Abstract
Effective HPLC-DAD and HPLC-ESI-MS/MS methods have been developed for the analysis of eight benzo[c]phenanthridine alkaloids (sanguinarine, chelirubine, macarpine, chelerythrine, dihydrosanguinarine, dihydrochelirubine, dihydromacarpine and dihydrochelerythrine), which are important metabolites in Eschscholtzia californica cell culture. By adopting a ternary gradient pump system, the dihydro-form alkaloids hardly separable from each other could be successfully separated, and all the target alkaloids could be simultaneously quantified with the LOD values of 0.01–0.79 μg/mL and the LOQ values of 0.03–3.59 μg/mL. This HPLC-DAD method was further confirmed by HPLC-ESI-MS/MS system in multiple reaction monitoring mode. Each separated HPLC peak was identified as the target alkaloid, showing its relevant ionized molecule and selected fragment ion. By applying the established method, alkaloid production during the E. californica cell culture could be successfully monitored and some valuable information on its metabolism could be deduced.
Graphical Abstract
HPLC-DAD and HPLC-ESI-MS/MS methods have been developed for the analysis of benzo[c]phenanthridine alkaloids and applied to Eschscholtzia californica cell culture monitoring.
Eschscholtzia californica Cham. (California poppy) is an ancient medicinal plant that has been widely used by Native Americans. This plant produces several benzo[c]phenanthridine alkaloids such as sanguinarine, chelerythrine, chelirubine, and macarpine, which could be used as raw materials in the pharmaceutical industry. Sanguinarine, chelirubine, and macarpine are known to show antimicrobial activity through inhibiting DNA synthesis of external pathogens.Citation1) Chelerythrine, a potential inhibitor of protein kinase C, also has an anti-inflammatory activity.Citation2−4) Along with increasing demand of benzo[c]phenanthridine alkaloids in the pharmaceutical field, there have been numerous studies on California poppy cell culture system, which is potentially capable of producing the target alkaloids in large scale.Citation5−7) As a natural consequence, analytical method to effectively determine the individual alkaloid contents has become important because their profiles during the cell culture might provide critical information to the metabolic pathway involved in the alkaloid production.
A number of chromatographic studies dealing with the benzo[c]phenanthridine alkaloid analysis are available in the literature.Citation8−11) More recently, there have been also intensive researches additionally exploiting electrospray ionization tandem mass spectroscopy (ESI-MS/MS) because of its excellent ability to identify unknown alkaloids that are hardly distinguishable with HPLC alone.Citation12,13) Most of these studies, however, mainly focused on the alkaloid contents of plant extracts themselves, and as such, little attention was given to dihydrobenzo[c]phenanthridines, which are usually present at low concentration. Considering the potential importance of the dihydro-precursors, accurate quantification is needed to help understand their role in cell culture.Citation14,15) Up until now, there are only a few reports that provide an analytical method to determine the dihydro-form alkaloids and their end products simultaneously.Citation16,17)
In this study, we present a series of methods to analyze four benzo[c]phenanthridine alkaloids (sanguinarine, chelerythrine, chelirubine, macarpine) and their dihydrobenzo[c]phenanthridine precursors, which are the major intracellular metabolites produced in California poppy cell culture. First, we developed an effective HPLC-DAD (photodiode array detector) method where nonconventional ternary pump system was adopted in order to achieve good peak separations. By modifying the HPLC method towards preparative HPLC, we also successfully fractionated two alkaloids (chelirubine and macarpine), of which standard chemicals cannot be purchased in commercial marketplace. Finally, we established a HPLC-ESI-MS/MS method that could confirm the target alkaloids with enhanced sensitivity and accuracy. The quantities of the target alkaloids were then determined throughout the growth period of the California poppy cell culture.
Experimental
Standard chemicals for benzo[c]phenanthridine alkaloids
Sanguinarine and chelerythrine were purchased from Sigma Chemical Co. (USA). Chelirubine and macarpine were obtained directly from California poppy cell extracts through the following procedures: the target alkaloids were first fractionated by Prep-LC as will be shown later; crystallization of alkaloid was induced by adding excess amount of sodium chloride into each fractionated solution; after overnight storage at −20 °C, the supernatant was removed by centrifugation; the precipitate containing excess sodium chloride as well as the target alkaloid was then dissolved into methanol solution (100%); the supernatant containing the alkaloid only was finally dried so as to obtain the pure alkaloid. Dihydrosanguinarine, dihydrochelerythrine, dihydrochelirubine, and dihydromacarpine were chemically synthesized from the above four standard compounds through the method proposed by Howell et al.Citation18)
The identity and purity of the six isolated or synthesized standard chemicals were checked using NMR (BRUKER AVANCE III 600 spectrometer, BRUKER Corporation, German). Each standard sample showed 1H-NMR spectra relevant to the target alkaloid and only negligible amounts of impurities were present in the sample. The detailed NMR spectra for the standard chemicals are as follows (see Fig. for carbon number): for chelirubine, 1H NMR (300 MHz, CD3OD) δ 4.21 (3H, O-Me, s), 4.84 (3H, N-Me, s), 6.26, 6.48 (2H, OCH2O, 2 x s), 7.75 (1H, H-1, s), 8.01 (1H, H-9, s), 8.17 (1H, H-4, s), 8.22 (1H, H-12, d, J = 9 Hz), 9.45 (1H, H-11, d, J = 9 Hz), 9.89 (1H, H-6, s); for macarpine, 1H NMR (600 MHz, CD3OD) δ 4.13 (3H, 10-OMe, s), 4.15 (1H, 12-OMe, s), 4.93 (3H, N-Me, s), 6.17, 6.32 (2H, OCH2O, 2 x s), 7.78 (1H, H-1, s), 7.97 (1H, H-9, s), 8.22 (1H, H-4, s); for dihydrosanguinarine, 1H NMR (600 MHz, CD3OD) δ 2.56 (3H, N-Me, s), 4.16 (1H, H2–6, s), 6.04, 6.06 (2H, OCH2O, 2 x s), 6.85 (1H, H-9, d, J = 8.4 Hz), 7.12 (1H, H-1, s), 7.48 (1H, H-12, d, J = 8.4 Hz), 7.69 (1H, H-11, d, J = 8.4 Hz); for dihydrochelerythrine, 1H NMR (600 MHz, CD3OD) δ 2.57 (3H, N-Me, s), 3.88 (3H, 7-OMe, s), 3.93 (3H, 8-OMe, s), 4.27 (1H, H2–6, s), 6.06 (2H, OCH2O, s), 7.11 (1H, H-1, s), 7.51 (1H, H-10, d, J = 8.4 Hz), 7.68 (1H, H-4, s) 7.75 (1H, H-11, d, J = 8.4 Hz); for dihydrochelirubine, 1H NMR (600 MHz, CD3OD) δ 2.57 (3H, N-Me, s), 3.87 (3H, O-Me, s), 4.07 (1H, H2–6, s), 5.98, 6.02 (2H, OCH2O, 2 x s), 6.71 (1H, H-9, s), 7.10 (1H, H-1, s), 7.43 (1H, H-12, d, J = 8.4 Hz), 7.71 (1H, H-4, s), 8.27 (1H, H-11, d, J = 8.4 Hz); for dihydromacarpine, 1H NMR (600 MHz, CD3OD) δ 2.52 (3H, N-Me, s), 3.87 (3H, 10-OMe, s), 4.03 (1H, 12-OMe, s), 4.06 (1H, H2–6, s), 5.98, 6.02 (2H, OCH2O, 2 x s), 6.69 (1H, H-9, s), 7.32 (1H, H-1, s), 7.62 (1H, H-4, s), 7.82 (1H, H-11, s).
Fig. 1. Biosynthetic Pathway and Chemical Structures of the eight Target Benzo[c]phenanthridine Alkaloids in California Poppy.
Notes: Solid arrow stands for a single-step enzyme reaction and dotted arrows represent a multiple-step one (adapted from Ref.Citation20).
![Fig. 1. Biosynthetic Pathway and Chemical Structures of the eight Target Benzo[c]phenanthridine Alkaloids in California Poppy.Notes: Solid arrow stands for a single-step enzyme reaction and dotted arrows represent a multiple-step one (adapted from Ref.Citation20).](/cms/asset/fa93f4bd-54c5-401c-be74-56e62363e4df/tbbb_a_917264_f0001_b.gif)
Metabolites analysis by HPLC-DAD
California poppy cells were sampled from suspended culture and lyophilized in an E-tube for 8 h. Then, each sample was resuspended in acidic methanol (0.2% (v/v) HCl), sonicated in an ultrasonic bath for 60 min, and centrifuged for 15 min to separate supernatant from cell debris. The supernatant containing alkaloids were filtered through a 0.2 μm sterile PES filter (Whatman International Ltd, England) and subjected to HPLC analysis.
HPLC analysis was conducted using a HPLC system (Waters 996 PDA HPLC system, Waters, Milford, MA) coupled with a reversed-phase C18 column (5 μm, 250 × 4.6 mm, DISOGEL 120 ODS-BP, DAISO, Japan) and photodiode array detector (DAD). In this study, a nonconventional ternary gradient pump system consisting of eluent A, B, and C was adopted in order to achieve good peak separations. Eluent A was prepared by mixing water and a commercial tetrabutylammonium hydroxide solution (40 wt% in water, Sigma–Aldrich, USA) [99.8:0.2 v/v, pH 2.5 with H3PO4]. Pure acetonitrile and methanol (HPLC grade) were used as eluent B and C, respectively. The following linear gradient was adopted: 70:10:20 [A:B:C v/v] at time 0; to 30:50:20 over 15 min; to 10:33:57 over 15 min; to 5:48:47 over 2 min; to 1:10:89 over 3 min, to 1:5:94 over 10 min, to 70:10:20 over 5 min. The flow rate was maintained at 1.0 ml/min and the sample injection volume was 30 μL. DAD spectral data were acquired from 210 to 580 nm but metabolite quantification was performed at 283 nm, which is the maximum UV absorbance wavelength of sanguinarine.Citation19) Each alkaloid was identified by comparing both retention time and spectral data with that of the authentic standard.
Preparative HPLC condition
Preparative HPLC conditions were modified from the aforementioned HPLC conditions. Eluent A composition was changed to H2O:tetrabutylammonium hydroxide solution [99.875:0.125, v/v]. Compounds were separated with a linear gradient starting at 70:20:10 [A:B:C, v/v] and changing to 30:50:20 over 20 min, to 20:40:40 over 15 min, to 13:50:37 over 5 min, to 5:65:30 over 5 min, to 2:88:10 over 10 min, to 70:20:10 over 20 min. Total flow rate of 25.0 ml/min was maintained.
Analytical conditions for HPLC-ESI-MS/MS
The aforementioned HPLC conditions were also applied to HPLC-ESI-MS/MS system (ACQUITY TQD, Waters Inc., USA) only with a change in eluent A composition: phosphoric acid used for pH adjustment was replaced with formic acid and the tetrabutylammonium hydroxide solution was not used. The positive ion electrospray ionization (ESI(+)) mass spectra were obtained with the following mass spectrometer source conditions of 1.9 kV electrospray voltage and 250 °C heated capillary temperature. Nitrogen gas (>99.999%) was used for nebulization (50 L/h flow rate, 120 °C source temperature, 0.1 V RF lens voltage, 3 V extractor voltage) and desolvation (350 L/h flow rate, 350 °C desolvation temperature). Argon (>99.999%) was used as the collision gas (collision pressure: 1.31 × 10−5 Torr) for fragmentation. The cone voltages and collision energies adopted to detect the target compounds are shown in Table . The measured mass range was m/z 100–450, which was determined by considering the molecular weights of benzylisoquinoline and benzo[c]phenanthridine alkaloids.
Plant cell and culture conditions
California poppy calli were induced on solid culture medium at room temperature of 25 °C with no illumination. The solid culture was maintained on Linsmier and Skoog (LS) medium (Duchefa Biochemie, Netherlands) containing 30 mg/L sucrose, 5 mg/L phytoagar (Duchefa Biochemie, Netherlands), Gamborg B5 vitamins (Duchefa Biochemie, Netherlands), 1.0 mg/L 2,4-dichlorophenoxy acetic acid (2,4-D), and 0.5 mg/L naphthalenacetic acid (NAA). Subcultures were carried out every 4 weeks. Callus was transferred to liquid medium containing 30 mg/L sucrose, LS medium with vitamins (Duchefa Biochemie, Netherlands), 1.0 mg/L 2,4-D, and 0.5 mg/L NAA.Citation16) Suspended cells were cultured at 120 rpm on a rotary shaker and room temperature. Subcultures were carried out every two weeks. All media were autoclaved at 121 °C for 30 min. All chemicals not mentioned above were purchased from Sigma-Aldrich (USA).
Results and Discussion
Chromatographic separation and DAD detection of target alkaloids
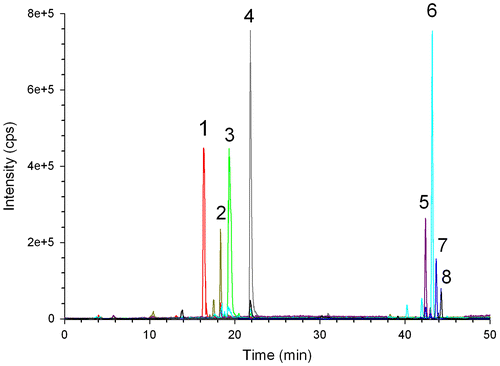
Fig. represents the structures of eight target alkaloids together with their locations in the metabolic pathway proposed by Oldham et al.Citation15,20) As can be seen, four dihydrobenzo[c]phenanthridines are the precursors of their relevant oxidized forms (end products) which have well known commercial values. The oxidized-forms of dihydrobenzo[c]phenanthridines could be easily separated using HPLC conditions described by Chen et al.Citation8) However, the separation of dihydrobenzo[c]phenanthridines was extremely difficult, which might be caused by their subtle differences in hydrophobicity. To improve peak separation, we adopted the ternary pump system where elution strength was more fine-tuned by independently adjusting the ratio of eluent A, B, and C. In this way, eight target alkaloids could be successfully separated both in a standard sample and in a real sample (Fig. ). It should be noted that tetrabutylammonium hydroxide was used in the eluent A in order to achieve improved peak separations.Citation21) Its pH was also adjusted to 2.5 so that all the benzo[c]phenanthridine alkaloids could be converted to their iminium forms, which would result in the increased UV absorbance intensities.Citation19)
Fig. 2. HPLC-DAD Analysis Results of a Standard Sample (A) and a Real Sample Obtained from California Poppy Cell Culture (B).
Notes: 1 sanguinarine; 2 chelerythrine; 3 chelirubine; 4 macarpine; 5 dihydrochelerythrine; 6 dihydrochelirubine; 7 dihydrosanguinarine; 8 dihydromacarpine.
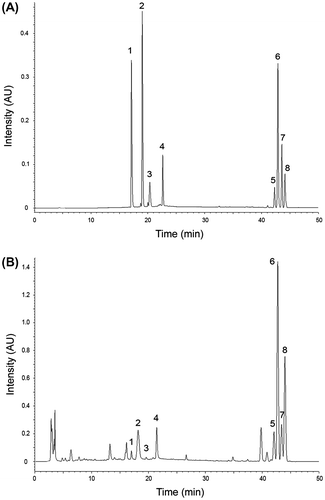
In HPLC-DAD system, each benzo[c]phenanthridine alkaloid peak was characterized by both the retention time and the characteristic peak wavelengths of UV absorbance spectra. The observed retention times and the characteristic peak wavelengths of the alkaloids were provided in Table . In general, the characteristic peak wavelengths of sanguinarine, chelerythrine, chelirubine and macarpine were coincident with the results of Chen et al.Citation8) However, it should be noted that the observed maximum UV wavelength (λmax = 273.9 nm) of sanguinarine was the value for its iminium form. With increasing pH, this iminium form sanguinarine is changed into alkanolamine form and shows a maximum peak at 283 nm.Citation19) The characteristic peak wavelengths of the dihydro-form alkaloids were as follows: dihydrochelerythrine, 236.0, 281.0 nm; dihydrochelirubine, 237.2, 278.6, 337.0 nm; dihydrosanguinarine, 232.4, 283.4, 321.5 nm; dihydromacarpine, 217.1, 283.4, 339.4 nm.
Table 1. Characteristics of the Alkaloids Determined by HPLC-DAD Analysis.
The calibration curves of the standards obtained by linear plots showed good linearities with the correlation coefficients over 0.99 for all the target alkaloids (Table ). The LOD values were 0.01, 0.61, 0.76, 0.79, 0.24, 0.11, 0.09, and 0.06 μg/mL for sanguinarine, chelerythrine, chelirubine, macarpine, dihydrosanguinarine, dihydrochelerythrine, dihydrochelirubine, and dihydromacarpine, respectively. The LOQs were found to range 0.03–3.59 μg/mL for the standard compounds.
Peak identification of the alkaloids by HPLC-ESI-MS/MS
In order to confirm the separated alkaloids, HPLC-ESI-MS/MS analysis was also performed using multiple reaction monitoring (MRM) mode, which could accurately discriminate our target compounds by detecting not only their ionized molecules but also their selected fragment ions. Table shows the operating conditions for the MRM mode, which was determined based on the MS or MS/MS spectral data of the standard alkaloids. First, we determined the optimum cone voltages to detect the ionized molecules using MS scan mode. The ionized molecules of the eight standard alkaloids were detected at the following m/z values: sanguinarine 332.3 [M]+; chelerythrine 348.5 [M]+; chelirubine 362.4 [M]+; macarpine 392.5 [M]+; dihydrosanguinarine 334.4 [M+H]+; dihydrochelerythrine 350.5 [M+H]+; dihydrochelirubine 364.6 [M+H]+; dihydromacarpine 394.6 [M+H]+. When cell extract samples were checked, MS spectra similar to those of the standards could be obtained at the relevant HPLC retention times (Supplemental Figure 1; see http://dx.doi.org/10.1080/09168451.2014.917264), which implies that each peak separated by HPLC at least contained a compound that has the same molecular weight with the target alkaloid. The given optimum cone voltage for each alkaloid was selected at the maximum intensity of the corresponding MS peak.
Table 2. Operating Conditions of ESI-MS/MS for the Detection of Eight Target Alkaloids.
The single MRM transitions adopted in the MRM mode were determined by inspecting the MS/MS spectra of the target alkaloids. After collision-induced dissociation (CID) of each ionized molecule, numerous fragment ions were generated depending on the molecular structure and detected in the MS/MS spectrum (Fig. ). In general, all our target alkaloids showed a CID mechanism corresponding to the neutral loss of their methyl group (15 Da) at the N position.Citation22) For example, demethylated sanguinarine ion [M-CH3]+ was detected at m/z 317.1 while demethylated dihydrosanguinarine ion [M+H-CH3]+ was detected at m/z 319.0 (Fig. (A) and (B)). Another important CID mechanism was the neutral loss of a methoxy group (31 Da).Citation22) Chelirubine can lose the methoxy-group at the carbon number of 10, macarpine can lose two methoxy-groups at the carbon number of 10 and 12, and chelerythrine can also lose two methoxy-groups at the carbon number of 7 and 8. All these alkaloids and their dihydro-forms showed a peak corresponding to their [M-OCH3]+ or [M+H-OCH3]+ ions in the MS/MS spectra (Fig. (C)-3H). In Fig. , three major fragment ions for each alkaloid were shown together with their corresponding CID mechanisms. Based on these results, the given MRM conditions were chosen so as to minimize the interferences among the same fragment ions which could originate from other unknown alkaloids when a real sample would be applied. The given collision voltages for the MRM modes were also obtained by the scanning method.
Fig. 3. MS/MS Spectrum of Eight Target Benzo[c]phenanthridine Alkaloids Analyzed by ESI-MS/MS.
Notes: (A) sanguinarine; (B) dihydrosanguinarine; (C) chelerythrine; (D) dihydrochelerythrine; (E) chelirubine; (F) dihydrochelirubine; (G) macarpine; (H) dihydromacarpine.
![Fig. 3. MS/MS Spectrum of Eight Target Benzo[c]phenanthridine Alkaloids Analyzed by ESI-MS/MS.Notes: (A) sanguinarine; (B) dihydrosanguinarine; (C) chelerythrine; (D) dihydrochelerythrine; (E) chelirubine; (F) dihydrochelirubine; (G) macarpine; (H) dihydromacarpine.](/cms/asset/a828d02d-0282-4fa3-93ac-ae34b4088b34/tbbb_a_917264_f0003_oc.gif)
Fig. represents the HPLC-ESI-MS/MS result of the California poppy cell culture, which were analyzed in the MRM mode. All the target alkaloids separated from HPLC have a peak relevant to the applied MRM conditions: sanguinarine separated at the retention time of 17.6 min was clearly detected in the MRM mode by the reaction of m/z 332.3 [M]+ → m/z 274.1 [M-CH2O-CO]+; chelerythrine at 19.5 min was detected by m/z 348.5 [M]+ → m/z 289.6 [M-CH2O-CO]+; chelirubine at 20.6 min was detected by m/z 362.4 [M]+ → m/z 317.4 [M-CO-CH3-H–H]+; macarpine at 22.9 min was detected by m/z 392.5 [M]+ → m/z 347.3 [M-CO-CH3-H–H]+; dihydrochelerythrine at 42.3 min was detected by m/z 350.5 [M+H]+ → m/z 303.7 [M+H-OCH2O]+; dihydrochelirubine at 43.6 min was detected by m/z 364.6 [M+H]+ → m/z 334.4 [M+H-CH2O]+; dihydrosanguinarine at 43.8 min was detected by m/z 334.4 [M+H]+ → m/z 319.0 [M+H-CH3]+; dihydromacarpine at 44.0 min was detected by m/z 394.6 [M+H]+ → m/z 361.5 [M+H-2CH3–2H]+.Citation9,22) These results clearly show that the applied HPLC method can effectively separate the target alkaloids, and the given analytical conditions described for ESI-MS/MS were adequate for the confirmation of the target alkaloids.
Production of benzo[c]phenanthridine alkaloids during the cell culture
The established method was then applied for the determination of the eight target alkaloids during the California poppy cell culture. Fig. represents the intracellular alkaloid content profiles together with the cell growth curve. All alkaloid contents in the media were also analyzed, but not shown in the figure because they are all negligible (< 1%) compared to those in the cells. The cell growth curve showed a sigmoid shape as other plant cell culturesCitation23,24) and reached maximum cell dry weight at day 12 (Fig. C). The most important alkaloid observed in our cell culture was dihydrochelirubine, which was increased after subculture and maintained over 4 mg/g-DCW (dried cell weight) until day 10 after subculture (Fig. (B)-◆-). During the stationary phase from day 11 to day 14, this dihydrochelirubine was converted into chelirubine and macarpine, which can be also deduced from the metabolic pathway given in the Fig. . No accumulation of dihydromacarpine during the stationary phase strongly supports that the conversion of dihydromacarpine into macarpine is more rapid compared to the conversion steps of dihydrochelirubine into dihydromacarpine. Chelerythrine was not produced during the whole cell culture even though its precursor dihydrochelerythrine was significantly accumulated and disappeared (Fig. A-□- vs. B-□-). From this observation, it might be hypothesized that there are unknown metabolic pathways converting dihydrochelerythrine into other alkaloids rather than chelerythrine.
Fig. 5. Benzo[c]phenanthridine Alkaloid Profiles during the California Poppy Cell Culture.
Notes: (A) sanguinarine (●); chelerythrine (□); chelirubine (◆); macarpine (△), (B) dihydrosanguinarine (●); dihydrochelerythrine (□); dihydrochelirubine (◆); dihydromacarpine (△). Data represent the mean and standard deviation of duplicate samples, (C) cell growth profiles in gram dry cell weight (gDCW, ●) and in gram fresh cell weight (gFCW, ■).
![Fig. 5. Benzo[c]phenanthridine Alkaloid Profiles during the California Poppy Cell Culture.Notes: (A) sanguinarine (●); chelerythrine (□); chelirubine (◆); macarpine (△), (B) dihydrosanguinarine (●); dihydrochelerythrine (□); dihydrochelirubine (◆); dihydromacarpine (△). Data represent the mean and standard deviation of duplicate samples, (C) cell growth profiles in gram dry cell weight (gDCW, ●) and in gram fresh cell weight (gFCW, ■).](/cms/asset/7caf9f05-7cc7-49cb-8e5d-323887ea3e2a/tbbb_a_917264_f0005_b.gif)
Conclusion
In this study, eight benzo[c]phenanthridine alkaloids that could be produced by the California poppy cell culture were analyzed using a liquid chromatography method coupled with photodiode array detector or tandem mass spectrometry. The given ternary gradient elution program was critical to the separation of dihydro-form alkaloids, which have usually been ignored in the previous researches despite their importance in the overall alkaloid production pathway. Each alkaloid peak separated by HPLC could be effectively quantified by photodiode array detector alone, being confirmed as the target alkaloid by tandem mass spectrometry also. The analysis results clearly revealed that both dihydrochelirubine and dihydrochelerythrine was the most important metabolites in our California poppy cell culture system. The determined alkaloid profiles also provided some insights into the metabolic flux information, which would be useful for metabolic engineering of the cell culture system.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.917264
Supplemental Fig 1.
Download PDF (121.8 KB)Funding
This work was supported by the Advanced Biomass R&D Center (ABC) of Korea Grant funded by the Ministry of Education, Science and Technology [ABC-2013059453]. This research was also partially supported by the WCU (World Class University) program through the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology [R31-30005].
References
- Schmeller T, Latz-Bruning B, Wink M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry. 1997;44(2):257–266.10.1016/S0031-9422(96)00545-6
- Muller-Jakic B, Muller M, Probstle A, Johns TA, Bauer R. Anti-inflammatory activity of Zanthoxylum chalybeum extracts and identification of protoberberine and benzophenanthridine alkaloids by GC-MS and HPLC. Planta Med. 1993;59:A664.10.1055/s-2006-959934
- Sacchetti B, Bielavska E. Chelerythrine, a specific PKC inhibitor, blocks acquisition but not consolidation and retrieval of conditioned taste aversion in rat. Brain Res. 1998;799:84–90.10.1016/S0006-8993(98)00460-0
- Vrba J, Doležel P, Vičar J, Modrianský M, Ulrichová J. Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells. Toxicol. in Vitro. 2008;22:1008–1017.10.1016/j.tiv.2008.02.007
- Tanahashi T, Zenk MH. New hydroxylated benzo[c]phenanthridine alkaloids from Eschscholtzia californica cell suspension cultures. J. Nat. Prod. 1990;53:579–586.10.1021/np50069a007
- Schumacher HM, Gundlach H, Fiedler F, Zenk MH. Elicitation of benzophenanthridine alkaloid synthesis in Eschscholtzia cell cultures. Plant Cell Rep. 1987;6(6):410–413.
- Kolewe ME, Gaurav V, Roberts SC. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008;5:243–256.10.1021/mp7001494
- Chen Y-Z, Liu G-Z, Shen Y, Chen B, Zeng J-G. Analysis of alkaloids in Macleaya cordata (Willd.) R. Br. using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J Chromatogr A. 2009;1216:2104–2110.10.1016/j.chroma.2008.08.066
- Fabre N, Claparols C, Richelme S, Angelin M-L, Fourasté I, Moulis C. Direct characterization of isoquinoline alkaloids in a crude plant extract by ion-pair liquid chromatography–electrospray ionization tandem mass spectrometry: example of Eschscholtzia californica. J. Chromatogr. A. 2000;904:35–46.10.1016/S0021-9673(00)00919-5
- Suchomelová J, Bochořáková H, Paulová H, Musil P, Táborská E. HPLC quantification of seven quaternary benzo[c]phenanthridine alkaloids in six species of the family Papaveraceae. J. Pharm. Biomed. Anal. 2007;44:283–287.10.1016/j.jpba.2007.02.005
- A-j Deng, H-l Qin. Cytotoxic dihydrobenzophenanthridine alkaloids from the roots of Macleaya microcarpa. Phytochemistry. 2010;71:816–822.
- Ahn MJ, Lee MK, Kim YC, Sung SH. The simultaneous determination of coumarins in Angelica gigas root by high performance liquid chromatography–diode array detector coupled with electrospray ionization/mass spectrometry. J Pharmaceut Biomed. 2008;46(2):258–266.10.1016/j.jpba.2007.09.020
- Sun Z, Song C, Xia L, Wang X, Suo Y, You J. Comprehensive comparisons between 1-Phenyl-3-methyl-5-pyrazolones, 1-(4-Methoxyphenyl)-3-methyl-5-pyrazolones and 1-(2-Naphthyl)-3-methyl-5- pyrazolones as labeling reagents used in LC-DAD-ESI-MS-MS analysis of neutral aldoses and uronic acids. Chromatographia. 2010;71:789–797.10.1365/s10337-010-1570-5
- Cho HY, Son SY, Rhee HS, Yoon SYH, Lee-Parsons CWT, Park JM. Synergistic effects of sequential treatment with methyl jasmonate, salicylic acid and yeast extract on benzophenanthridine alkaloid accumulation and protein expression in Eschscholtzia californica suspension cultures. J. Biotechnol. 2008;135:117–122.10.1016/j.jbiotec.2008.02.020
- Weiss D, Baumert A, Vogel M, Roos W. Sanguinarine reductase, a key enzyme of benzophenanthridine detoxification. Plant Cell Environ. 2006;29:291–302.10.1111/pce.2006.29.issue-2
- Park JJ, Yoon SYH, Cho HY, Son SY, Rhee HS, Park JM. Patterns of protein expression upon adding sugar and elicitor to the cell culture of Eschscholtzia californica. Plant Cell Tiss. Org. Cult. 2006;86:257–269.10.1007/s11240-006-9115-1
- Psotova J, Klejdus B, Vecera R, Kosina P, Kuban V, Vicar J, Simanek V, Ulrichova J. A liquid chromatographic–mass spectrometric evidence of dihydrosanguinarine as a first metabolite of sanguinarine transformation in rat. J Chromatogr B. 2006;830:165–172.10.1016/j.jchromb.2005.10.030
- Howell CR, Stipanovic RD, Bell AA. Dihydrosanguinarine, a product of sanguinarine detoxification by Verticillium dahliae. Pestic Biochem Physiol. 1972;2:364–370.10.1016/0048-3575(72)90041-7
- Das A, Nandi R, Maiti M. Photophysical property of sanguinarine in the excited singlet state. Photochem. Photobiol. 1992;56:311–317.10.1111/php.1992.56.issue-3
- Oldham JT, Hincapie M, Rejtar T, Wall PK, Carlson JE, Lee-Parsons CWT. Shotgun proteomic analysis of yeast-elicited California poppy (Eschscholzia californica) suspension cultures producing enhanced levels of benzophenanthridine alkaloids. J. Proteome Res. 2010;9:4337–4345.10.1021/pr1000412
- Han L-F, Nowicky W, Gutmann V. Reversed-phase high-performance liquid chromatographic separation of tertiary and quaternary alkaloids from Chelidonium majus L. J. Chromatogr. A. 1991;543:123–128.10.1016/S0021-9673(01)95760-7
- Gathungu RM, Oldham JT, Bird SS, Lee-Parsons CWT, Vouros P, Kautz R. Application of an integrated LC-UV-MS-NMR platform to the identification of secondary metabolites from cell cultures: benzophenanthridine alkaloids from elicited Eschscholzia californica (california poppy) cell cultures. Anal. Methods. 2012;4:1315–1325.10.1039/c2ay05803k
- Archambault J, Williams RD, Bédard C, Chavarie C. Production of sanguinarine by elicited plant cell culture I. Shake flask suspension cultures. J. Biotechnol. 1996;46:95–105.10.1016/0168-1656(95)00184-0
- Rho D, Chauret N, Laberge N, Archambault J. Growth characteristics of Sanguinaria canadensis L. Cell suspensions and immobilized cultures for production of benzophenanthridine alkaloids. Appl. Microbiol. Biot. 1992;36(5):611–617.