Abstract
In this paper we report here a hydrogen/deuterium exchange (H/D exchange) of cross-linkable α-amino acid derivatives with deuterated trifluoromethanesulfonic acid (TfOD). H/D exchange with TfOD was easily applied to o-catechol containing phenylalanine (DOPA) within an hour. A partial H/D exchange was observed for trifluoromethyldiazirinyl (TFMD) phenylalanine derivatives. N-Acetyl-protected natural aromatic α-amino acids (Tyr and Trp) were more effective in H/D exchange than unprotected ones. The N-acetylated TFMD phenylalanine derivative afforded slightly higher H/D exchange than unprotected derivatives. An effective post-deuteration method for cross-linkable α-amino acid derivatives will be useful for the analysis of biological functions of bioactive peptides and proteins by mass spectrometry.
Graphical Abstract
Hydrogen-deuterium exchange of cross-linkable α-amino acid derivatives in deuterated triflic acid proceeded smoothly at low temperature.
Introduction
Cross-linking reagents are valuable tools for investigating ligand–biomolecule interactions.Citation1) Based on the progress of the mass analysis, stable isotope-labeled bioactive ligands are attractive tools in the field.Citation2) Deuterium is one of the simplest stable isotopes and can be utilized for post-introductions to bioactive compounds. Many attempts for deuterium introduction in the aromatic portions of compounds have been reported.
Hydrogen/deuterium exchange (H/D exchange)Citation3–5) at a specific position and universal H/D exchange in acidic conditionCitation6) and/or metal catalysisCitation7) on aromatics have been reported in the literature. Although aromatic α-amino acids were considered to be the substrate for H/D exchange, the α-amino acid skeleton sometimes inhibited to apply previous H/D exchange methods due to their solubility in many organic solvent.Citation8,9)
Recently we developed a method for effective H/D exchange for natural aromatic α-amino acids and their containing peptides with trifluoromethanesulfonic acid-d (TfOD) at a temperature lower than room temperature without a side effect on stereochemistry. The exchange can be monitored regularly and controlled by adjusting the reaction temperature and reagent ratio.Citation10) We report in this paper the post-H/D exchange for cross-linkable aromatic α-amino acid derivatives with TfOD under mild conditions.
Results and discussion
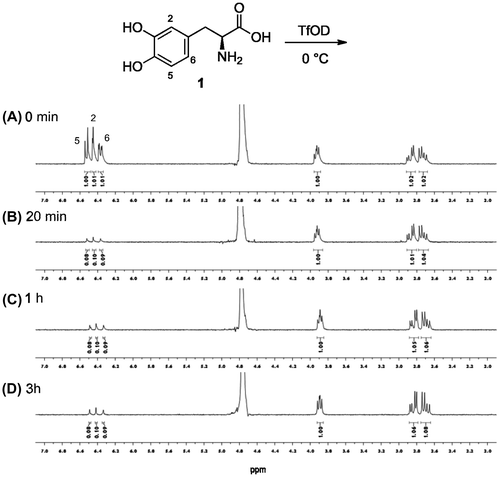
3,4-Dihydroxy-l-phanylalanine (l-DOPA, 1) has catechol moiety on the benzene ring, which can be utilized as a cross-linkable functional group in the presence of periodate.Citation11–13) The hydroxyl groups in the molecule might enhance the H/D exchange due to their electron-donating properties. H/D exchange of about 90% takes place at three exchangeable sites on the benzene ring at 0 °C or room temperature within 20 min in TfOD as see in the 1H-NMR measurement (Fig. ). The degree of exchange did not change with longer incubation time. ESI-TOF mass analysis also indicated that three hydrogen atoms exchanged with deuterium (m/z 201 and 198 with and without deuterium exchange, respectively). It has been reported that the H/D exchange of 1 in 37%DCl/D2O was conducted at 50 °C for 24 h.Citation14) Our H/D exchange with TfOD proceeded very swiftly even though it was conducted at low temperature. The H/D exchange ratio for 1 was enhanced significantly than that for l-tyrosine, which favored H/D exchange at the 3-position. All C–H bonds on the aromatic moiety of 1 were placed at o- or p- positions against two hydroxyl groups. These characters promoted H/D exchange for DOPA swiftly even though the reaction was conducted at 0 °C.
Fig. 1. Time-course H/D exchange for l-DOPA (1) treated with TfOD at 0 °C by 1H-NMR spectrum in D2O.
Notes: 1H-NMR of the reaction mixture at 0 min, 20 min, 1 h and 3 h are (A), (B), (C) and (D), respectively.
Photoaffinity labeling is the most attractive method for creating a cross-link between ligand and biomolecule interactions.Citation15,16) Photoreactive aromatic α-amino acid derivatives have been used to investigate bioactive peptides interactions.Citation17–20) Three common photophores, arylazide, benzophenone, and diazirine, were utilized for the photoaffinity labeling. The H/D or tritium exchange for photoreactive aromatic α-amino acid derivatives has not yet been reported.
l-Phe(4-azido) (2) was treated with TfOD at room temperature and the reaction mixture afforded a complicated mixture within a half hour. It has been reported that the azide group easily reacts in TfOHCitation21) and no improvement was observed in the exchange reaction conducted at 0 °C (Fig. (A)).
Fig. 2. H/D exchange of photophore containing aromatic α-amino acid.
Notes: (A) l-Phe(4-azido) (2), (B) l-Phe(4-benzoyl) (3), and (C) l-Phe(4-TFMD) (4) at 0 °C.
Time-course H/D exchange ratio for 2- and 3- position of compound 4 was presented by open square and circle, respectively.
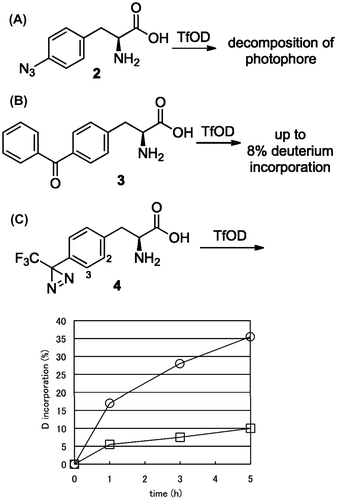
l-Phe(4-benzoyl) (3) was stable in the treatment with TfOD at room temperature for a day. However, low incorporation (less than 8% per aromatic proton) was observed in 1H-NMR measurement. The low incorporation resulted in no significant detectable differences on the mass spectrum compared to untreated samples. These results indicated that the carbonyl group acted as the electron withdrawing group to the benzene ring and hampered the H/D exchange reaction at room temperature (Fig. (B)).
l-Phe(4-TFMD) (4) was subjected to H/D exchange with TfOD. It was observed that the diazirinyl ring was decomposed within 1 h at room temperature. The result was consistent with the previous reports of instability of the diazirinyl ring in strong acidic conditions over room temperature.Citation22,23) No decomposition of the diazirine ring was observed when the reaction was conducted at 0 °C for 5 h. The H/D exchange proceeded in a time-dependent manner and the H/D exchange ratio was observed up to 35% (35 and 10% for the 2- and 3- position, respectively) in 1H-NMR measurement (Fig. (C)). Further reaction at the same temperature caused decomposition of diazirinyl ring.
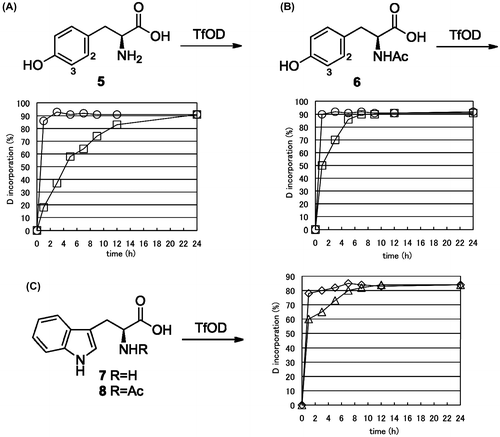
To improve the H/D exchange ratio for compound 4, we used N-acetyl-protected aromatic α-amino acid derivatives in the H/D exchange. N-Ac-l-Tyr (6) was subjected to treatment with TfOD at room temperature. H/D exchange at both, the 2- and 3- position of 6 was more rapid than that of unprotected l-Tyr (5). It was observed that 90% H/D exchange occurred within 3 h at the 3-position and over 24 h at the 2-position for 5 at room temperature (Fig. (A)). The exchange rate was drastically improved using 6 for over 90% exchange, within 1 and 6 h at the 3- and 2- position, respectively (Fig. (B)). The same tendencies were observed for l-Trp derivatives. The H/D exchange for aromatic protons of Trp was observed to be up to 80% at room temperature. Although unprotected, l-Trp (7) took over 8 h and N-Ac-l-Trp (8) took less than 3 h (Fig. (C)). These results indicate that the exchange rate for N-acetyl-protected aromatic α-amino acids is faster than that for unprotected compounds.
Fig. 3. Time-course H/D exchange of l-Tyr (5), N-Ac-l-Tyr (6), l-Trp (7), and N-Ac-l-Trp (8).
Notes: ((A), (B)) Time-course H/D exchange ratio for 2- and 3- position of compound 5 or 6 was presented by open square and circle, respectively. (C) Time-course H/D exchange ratio for indole protons of compound 7 or 8 was presented by open triangle and diamond, respectively.
Based on the above observations, N-Ac-l-DOPA (9), N-Ac-l-Phe(4-benzoyl) (10), and N-Ac-l-Phe(4-TFMD) (11) were subjected to H/D exchange in TfOD. For DOPA derivative 9, the N-protection did not cause improvement in H/D exchange drastically because unprotected 1 is a good substrate for our H/D exchange. Compound 9 was easily deuterated by over 90% at 0 °C within 20 min (Fig. (A)). No improvement was observed for the treatment of 10 at room temperature with 1H-NMR analysis (less than 8% exchange per aromatic proton) (Fig. (B)). The results indicated that the electron-withdrawing properties of the carbonyl group were the main factor for a lower H/D exchange ratio.
Fig. 4. H/D exchange of photophore containing N-acetyl aromatic α-amino acid.
Notes: (A) N-Ac-l-DOPA (9), N-Ac-l-Phe(4-benzoyl) (10), and (B) N-Ac-l-Phe(4-TFMD) (11) at 0 °C.
(A) Time-course H/D exchange ratio for 2-, 5-, and 6- position of compound 9 was presented by open square, triangle, and circle, respectively. (C) Time-course H/D exchange ratio for 2- and 3- position of compound 11 was presented by open square and circle, respectively.
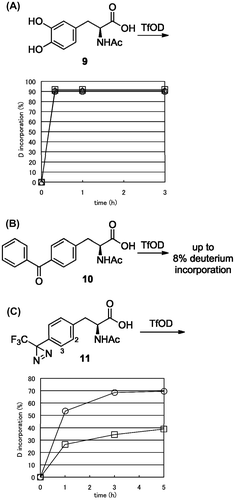
For trifluoromethyldiazirinyl (TFMD) derivative 11, H/D exchange at room temperature promoted decomposition of the diazirinyl ring within 1 h, which was consistent with previous results. On the other hand, H/D exchange conducted at 0 °C promoted higher H/D exchange at both the 2- (up to 35%) and 3- (up to 70%) position for 5 h than unprotected l-Phe(4-TFMD) (4) (Fig. (C)). N-Protection improved H/D exchange with TfOD for the unfavored substrate due to the prevention of neutralization of TfOD by amino groups.
We described post-deuteration (H/D exchange) of cross-linkable aromatic α-amino acid derivatives in TfOD at low temperature. The exchange conditions were beneficial due to the introduction of deuterium at the final steps, reaction at low temperature, and the detectable progress of the reaction. These reactions were not easy to perform using previously used methods. The advanced synthetic methods will be utilized to elucidate ligand–biomolecule interaction with mass spectrometry.
Experimental
General methods
1H-NMR spectra were measured by a Jeol EX 270 spectrometer for determining the H/D exchange ratio. MS data were obtained with a Waters LCT Premier XE instrument. ESI-TOF-MS data were obtained with a Waters UPLC ESI-TOF mass spectrometer. TfOD (98 atom % D) was purchased from Sigma–Aldrich. l-DOPA was purchased from Wako chemicals. Photoreactive l-phenylalanine derivatives were synthesized by methods described in a previous study.Citation24)
General procedures for H/D exchange with TfOD
Cross-linkable aromatic α-amino acid derivatives (0.25 mM) were dissolved in TfOD (0.9 mL, 10 mM) at a specific temperature. The reaction mixture was diluted with H2O (0.6 mL) followed by 1H-NMR and ESI-TOF mass analysis.
General procedures for acetylation of aromatic α-amino acid derivatives
Aromatic α-amino acid derivatives (0.4 mM) were dissolved in methanol (2 mL) and acetic anhydride (0.8 mM) was added dropwise at 40 °C. The reaction mixture was stirred at same temperature for overnight and concentrated. The residue was subjected to silica column chromatography (CH2Cl2:CH3OH = 4:1, then ethyl acetate:CH3OH = 1:1) to afford pure N-acetyl compounds in 60–75% yield.
Acknowledgments
This research was partially supported by the Fugaku Foundation, Noastec Foundation and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” for financial support.
References
- Paramelle D, Miralles G, Subra G, Martinez J. Chemical cross-linkers for protein structure studies by mass spectrometry. Proteomics. 2013;13:438–456.10.1002/pmic.v13.3-4
- Arsene CG, Ohlendorf R, Burkitt W, Pritchard C, Henrion A, O’Connor G, Bunk DM, Güttler B. Protein quantification by isotope dilution mass spectrometry of proteolytic fragments: cleavage rate and accuracy. Anal. Chem. 2008;80:4154–4160.10.1021/ac7024738
- Dormán G, Olszewski JD, Prestwich GD, Hong Y, Ahern DG. Synthesis of highly tritiated 4-benzoyl-L-phenylalanine, a photoactivatable amino acid. J. Org. Chem. 1996;60:2292–2297.
- Ambroise Y, Mioskowski C, Djéga-Mariadassou G, Rousseau B. Consequences of affinity in heterogeneous catalytic reactions: highly chemoselective hydrogenolysis of iodoarenes. J. Org. Chem. 2000;65:7183–7186.10.1021/jo0012243
- Faucher N, Ambroise Y, Cintrat JC, Doris E, Pillon F, Rousseau B. Highly chemoselective hydrogenolysis of iodoarenes. J. Org. Chem. 2002;67:932–934.10.1021/jo0109669
- Atzrodt J, Derdau V, Fey T, Zimmermann J. The renaissance of H/D exchange. Angew. Chem. Int. Ed. 2007;46:7744–7765.10.1002/(ISSN)1521-3773
- Ito N, Watahiki T, Maesawa T, Maegawa T, Sajiki H. Synergistic effect of a palladium-on-carbon/platinum-on-carbon mixed catalyst in hydrogen/deuterium exchange reactions of alkyl-substituted aromatic compounds. Adv. Synth. Catal. 2006;348:1025–1028.10.1002/(ISSN)1615-4169
- Benfenati E, Icardi G, Chen S, Fanelli R. Syntheses of deuterated leu-enkephalins and their use as internal standards for the quantification of leu-enkephalin by fast atom bombardment mass spectrometry. J. Labelled Compd. Radiopharm. 1990;28:411–419.10.1002/jlcr.v28:4
- Faull KF, Barchas JD, Murray S, Halpern B. Deuterated peptides: platinum catalyzed exchange labeling of leucine enkephalin and related peptides. Biomed. Mass Spectrom. 1983;10:463–470.10.1002/(ISSN)1096-9888
- Murai Y, Wang L, Masuda K, Sakihama Y, Hashidoko Y, Hatanaka Y, Hashimoto M. Rapid and controllable hydrogen-deuterium exchange on aromatic rings of α-amino acids and peptides. Eur. J. Org. Chem. 2013;5111–5116.10.1002/ejoc.201300405
- Umanah GKE, Son C, Ding FX, Naider F, Becker JM. Cross-linking of a dopa-containing peptide ligand into its g protein-coupled receptor. Biochemistry. 2009;48:2033–2044.10.1021/bi802061z
- Burdine L, Gillette TG, Lin HJ, Kodadek T. Periodate-triggered cross-linking of dopa-containing peptide−protein complexes. J. Am. Chem. Soc. 2004;126:11442–11443.10.1021/ja045982c
- Liu B, Burdine L, Kodadek T. Chemistry of periodate-mediated cross-linking of 3,4-dihydroxylphenylalanine-containing molecules to proteins. J. Am. Chem. Soc. 2006;128:15228–15235.10.1021/ja065794h
- Pająk M, Kańska M. Enzymatic synthesis of dopamine ring labeled with hydrogen isotopes. J. Radioanal. Nucl. Chem. 2009;279:455–458.10.1007/s10967-008-7335-z
- Brunner J. New photolabeling and crosslinking methods. Annu. Rev. Biochem. 1993;62:483–514.10.1146/annurev.bi.62.070193.002411
- Kotzyba-Hibert F, Kapfer I, Goeldner M. Recent trends in photoaffinity labeling. Angew. Chem. Int. Ed. Engl. 1995;34:1296–1312.10.1002/(ISSN)1521-3773
- Nassal M. 4′-(1-Azi-2,2,2-trifluoroethyl)phenylalanine, a photolabile carbene-generating analog of phenylalanine. J. Am. Chem. Soc. 1984;106:7540–7545.10.1021/ja00336a038
- Shih LB, Bayley H. A carbene-yielding amino acid for incorporation into peptide photoaffinity reagents. Anal. Biochem. 1985;144:132–141.10.1016/0003-2697(85)90094-6
- Masuda K, Koizumi A, Misaka T, Hatanaka Y, Abe K, Tanaka T, Ishiguro M, Hashimoto M. Photoactive ligands probing the sweet taste receptor. Design and synthesis of highly potent diazirinyl D-phenylalanine derivatives. Bioorg. Med. Chem. Lett. 2010;20:1081–1083.10.1016/j.bmcl.2009.12.029
- Murai Y, Masuda K, Sakihama Y, Hashidoko Y, Hatanaka Y, Hashimoto M. Comprehensive synthesis of photoreactive (3-trifluoromethyl)diazirinyl indole derivatives from 5- and 6- trifluoroacetylindoles for photoaffinity labeling. J. Org. Chem. 2012;77:8581–8587.10.1021/jo301552m
- Olah GA, Ramaiah P, Wang Q, Prakash GKS. Synthetic methods and reactions. 188. Triflic acid catalyzed phenylamination of aromatics with phenyl azide. J. Org. Chem. 1993;58:6900–6901.10.1021/jo00076a063
- Moss RA, Fedé JM, Yan S. SbF5-mediated reactions of oxafluorodiazirines. Org. Lett. 2001;3:2305–2308.10.1021/ol010091k
- Murai Y, Masuda K, Ogasawara Y, Wang L, Hashidoko Y, Hatanaka Y, Iwata S, Kobayashi T, Hashimoto M. Synthesis of photoreactive 2-phenethylamine derivatives. –Synthesis of adenosine derivatives enabling functional analysis of adenosine receptors via photoaffinity labeling–. Eur. J. Org. Chem. 2013;2428–2433.10.1002/ejoc.201201657
- Wang L, Hisano W, Murai Y, Sakurai M, Muto Y, Ikemoto H, Okamoto M, Murotani T, Isoda R, Kim D, Sakihama Y, Sitepu IR, Hashidoko Y, Hatanaka Y, Hashimoto M. Distinct metabolites for photoreactive L-phenylalanine derivatives in Klebsiella sp. CK6 isolated from rhizosphere of a wild dipterocarp sapling. Molecules. 2013;18:8393–8401.10.3390/molecules18078393
