. 2007;13:2053–2060.
- Loguercio C, Taranto D, Beneduce F, del Vecchio Blanco C, de Vincentiis A, Nardi G, Romano M. Glutathione prevents ethanol induced gastric mucosal damage and depletion of sulfhydryl compounds in humans. Gut. 1993;34:161–165.
- Nagy L, Morales RE, Beinborn M, Vattay P, Szabo S. Investigation of gastroprotective compounds at subcellular level in isolated gastric mucosal cells. Am. J. Physiol. Gastrointest Liver Physiol. 2000;279:G1201–G1208.
