Abstract
Acid rock drainage (ARD) originating from the Yasumi-ishi tunnel near the main tunnel of the Yanahara mine in Japan was characterized to be moderately acidic (pH 4.1) and contained iron at a low concentration (51 mg/L). The composition of the microbial community was determined by sequence analysis of 16S rRNA genes using PCR and denaturing gradient gel electrophoresis. The analysis of the obtained sequences showed their similarity to clones recently detected in other moderately acidic mine drainages. Uncultured bacteria related to Ferrovum- and Gallionella-like clones were dominant in the microbial community. Analyses using specific primers for acidophilic iron- or sulfur-oxidizing bacteria, Acidithiobacillus ferrooxidans, Leptospirillum spp., Acidithiobacillus caldus, Acidithiobacillus thiooxidans, and Sulfobacillus spp. revealed the absence of these bacteria in the microbial community in ARD from the Yasumi-ishi tunnel. Clones affiliated with a member of the order Thermoplasmatales were detected as the dominant archaea in the ARD microbial population.
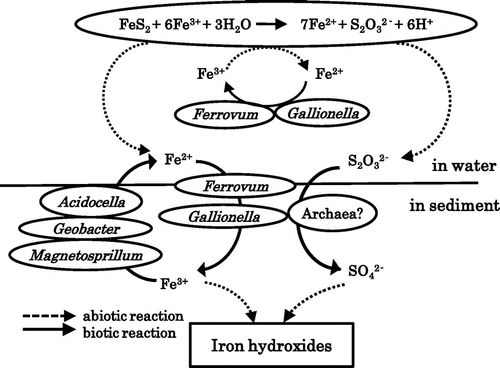
Graphical Abstract
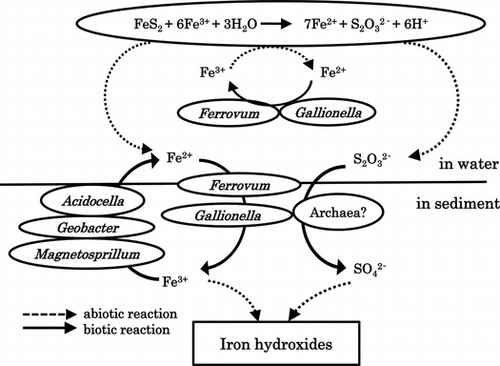
Model Showing the Roles of the Different Microorganisms Identified in the Pond of Yasumi-Ishi tunnel. Microorganisms are shown with their roles in the iron and sulfur cycles.
Acid mine drainage (AMD) and acid rock drainage (ARD) are acidic and generally enriched with iron, sulfate, and heavy metals such as lead and cadmium.Citation1,2) ARD or AMD is formed when the sulfide mineral ore comes in contact with oxygen and atmospheric humidity. Then, a complex mechanism begins on the mineral ore surface, starting with the oxidation of insoluble sulfides into sulfates followed by acid production. Although this oxidation kinetics is slow, the speed can be accelerated up to a hundred times in the presence of ferric ion and by the activity of catalyzing bacteria.Citation3,4) Because Acidithiobacillus ferrooxidans, which is a chemolithotrophic prokaryote that obtains energy for growth from the oxidation of ferrous iron and/or inorganic reduced sulfur compounds, has often been isolated from AMD and ARD, this bacterium has been used as a model micro-organism for analyzing the mechanism underlying acid production in metal-leaching environments.Citation3–6) In addition to A. ferrooxidans, the presence of various prokaryotic chemolithotrophs, such as A. thiooxidans, A. caldus, and Leptospirillum ferrooxidans, has been reported in mining environments. The presence of archaea, including a group of sulfur and/or iron oxidizers, such as members of the Sulfolobus, Acidianus, Metallosphaera, Sulfurisphaera, and Ferroplasma genera, has also been reported in acidic environments.Citation7–10) A diversity of culturable acidophilic bacteria, particularly heterotrophs belonging to the genera Acidiphilium and Acidocella, has also been reported in acidic environments.Citation11,12)
The Yanahara mine in Misaki Town, Okayama, Japan, has mainly produced pyrite for the manufacture of sulfuric acid.Citation13) The treatment of ARD or AMD is indispensable for preventing environmental pollution. Although the Yanahara mine has already been abandoned, ARD is still being generated there. Therefore, an ARD treatment system using A. ferrooxidans as iron-oxidizer has been developed and is now actively operating.Citation14,15) Economic factors have a significant impact on the viability of methods for treating ARD or AMD because abandoned mine sites require long-term maintenance. The cost incurred by the mining industry for the treatment of such wastes depends on the treatment system used to manage abandoned sites. Once ARD formation begins, it is generally difficult to control or suppress, and therefore development of cost-efficient methods to treat ARD-contaminated waters is still required. Since A. ferrooxidans has the optimum pH of around 2 for iron oxidation, the system for ARD treatment of the Yanahara mine is operated at pH 2.3–2.6. From the economic point of view, we think that a treatment system operating under moderately acidic conditions (pH 3–4) is suitable. To develop such a system, an iron-oxidizing bacterium having an optimum pH of around 4 for iron oxidation would be favorable. In this study, we analyzed the microbial community in moderately acidic ARD from the Yanahara mine. To our knowledge, this is the first report on a microbial community structure in moderately acidic ARD from a Japanese pyrite mine. The microbial community structure has been discussed in the context of local geochemical conditions.
Materials and methods
Site description, sample collection, and physicochemical analysis
The Yanahara pyrite mine is located in Misaki town, approximately 50 km northeast of Okayama city, Japan (N34.962040, E134.072494) (Supplemental Fig. 1.; see Biosci. Biotechnol. Biochem., Web site). To analyze physicochemical properties of ARD, ARD seeping from rock and flowing into a pond at the Yasumi-ishi tunnel, a small tunnel near the Yanahara main tunnel (Supplemental Figs. 2 and 3), was collected in July 2009. The properties were examined as described previously.Citation13) To analyze microbial community structure, ARD-containing sediments were also collected from the pond (Supplemental Fig. 3(C)). The sediments were obtained from the surface layer (0–5 cm depth).
Bacterial strains and DNA extraction
DNA was extracted from ARD samples by using the Soil DNA Kit UltracleanTM (MO BIO, Solana Beach, CA, USA) as described previously.Citation13) Chromosomal DNA from A. ferrooxidans ATCC 23270, A. thiooxidans NB1-3, and A. caldus GO-1 was prepared as described previously and used as a control template DNA for analysis of the amplified specific 16S rRNA genes.Citation15)
Polymerase chain reaction and denaturing gradient gel electrophoresis
Polymerase chain reaction (PCR) was carried out to amplify the V3 region of the bacterial 16S rRNA gene with the GC-clamp using primers: forward 341F-GC and reverse 518R (Supplemental Table 1). PCR reaction mixtures contained 25 μL of AmpliTaq Gold PCR Master Mix (Applied Biosystems, Tokyo, Japan), 5 μL of extracted DNA, and 2.5 μL of both primers (10 μM); sterile distilled water was added to a final volume of 50 μL. DNA extracted from the ARD sample was used without dilution. PCR was performed using a thermal cycler (Takara Bio Inc., Shiga, Japan) and the cycling conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 30 cycles of denaturation at 95 °C for 15 s, primer annealing at 55 °C for 15 s, and primer extension at 72 °C for 15 s, with final extension at 72 °C for 7 min. To analyze the archaeal community in the ARD sample, 16S rRNA gene fragments were amplified by PCR using the primers ARC519R and ARC344F-GC (Supplemental Table 1), using the same composition of the PCR mixture and PCR cycling conditions as those described above. PCR products were visualized by agarose gel electrophoresis and purified using the GeneClean kit (Qbiogene, Carlsbad, CA, USA).
GC-clamp PCR products were separated according to their sequences using the DCode Universal Mutation System (Bio-Rad Ltd, Tokyo, Japan). Samples were applied onto 10% polyacrylamide gradient gels in the buffer containing 20 mM Tris, 10 mM acetate, and 0.5 mM EDTA-2Na (pH 8.0). Denaturing gradient gels were prepared with 30–60% urea. Denaturing gradient gel electrophoresis (DGGE) was conducted using constant voltage of 130 V for 12 h at 60 °C. After electrophoresis, the gels were stained with SYBR Green I (Cambrex Bio Science Inc., Rockland, ME, USA) and photographed under UV illumination. Select bands were excised from the gels, and each band was incubated in 10 μL of sterile water at 4 °C overnight to allow DNA diffusion. PCR reactions were carried out with the extracted DNA as a template and the primer pairs 341F (without GC-clamp) and 518R for bacterial 16S rDNA sequencing, or ARC344F (without GC-clamp) and ARC519R for archaeal 16S rDNA sequencing. The reaction products were resolved by agarose gel electrophoresis and purified using the GeneClean kit. Nucleotide sequences of the purified PCR products were determined by using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems Inc., Foster City, CA, USA).
PCR with specific primers
Specific primers were used to amplify fragments of 16S rRNA genes of A. ferrooxidans, A. thiooxidans, A. caldus, Leptospirillum spp., or Sulfobacillus spp. in the ARD sample. Oligonucleotide sequences used for amplifying the specific bacterial 16S rRNA genes are listed in Supplemental Table 1. PCR reaction mixtures contained 5 μL of AmpliTaq Gold PCR Master Mix, 1 μL of extracted DNA, 0.5 μL of both primers (10 μM), and sterile distilled water (up to 10 μL). Five different annealing temperatures were used to amplify rRNA genes and the cycling conditions were as follows: initial denaturation for 10 min at 95 °C; followed by 30 cycles each of 15 s at 95 °C, 15 s at 42, 48, 52, 55, or 58 °C, and 15 s at 72 °C; and final extension for 7 min at 72 °C. Amplification products were visualized by agarose gel electrophoresis as described above.
Sequence analysis
Partial 16S rDNA sequences were analyzed using BLAST search algorithm to estimate the degree of similarity to other rRNA gene sequences obtained from the BLAST program of the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov). Least-squares distance matrix analysis based on evolutionary distances was performed using Kimura’s correction.Citation16) The initial sequence alignment was performed using the DNA Data Bank of Japan (DDBJ) ClustalW System. The phylogenetic tree was constructed from evolutionary distance data by the neighbor-joining method using the DDBJ ClustalW. Bootstrap analyses of 1000 replicates were used to estimate the reproducibility of tree topologies.
Nucleotide sequence accession numbers
Partial 16S rRNA gene sequences obtained from DNA fragments by DGGE analysis in this study were deposited at DDBJ/EMBL/GenBank under the accession numbers AB858435–AB858450.
Results
Characterization of ARD
Our previous research analyzing microbial community in ARD from the main tunnel of the Yanahara mine revealed that the microbial population was highly contaminated with micro-organisms from Yoshii River streaming over the tunnel of the Yanahara mine.Citation13) Therefore, the structure of microbial community involved in the generation of ARD in the Yanahara mine was not clearly determined. To elucidate the microbial community involved in the generation of ARD, we collected an ARD sample from the Yasumi-ishi tunnel–one of the tunnels in the Yanahara mine located higher than Yoshii River (Supplemental Figs. 1–3). Physicochemical characteristics of ARD are shown in Table . ARD from the main tunnels of the Yanahara mine was previously characterized to be moderately acidic (pH 3.6) and to contain high iron concentration (1200 mg/L).Citation13) ARD from the Yasumi-ishi tunnel was also characterized to be moderately acidic (pH 4.1), but the concentration of iron (51 mg/L) was significantly lower than that in ARD from the Yanahara mine main tunnels.
Table 1. The composition of the ARD sample of Yasumi-Ishi tunnel.
Phylogenetic affiliation of 16S rRNA genes isolated from ARD
The PCR products amplified using 341F-GC and 518R primers were used to analyze the microbial community in the ponds of the Yasumi-ishi tunnel. The ARD microbial community was profiled by DGGE of the amplified 16S rRNA gene fragments (Fig. (A)). According to the DGGE profile, the community structure was relatively simple. We tried to determine nucleotide sequences of all the DNA fragments detected by DGGE, but minor or insufficiently separated bands could not be sequenced. Using BLAST search based on 8 nucleotide sequences, the closest relative of each of the clones was identified (Table ). The phylogenetic tree based on comparative sequence analysis of the clones and their close relatives is shown in Fig. .
Fig. 1. DGGE profiles of the V3 variable region of bacterial (A) and archaeal (B) 16S rRNA genes from the ARD sample of the Yasumi-Ishi tunnel.
Note: Bands with a name were identified by sequencing and sequence comparison. Results of the identification are summarized in Table
Table 2. BLAST results of the bacterial 16S rRNA gene sequences from the ARD of Yasumi-Ishi tunnel.
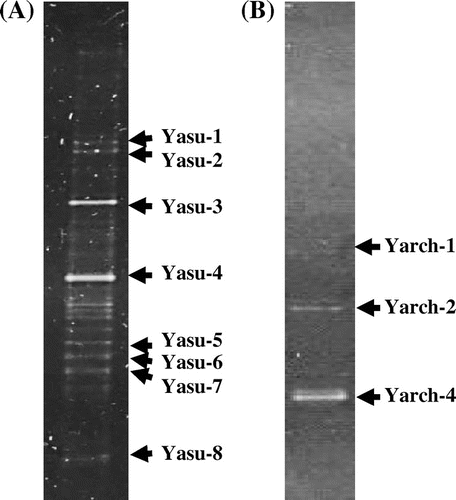
Fig. 2. Phylogenetic relationships of bacterial 16S rRNA gene sequences from the ARD sample of the Yasumi-Ishi tunnel to closely related sequences from GenBank.
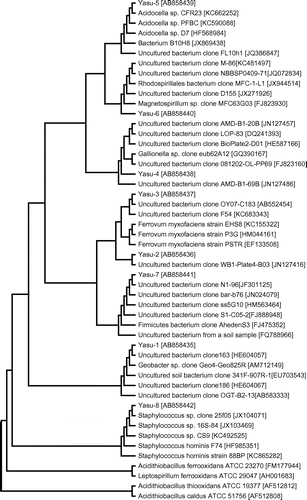
The DGGE analysis revealed that Yasu-4 was the most dominant clone in the ARD bacterial community closely related to uncultured bacterium clones; AMD-B1-20B (JN127457; 99%) found in AMD of the Tab-Simco coal mine (pH 3.09) located near Carbondale, IL, USACitation17); BioPlates2-D01 (HE587166; 99%) detected in an AMD site of the California Coast Range, USA; and LOP-83 (DQ241393; 99%) isolated in a laboratory reactor removing arsenic from mine drainage water.Citation18) Yasu-4 was loosely related to Gallionella sp. clone eub62A12 (GQ390167; 98%) detected in Lake Pavin, a low-sulfate-containing lake in the French Massif central area (Fig. ).Citation19)
The second most dominant clone in the ARD bacterial community was Yasu-3, which was closely related to an uncultured clone OY07-C183 (AB552454; 100%) detected in bacterial communities of volcanic deposits (pH 3.0–3.6) on Miyake Island, Japan,Citation20) and was also loosely affiliated with Ferrovum myxofaciens EHS8 (KC155322, 96%) (Fig. ).
Yasu-2 showed 96% identity to Yasu-3 and was also found to belong to this cluster. Yasu-2 was closely related to the environmental clone (JN127416, 99%) found in AMD from Well B-1 (pH 3.12; Fe, 33.5 mg/L) of the Tab-Simco coal mine located near Carbondale, IL, USA,Citation18) and loosely related to F. myxofaciens EHS8 (KC155322, 95%) and F. myxofaciens PSTR (EF133508; 95%), which were detected in acidic metal-rich water in North Wales, UK (Fig. ).Citation21)
Yasu-1 showed high sequence identity (100%) with a Geobacter sp. clone (HE604057) detected in the iron snow from an acidic lake (pH 3.3–4.0) in the Lusatian mining area in east-central Germany, and Geobacter sp. clone (AM712149; 99%) detected in slightly acidic zones (pH 5.0) of coal mining-associated lake sediments.Citation22) Geobacter sp. is thought to be involved in the reduction in Fe(III) at a broad pH range (pH 5.5–8.1).Citation23)
Yasu-5 was closely related to Acidocella sp. D7 (HF568984; 100%) detected in AMD of the Carnoulès Pb–Zn mine in southern France (pH 2.39–5.5; Fe, 12–25 mM),Citation24) and B10H8 (JX869438, 100%) detected in acid sulfate soil (pH 3.7–4.2) from the Risöfladan experimental field, Ostrobothnia, Finland.Citation25)
Yasu-6 was related to Magnetospirillum sp. clone MFC63G03 (FJ823930, 98%), which has beendetected earlier in the microbial consortium enriched in the anode chamber of microbial fuel cells.Citation26) Yasu-6 was also related to a cultured clone (KC481497, 98%) detected in microbial fuel cells used for sulfide removal and electricity generation and a Rhodospirillales sp. clone MFC-1-L1 (JX944514, 98%), which has been detected in an iron-reducing enrichment culture.
Yasu-7 was related to a clone (FQ788966; 99%) isolated from ferralsol in Madagascar by 16-day laboratory incubation in the presence of wheat-straw and tropical peregrine endogenic earthworms. Although Firmicutes sp. clone (FJ475352; 99%) belongs to this cluster, its affiliation with Firmicutes may be uncertain. Almost all the clones showing high sequence identity with Yasu-7 were not affiliated with any genus and originated from non-acidic soil environments.
Yasu-8 was closely related to Staphylococcus hominis strain 88BP (KC865282; 100%), a heterotrophic and neutrophilic bacterium.
Detection of iron- or sulfur-oxidizing bacteria by PCR using specific primers
Iron-oxidizing A. ferrooxidans and L. ferrooxidans and sulfur-oxidizing A. thiooxidans and A. caldus have been frequently detected in ARD. However, the 16S rRNA gene sequences of these bacteria were not found among the samples examined. Since minor or insufficiently resolved bands in denatured gradient gel were not sequenced, specific primer pairs for iron- or sulfur-oxidizing bacteria were used to examine the presence of these bacteria in ARD. When DNA extracted from ARD was directly used for amplification of the 16S rRNA genes of A. ferrooxidans, A. thiooxidans, and A. caldus, no corresponding DNA fragments were detected by agarose gel electrophoresis, regardless of the different annealing temperatures used in PCR reactions (Supplemental Fig. 4). It was possible that the concentration of these bacteria in ARD was too low for detection by PCR. However, no fragments were also amplified when the first PCR reaction was carried out using the primer pair 27F and 1492R, and then used as the template for the second PCR reaction with specific primer pairs.
Phylogenetic analysis of archaea and community structure
To determine the phylogenetic diversity of archaea in the ARD sample, 16S rRNA gene fragments were amplified using the primer pair ARC344F-GC and ARC519R, and analyzed by DGGE (Fig. (B)). Comparative analysis of the three sequences determined is summarized in Table and presented as phylogenetic tree shown in Fig. . The major archaeon clone Yarch-4 was closely related to the following: the clone ArCoSdN9H67 (HE653795; 100%), which was isolated from arsenic-rich creek sediments of the Carnoulès mine, FranceCitation10); an uncultured archaeon (KC537594; 100%), detected in AMD of the TongLing pyrite mine, Anhui Province, China; and the clone DAAP3A2 (KC208501; 100%), detected in acidic metal-contaminated stream drainage from an abandoned underground copper mineCitation27); and was affiliated with Thermoplasmatales spp (Fig. ).
Table 3. BLAST results of the archaeal 16S rRNA gene sequences from the ARD of Yasumi-Ishi tunnel.
Fig. 3. Phylogenetic relationships of archaeal 16S rRNA gene sequences from the ARD sample of the Yasumi-Ishi tunnel to closely related sequences from GenBank.
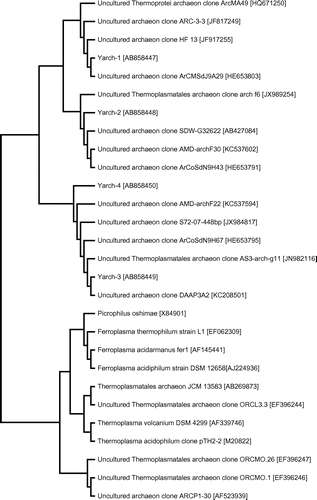
Yarch-2 was the second dominant archaeon related to the following clones: SDW_G32622 (AB427084; 98%) found in nitrifying acid-sulfate soil (pH 3.5) from an abandoned paddy field in ThailandCitation28); AMD-archF30 (KC537602; 98%) detected in AMD of the TongLing pyrite mine, Anhui Province, China; ArCoSdN9H43 (HE653791; 98%) detected in arsenic-rich creek sediments of the Carnoulès mine.Citation10) These clones were also loosely related to Thermoplasmatales spp (Fig. ).
Yarch-1 was closely related to the clone HF13 (JF91255; 100%) detected in strongly acidic agricultural soils (pH 4.20–4.47) across southern China,Citation29) clone ArCMSdJ9A29 (HE653803; 100%) detected in arsenic-rich creek sediments of the Carnoulès mine, France,Citation10) and clone GBX-ACOQ1-14 (JF280342; 100%) from an acidic hot spring in Colombian Andes.Citation30) These clones have not been related to any known species of archaea.
Microscopic observation of sediment from the pond
A Gallionella-like clone was detected as the dominant bacteria in the microbial community from ARD. Gallionella is known to produce uniquely twisted extracellular iron oxide-encrusted bundle of fibers (commonly called a twisted stalk).Citation31) When sediment sample from the pond was analyzed by light microscopy, some twisted stalk-like structures were observed (Supplemental Fig. 5).
Discussion
The microbial community from the ARD pond of the Yasumi-ishi tunnel that was not contaminated by the bacteria from Yoshii River was analyzed by PCR-DGGE to determine micro-organisms involved in the generation of moderately acidic ARD. The DDGE analysis revealed that 16S rRNA gene sequences from Acidithiobacillus strains (A. ferrooxidans, A. thiooxidans, and A. caldus) and Leptospirillum species were not detected in the samples. A. ferrooxidans and Leptospirillum species are well-known acidophiles contributing to ARD or AMD generation and are known to be dominant in strongly acidic environments. It has been reported that Leptospirillum species constitute a minor component of the microbial community or are absent in AMD with pH above 2.5.Citation32–34) Therefore, the absence of these bacteria in the ARD sample with pH 4.1 from the Yasumi-ishi tunnel was not surprising.
The most dominant 16S rRNA gene clone Yasu-4 was related to a Gallionella-like clone. The Gallionella 16S rRNA gene-containing clone was initially reported in an in situ reactor system treating monochlorobenzene-contaminated groundwater.Citation35) To date, many studies reporting the detection of Gallionella 16S rRNA gene-containing clones in acidic environments have been published,Citation21,36–38) but the isolation of Gallionella-like species from acidic environments has not yet been successful. Since Gallionella spp. are known as neutrophilic and microaerophilic iron-oxidizing bacteria,Citation39) Hallberg suggested that the Gallionella-like species detected in mine waters were a new species of acidophilic iron-oxidizing bacteria.Citation40) Our preliminary experiments showed the oxidation of ferrous iron and the enrichment of both Ferrovum- and Gallionella-like clones in the medium for autotrophic iron-oxidizers, strongly suggesting the iron-oxidizing activity of the Gallionella-like species. Since microscopic observation revealed the presence of twisted stalk-like structures in the sediment from the pond (Supplemental Fig. 5), it can be suggested that the Gallionella-like species detected in ARD may also produce such a twisted stalk.
The second most abundant group of micro-organisms in the pond is closely related to F. myxofaciens PSTR.Citation21) It has been shown that this organism could grow autotrophically using ferrous iron as an electron donor and oxygen as an electron acceptor.Citation41,42) Since clones closely related to F. myxofaciens or Gallionella-like bacteria were detected in the ARD sample from the Yasumi-ishi tunnel, these organisms might be the main bacterial groups involved in ARD generation in the Yasumi-ishi tunnel.
The presence of archaea including sulfur- and/or iron-oxidizers, such as members of Sulfolobus, Acidianus, Metallosphaera, Sulfurisphaera, or Ferroplasma genera, has also been reported in ARD, AMD, and other acidic environments. Although these archaea were not present among the archaeal clones of the Yasumi-ishi tunnel ARD, members of the order Thermoplasmatales were detected as the major constituent of the archaeal community. This order is represented by thermoacidophilic micro-organisms, which often derive energy from iron oxidation, sulfur oxidation, or reduction.Citation43) This order contains three families: Thermoplasmataceae, Picrophilaceae, and Ferroplasmaceae.Citation44) Thermoplasmataceae comprises of species like Thermoplasma acidophilum, which couples the oxidation of organic carbon with the reduction in elemental sulfur, whereas Ferroplasmaceae comprises of iron-oxidizing chemolithotrophs such as Ferroplasma acidiphilium. These archaea have been detected in highly acidic environments (pH 1.0).Citation45) Yarch-2 and Yarch-4 were loosely affiliated with the Thermoplasmatales clones, which have been also detected in moderately acidic environments (pH 2.91–3.28).Citation10) Because the isolation of many environmental archaea is currently not possible, the physiological features and ecological significance of Yarch-2 and Yarch-4 detected in this study remain to be determined. These archaeal sequences were only loosely related to the known archaea species, suggesting that these archaea are highly adaptive to moderately acidic environments and are involved in iron/sulfur oxidation or reduction in their habitats. Recently, analysis of microbial diversity patterns in physically and geochemically diverse AMD sites across Southeast China showed that overall microbial diversity largely correlated with pH condition.Citation46) β-Proteobacteria affiliated with a Ferrovum-like clone was predominant in microbial communities existing under moderate pH conditions (pH > 2.4), and our results are consistent with these findings. In addition to the presence of Ferrovum-like and Gallionella-like bacteria in moderately acidic environments, the existence of Thermoplasmatales spp. specifically adapted to moderately acidic conditions are strongly suggested in this study.
On the basis of the deduced physiological characteristics of the various clones detected by DGGE analysis, a model for iron and sulfur cycling pathways occurring in the microbial community structure in the pond of the Yasumi-ishi tunnel may be drawn (Fig. ). Since our preliminary experiment for the enrichment of autotrophic iron-oxidizers revealed the enrichments of both Ferrovum- and Gallionella-like species, we propose that these bacteria are iron oxidizers in the pond. The ferric iron can be reduced to a certain extent, either inorganically by pyrite or biologically by iron-reducing Acidocella, Geobacter, and Magnetospirillum spp. Chemolithotrophic iron oxidizers could provide the organic carbon sources for the heterotrophic iron and sulfate reducers via CO2 fixation. Judging by the composition of micro-organisms detected in the ARD sample, there was no clear candidate for a sulfur oxidizer and the reaction is likely to proceed abiotically. Yarch-4 and Yach-2 affiliated with Thermoplasmatales archaea may be involved in the oxidation of sulfur compounds. The role of heterotrophic neutrophilic clones Yasu-7 or Yasu-8 in the generation of the ARD remains unclear.
Fig. 4. Model showing the roles of the different microorganisms identified in the pond of Yasumi-Ishi tunnel.
Note: Microorganisms are shown with their roles in the iron and sulfur cycles.
Ferrovum or Gallionella bacteria in moderately acidic environments are thought to be useful microbial candidates for developing cost-effective treatment system for ARD and AMD. A pilot plant where Ferrovum and Gallionella dominated the microbial community and accelerated the oxidation of ferrous iron has been described.Citation38) Although pure cultures of Ferrovum-like or Gallionella-like bacteria from moderately acidic environments have not been established, a new cultivation medium for enrichment of these strains has recently been reported.Citation47) We are also trying to isolate Ferrovum-like or Gallionella-like species from ARD of the Yasumi-ishi tunnel. In future, the system based on Ferrovum spp. and Gallionella spp. may be developed and applied in mine sites for the treatment of ARD and AMD.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.915735.
Supplementary Figures
Download PDF (842.1 KB)Acknowledgments
We are grateful to Fumio Akahori from Unekura Mining Co. Ltd., Akita, Japan, for his cooperation in sampling ARD from the Yanahara mine. This study was financially supported by the Special Grant for Education and Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan [grant number 23580458].
References
- Banks D, Younger PL, Arnesen R-T, Iversen ER, Banks SB. Mine-water chemistry: the good, the bad and the ugly. Environ. Geol. 1997;32:157–174.10.1007/s002540050204
- Grande JA, Beltrán R, Sáinz A, Santos JC, de la Torre ML, Borrego J. Acid mine drainage and acid rock drainage processes in the environment of Herrerías mine (Iberian pyrite belt, Huelve-Spain) and impact on the Andevalo dum. Environ. Geol. 2005;47:185–196.10.1007/s00254-004-1142-9
- Baker BD, Banfield JF. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003;44:139–152.10.1016/S0168-6496(03)00028-X
- Fowler TA, Holmes PR, Crundwell FK. Mechanism of pyrite dissolution in the presence of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1999;65:2987–2993.
- Rawlings DE. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Fact. 2005;4:13. Available from: http://www.microbialcellfactories.com/content/4/1/13.10.1186/1475-2859-4-13
- Rohwerder T, Gehrke T, Kinzler K, Sand W. Bioleaching review part A: progress in bioleaching: fundamentals and mechanism of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003;63:239–248.10.1007/s00253-003-1448-7
- Dopson M, Baker-Austin C, Hind A, Bowman JP, Bond PJ. Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl. Environ. Microbiol. 2004;70:2079–2088.10.1128/AEM.70.4.2079-2088.2004
- Edwards KJ, Bond PL, Gihring TM, Banfield JF. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science. 2000;287:1796–1799.10.1126/science.287.5459.1796
- Golyshina OV, Pivovarova TA, Karavaiko GI, Kondrateva TF, Moore ER, Abraham WR, Lünsdorf H, Timmis KN, Yakimov MM, Golyshin PN. Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the Archaea. Int. J. Syst. Evol. Microbiol. 2000;50:997–1006.10.1099/00207713-50-3-997
- Volant A, Desoeuvre A, Casiot C, Lauga B, Delpoux S, Morin G, Personné JC, Héry M, Elbaz-Poulichet F, Bertin PN, Bruneel O. Archaeal diversity: temporal variation in the arsenic-rich creek sediments of Carnoulès Mine, France. Extremophiles. 2012;16:645–657.10.1007/s00792-012-0466-8
- Goebel BM, Stackebrandt E. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl. Environ. Microbiol. 1994;60:1614–1621.
- Johnson DB, Rolfe S, Hallberg KB, Iversen E. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine. Environ. Microbiol. 2001;3:630–637.10.1046/j.1462-2920.2001.00234.x
- Okabayashi A, Wakai S, Kanao T, Sugio T, Kamimura K. Diversity of 16S ribosomal DNA-defined bacterial population in acid rock drainage from Japanese pyrite mine. J. Biosci. Bioeng. 2005;100:644–652.10.1263/jbb.100.644
- Imai K. Environment cleaning by iron-oxidizing bacteria. KASEAA (in Japanese). 1977;15:299–300.
- Kamimura K, Okabayashi A, Kikumoto M, Manchur MA, Wakai S, Kanao T. Analysis of iron- and sulfur-oxidizing bacteria in a treatment plant of acid rock drainage from a Japanese pyrite mine by use of ribulose-1, 5-bisphosphate carboxylase/oxygenase large-subunit gene. J. Biosci. Bioeng. 2010;109:244–248.10.1016/j.jbiosc.2009.08.007
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequence. J. Mol. Evol. 1980;16:111–120.10.1007/BF01731581
- Burns AS, Pugh CW, Segid YT, Behum PT, Lefticariu L, Bender KS. Performance and microbial community dynamics of a sulfate-reducing bioreactor treating coal generated acid mine drainage. Biodegradation. 2012;23:415–429.10.1007/s10532-011-9520-y
- Fabienne BB, Yann I, Francis G, Fabian D, Catherine C, Catherine G, Catherine J. A Simple biogeochemical process removing arsenic from a mine drainage water. Geomicrobiol. J. 2006;23:201–211.
- Biderre-Petit C, Boucher D, Kuever J, Alberic P, Jézéquel D, Chebance B, Borrel G, Fonty G, Peyret P. Identification of sulfur-cycle prokaryotes in a low-sulfate lake (Lake Pavin) using aprA and 16S rRNA gene markers. Microb. Ecol. 2011;61:313–327.10.1007/s00248-010-9769-4
- Fujimura R, Sato Y, Nishizawa T, Nanba K, Oshima K, Hattori M, Kamijo T, Ohta H. Analysis of early bacterial communities on volcanic deposits on the island of Miyakew (Miyake-jima), Japan: a 6-year study at a fixed site. Microbes Environ. 2012;27:19–29.10.1264/jsme2.ME11207
- Hallberg KB, Coupland K, Kimura S, Johnson DB. Macroscopic streamer growths in acidic, metal-rich mine waters in north Wales consist of novel and remarkably simple bacterial communities. Appl. Environ. Microbiol. 2006;72:2022–2030.10.1128/AEM.72.3.2022-2030.2006
- Blöthe M, Akob DM, Kostka JE, Göschel K, Drake HL, Küsel K. pH gradient-induced heterogeneity of Fe(III)-reducing microorganisms in coal mining-associated lake sediments. Appl. Environ. Microbiol. 2008;74:1019–1029.10.1128/AEM.01194-07
- Cummings DE, March AW, Bostick BS, Spring S, Fendorf S, Rosenzweig RF. Evidence for microbial Fe(III) reduction in anoxic, mining impacted lake sediments (Lake Coeur d’Alene, Idaho). Appl. Environ. Microbiol. 2000;66:154–162.10.1128/AEM.66.1.154-162.2000
- Delavat F, Lett MC, Lièvremont D. Yeast and bacterial diversity along a transect in an acidic, As-Fe rich environment revealed by cultural approaches. Sci. Total Environ. 2013;463–464:823–828.10.1016/j.scitotenv.2013.06.023
- Wu X, Wong ZL, Sten P, Engblom S, Österholm P, Dopson M. Microbial community potentially responsible for acid and metal release from an Ostrobothnian acid sulfate soil. FEMS Microbiol. Ecol. 2013;84:555–563.10.1111/femsec.2013.84.issue-3
- Borole AP, Mielenz JR, Vishnivetskaya TA, Hamilton CY. Controlling accumulation of fermentation inhibitors in biorefinery recycle water using microbial fuel cells. Biotechnol. Biofuels. 2009;2:7. doi: 10.1186/1754-6834-2-7.10.1186/1754-6834-2-7
- Kay CM, Rowe OF, Rocchetti L, Coupland K, Hallberg KB, Johnson DB. Evolution of Microbial ‘Streamer’ Growths in an Acidic, Metal-contaminated Stream Draining an Abandoned Underground Copper Mine. Life. 2013;3:189–211.10.3390/life3010189
- Nakaya A, Onodera Y, Nakagawa T, Satoh K, Takahashi R, Sasaki S, Tokuyama T. Analysis of ammonia monooxygenase and archaeal 16S rRNA gene fragments in nitrifying acid-sulfate soil microcosms. Microbes Environ. 2009;24:168–174.10.1264/jsme2.ME09104
- Zhang L, Hu H, Shen J, He J. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012;6:1032–1045.10.1038/ismej.2011.168
- Bohorquez LC, Delgado-Serrano L, López G, Osorio-Forero C, Klepac-Ceraj V, Kolter R, Junca H, Baena S, Zambrano MM. In-depth characterization via complementing culture-independent approaches of the microbial community in an acidic hot spring of the Colombian Andes. Microb. Ecol. 2012;63:103–115.10.1007/s00248-011-9943-3
- Suzuki T, Hashimoto H, Matsumoto N, Furutani M, Kunoh H, Takada J. Nanometer-scale visualization and structural analysis of the inorganic/organic hybrid structure of Gallionella ferruginea twisted stalks. Appl. Environ. Microbiol. 2011;77:2877–2881.10.1128/AEM.02867-10
- Schrenk MO, Edwards KJ, Goodman RM, Hamers RJ, Banfield JF. Distribution of Thiobacillus ferrooxidans and Leptosprillum ferrooxidans: implication for generation of acid mine drainage. Science. 1998;279:1519–1522.10.1126/science.279.5356.1519
- Tan GL, Shu WS, Zhou WH, Li XL, Lan CY, Huang LN. Seasonal and spatial variations in microbial community structure and diversity in the acid stream draining across an ongoing surface mining site. FEMS Microbiol. Ecol. 2009;70:121–129.
- Kuang JL, Huang LN, Chen LX, Hua ZS, Li SJ, Hu M, Li JT, Shu WS. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J. 2013;7:1038–1050.10.1038/ismej.2012.139
- Alfreider A, Vogt C, Babel W. Microbial diversity in an in situ reactor system treating monochlorobenzene contaminated groundwater as revealed by 16S ribosomal DNA analysis. Syst. Appl. Microbiol. 2002;25:232–240.10.1078/0723-2020-00111
- Bruneel O, Duran R, Casiot C, Elbaz-Poulichet F, Personné JC. Diversity of microorganisms in Fe-As-rich acid mine drainage waters of Carnoulès, France. Appl. Environ. Microbiol. 2006;72:551–556.10.1128/AEM.72.1.551-556.2006
- Kimura S, Bryan CG, Hallberg KB, Johnson DB. Biodiversity and geochemistry of an extremely acidic, low-temperature subterranean environment sustained by chemolithotrophy. Environ. Microbiol. 2011;13:2092–2104.10.1111/j.1462-2920.2011.02434.x
- Heinzel E, Janneck E, Glombitza F, Schlömann M, Seifert J. Population dynamics of iron-oxidizing communities in pilot plants for the treatment of acid mine waters. Environ. Sci. Technol. 2009;43:6138–6144.10.1021/es900067d
- Emerson D, Moyer C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl. Environ. Microbiol. 1997;63:4784–4792.
- Hallberg KB. New perspectives in acid mine drainage microbiology. Hydrometallurgy. 2010;104:448–453.10.1016/j.hydromet.2009.12.013
- Rowe OF, Johnson DB. Comparison of ferric iron generation by different species of acidophilic bacteria immobilized in packed-bed reactors. Syst. Appl. Microbiol. 2008;31:68–77.10.1016/j.syapm.2007.09.001
- Heinzel E, Hedrich S, Janneck E, Glombitza F, Seifert J, Schlömann M. Bacterial diversity in a mine water treatment plant. Appl. Environ. Microbiol. 2009;75:858–861.10.1128/AEM.01045-08
- Reysenbach AL, Cady SL. Microbiology of ancient and modern hydrothermal systems. Trends Microbiol. 2001;9:79–86.10.1016/S0966-842X(00)01921-1
- Itoh T, Yoshikawa N, Takashina T. Thermogymnomonas acidicola gen. nov., sp. nov., a novel thermoacidophilic, cell wall-less archaeon in the order Thermoplasmatales, isolated from a solfataric soil in Hakone, Japan. Int. J. Syst. Evol. Microbiol. 2007;57:2557–2561.10.1099/ijs.0.65203-0
- Edwards KJ, Bond PL, Gihring TM, Banfield JF. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science. 2000;287:1796–1799.10.1126/science.287.5459.1796
- Kuang JL, Huang LN, Chen LX, Hua ZS, Li SJ, Hu M, Li JT, Shu WS. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J. 2013;7:1038–1050.10.1038/ismej.2012.139
- Tischler JS, Jwair RJ, Gelhaar NG, Drechsel A, Skirl AM, Wiacek C, Janneck E, Schlömann M. New cultivation medium for “Ferrovum” and Gallionella-related strains. J. Microbiol. Methods. 2013;95:138–144.10.1016/j.mimet.2013.07.027
