Abstract
The cyanobacterium Nostoc verrucosum occurs in cool, clear streams and its gelatinous colonies, called “ashitsuki,” have been eaten in ancient Japan. Its ethanolic extract was found to inhibit the growth of Gram-positive bacteria and activity-guided fractionation yielded an unusual n-1 fatty acid, (9Z,12Z)-9,12,15-hexadecatrienoic acid (1), as one of the active principles. It inhibited the growth of Staphylococcus aureus at MIC 64 μg/mL.
Cyanobacteria are known as a prolific source of bioactive secondary metabolites,Citation1) but those forming macroscopic colonies are also consumed as food sources in many parts of the world. During the Age of Exploration, members of conquistadors observed harvesting and cooking of Arthrospira platensis and A. maxima, now aquacultured as ingredients of a dietary supplement “Spirulina,” by the Aztecs.Citation2) In our times, the population surrounding Lake Chad annually trades and consumes 40 t of dihé, which is a dried cake of the same organisms harvested from the lakeCitation3); the Peruvian highlanders value spherulous Nostoc pruniforme colonies, emerging in pools and lakes in spring, as preservable food and medicine.Citation4) The same organism is also consumed in Tartary, Mogolia, and ChinaCitation5); N. flagelliforme, a hairy species grown on dry deserts, is a special soup ingredient for New Year’s cuisine in ChinaCitation6); a cosmopolitan terrestrial species, N. commune, has been eaten in Philippines,Citation7) Indonesia,Citation8) Japan, China, Mongolia, Ecuador, and FijiCitation4); Aphanothece sacrum, endemic to the Aso water system in Kyushu District, Japan, has been prized since the Edo Period.Citation9) However, the chemical studies of these species have only sparsely been reported: two pterin derivativesCitation10) and an antiviral sulfated polysaccharideCitation11,12) from A. maxima; an antiviral polysaccharide from N. flagelliformeCitation13); seven meroditerpenes,Citation14−16) an anthraquinone derivative,Citation16) three polyaromatics with amino acid origin,Citation16−18) and an antifungal lipopeptideCitation19) from N. commune; pseudovitamin B12Citation20) and a sulfated polysaccharide from A. sacrum.Citation21)
Nostoc verrucosum, called ashitsukiCitation22) in Japanese, is another edible cyanobacterium that has been eaten at least since the eighth century in Japan and also reportedly in Thailand.Citation4) It grows attached to well-lit riverbed in cool (13–14 °C) limpid water and only persists during a limited time of the season. Its past prevalence and familiarity as foodstuff are represented by many local names such as kotobuki-nori or -dake in Okayama,Citation23) mitoku-nori in Tottori,Citation24) shiga-nori in Shiga,Citation25) and so on (Table S1; see http://dx.doi.org/10.1080/09168451.2014.918484). However, as a result of river development that surged across the country during the last half century, a significant loss of habitat is now evident, though no formally published data are available.
As part of our program to tap unexploited bacterial taxa as new drug discovery resources, we are interested in cyanobacteria that are consumed as delicacies in Japan. Specimens of N. verrucosum grown in three major habitats are protected by Toyama Prefecture Ordinance for the Protection of Cultural Properties and thus not accessible. Fortunately, one of the authors (Sugawa T.) found a new habitat in Toga River with substantial abundance,Citation26) which enabled the first chemical study on this microbe.
N. verrucosum, grown on the surface of riverbed concrete blocks was scraped off with a square-shaped aquarium fish net in June 2011. The collected material was immediately transported to the laboratory and kept frozen until extraction.
The frozen specimen (4.2 kg) was mixed with an approximately equal volume of Celite® and homogenized in EtOH (2 L). The homogenate was filtered through No.2 filter paper in vacuo, and the residue was extracted four more times with EtOH. The extracts were combined and concentrated to a water suspension, which was diluted to 60% aqueous MeOH and extracted with CH2Cl2. The lipophilic extract was further partitioned between 90% aqueous MeOH and n-hexane, while the polar layer was partitioned between n-BuOH and water. The resulting four layers were tested for antimicrobial activity against five bacteria (Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus, Streptomyces lividans, and Escherichia coli), two yeasts (Candida albicans and Saccharomyces cerevisiae), and two fungi (Penicillium chrysogenum and and Aspergillus oryzae) with the paper disk diffusion method, which detected growth inhibitory activity against Gram-positive bacteria from the 90% aqueous MeOH and n-BuOH layers.
The more potent 90% MeOH layer was fractionated by flash chromatography on silica gel (solvent: n-hexane/Et2O 5:1, 3:1, 1:1, and 1:3; CHCl3/MeOH 99:1, 97:3, 95:5, and 9:1; CHCl3/MeOH/H2O 8:2:0.1, 7:3:0.5, and 6:4:1), gel-filtration on Sephadex LH-20 (solvent: CHCl3/MeOH 1:1), and ODS-HPLC (column: Cosmosil AR-II 20 × 250 mm, solvent: 83% MeCN containing 4% HCO2H) to give several active peaks. Among these, a predominant one was identified to be linoleic acid (98.9 mg, 2.3 × 10−3%wt) based on NMR and MS spectral analysis, whereas the second most prominent was further purified by reversed phase recycle HPLC (column: Cholester 10 × 250 mm, solvent: 85% MeCN containing 4% HCO2H) to give 1 (8.7 mg, 2.1 × 10−4%wt): UV λmax (MeOH) nm (ε): 202 (9200); IR νmax (ATR) cm−1: 3011, 2926, 2854, 1706, 1638, 1411, 991, 910, 723.
A molecular formula of C16H26O2 was determined for 1 based on the molecular ion observed at m/z 273.1816 [M + Na]+ (calcd. for C16H26NaO2 273.1825) in a HRESITOFMS measurement. The 1H NMR resonances were observed only at the olefinic and aliphatic regions, exhibiting three isolated (δH 5.81, 5.03, and 4.95) and a cluster of olefinic signals (δH 5.40–5.30, 4H), five methylene signals (δH 2.83, 2.80, 2.28, 2.07, and 1.60), and a methylene envelope (δH 1.36–1.32, 8H) (Table ). The three olefinic signals were mutually coupled [δH 5.81 ddt (17.0, 10.0, and 6.1), 5.03 ddt (17.2, 1.5, 1.5), 4.96 ddt (10.2, 1.5, 1.5)] to form a vinyl group, while the methylene signals all appeared coalesced with rather simpler multiplicity, which indicated a polyunsaturated chain structure. The 13C NMR spectrum showed one carboxyl (δC 177.9), six olefinic (δC 138.2, 131.3, 130.5, 129.0, 128.0, and 115.2), and nine aliphatic resonances (δC 35.4, 32.7, 30.9, 30.5, 30.42, 30.37, 28.3, 26.6, and 26.4), suggesting that 1 is a hexadecatrienoic acid. The lack of a methyl group was endorsed by an HSQC spectrum, which in turn proved that 1 is an unusual n-1 fatty acid. Five COSY cross-peaks from deshielded methylenes to olefinic protons (δH 2.07/5.36, 2.80/5.41, 2.83/5.81, 2.83/4.96, and 2.83/5.03) assembled a methylene-intervened triene unit, whereas a cross-peak between the remaining two methylene signals (δH 2.28/1.60) evidenced an ethylene system. Substitution of the latter fragment by a carboxylate group was supported by HMBC cross-peaks δH 1.60/δC 177.9 and 2.28/177.9. The remaining parts must constitute a C4-methylene chain flanked by the propionate and the triene units, which concluded a 16:3n-1 structure for 1 and thus satisfied the molecular formula.
Table 1. NMR data for (9Z,12Z)-9,12,15-hexadecatrienoic acid (1) in CD3OD (500 MHz).
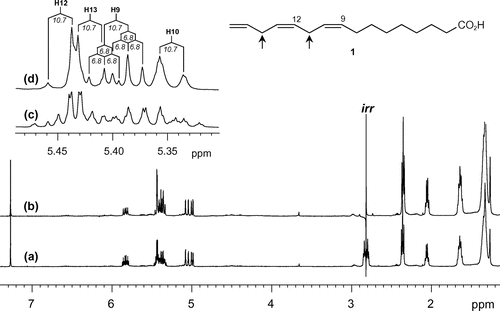
The geometry of two internal double bonds was not deducible from the magnitude of 1H-1H coupling constants because of their mutual overlapping. A NOESY experiment only helped establish a Z-geometry for the C9=C10 double bond by a cross-peak between allylic protons H8 and H11 (δH 2.07/2.80). A similar correlation between H11 and H14 (δH 2.80/2.83) was not discernible because of its proximity to the diagonal peaks. To specify the geometry for C12=C13, a homodecoupling experiment was conducted irradiating in the middle frequency between H11 and H14. Gratifyingly, a spectrum measured in CDCl3 (Table S2; see http://dx.doi.org/10.1080/09168451.2014.918484) uncovered an AB spin system for H12 and H13 with a coupling constant of 10.7 Hz (Fig. ), revealing a Z-geometry for this double bond. Thus, 1 was concluded to be (9Z,12Z)-9,12,15-hexadecatrienoic acid.
Fig. 1. 1H NMR (500 MHz) spectra in CDCl3 of (a) 9,12,15-hexadecatrienoic acid (1) and (b) double-frequency irradiation on H11 and H14 (positions indicated by arrow in the structure).
Notes: Olefinic regions magnified in (c) and (d) show simplification of the splitting patterns of H9, H10, H12, and H13, allowing measurement of coupling constants denoted in italics. Signals are referenced by a residual solvent peak at δH 7.27.
n-1 Fatty acids are a minor group of lipids in nature. Only two records exist on the isolation of this group, both of which deal with (6Z,9Z,12Z)-6,9,12,15-hexadecatetraenoic acid from diatoms.Citation27,28) Although the precedent isolation of 1 has been achieved as the product of fungal bioconversion from palmitolinoleic acid,Citation29) its spectroscopic assignments and physicochemical properties were not provided. Thus, the current study describes the first isolation from nature and rigorous spectroscopic characterization of the same fatty acid. Detection from natural resources has been reported from herring oil,Citation30) rapeseed oil,Citation31) and leaves and nuts of the ginkgo tree.Citation32)
(6Z,9Z,12Z)-6,9,12,15-Hexadecatetraenoic acid has been discovered as one of the antibacterial constituents of a marine diatom Parlibellus delognei.Citation27) In analogy with this, 1 inhibited the growth of S. aureus, M. luteus, and B. subtilis at MIC values of 64, 256, and 256 μg/mL, respectively, showing a slight modification of the activity relative to other structurally related unsaturated fatty acids (Table ). Antibacterial activity of fatty acids are known for more than a hundred years, and some of the common fatty acids have repeatedly been isolated as antibacterial principles during natural products drug discovery attempts.Citation33) As exemplified by sapienic acid (C16:1n-10), which is a unique constituent of human sebum and shown to inhibit the growth of S. aureus,Citation34,35) these fatty acids are potentially the innate antibacterial agents in the host organisms. Their surface-active property is attributed to the basis of antibacterial action, but the exact mode of action is yet to be established.Citation36,37)
Table 2. Antimicrobial activity of compound 1 and related fatty acids.
Because fatty acids are substrates of energy production and are expected to be much safer than antibiotics for human consumption, 1 could be used as an ingredient of fatty acid-based antibacterial formulation or therapy where the use of conventional antibiotics is prohibited.Citation37)
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.918484
bbb-131014-File005_1_.pdf
Download PDF (1.2 MB)Acknowledgments
We thank Imizu City Board of Education, Toyama, and Toga Administration Center, Nanto City, Toyama, for providing information on the inhabitation of N. verrucosum in Toga River.
Funding
This work is supported by Grant-in-aid for Scientific Research [grant number 23710259] to N. O. from the Ministry of Education, Culture, Sports, and Technology of Japan, and FY2011 Intramural Research Fund for Regional Challenges from TPU.
References
- Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC. Tetrahedron. 2001;57:9347–9377.10.1016/S0040-4020(01)00931-0
- Sánchez M, Bernal-Castillo J, Rozo C, Rodríguez I. Univ. Sci. 2003;8:7–24.
- Abdulquader G, Barsanti L, Tredici MR. J. Appl. Phycol. 2000;12:439–498.
- Johnson HE, King SR, Banack SA, Webster C, Callanaupa WJ. J. Ethnopharmacol. 2008;118:159–165.10.1016/j.jep.2008.04.008
- Soeder CJ. In: Shelef G, Soeder CJ, editors. Algal Biomass. Amsterdam: Elsevier; 1980. p. 9–20.
- Gao K. J. Appl. Phycol. 1998;10:37–49.10.1023/A:1008014424247
- Martinez MR. Philipp. Agriculturist. 1988;71:295–307.
- Zaneveld JS. Econ. Bot. 1959;13:89–131.10.1007/BF02859244
- Kabata K, Kaneko T. Shinhakken ‘sacran’ to dento no suizenjinori (in Japanese). In: Kabata K, Kaneko T, editors. Hahto Shuppan, Tokyo; 2009.
- Noguchi Y, Ishii A, Matsushima A, Haishi D, Yasumuro K, et al. Mar. Biotechnol. 1999;1:207–210.10.1007/PL00011769
- Hayashi T, Hayashi K, Maeda M, Kojima I. J. Nat. Prod. 1996;59:83–87.10.1021/np960017o
- Lee J, Hayashi T, Hayashi K, Sankawa U. J. Nat. Prod. 2000;63:136–138.10.1021/np990348b
- Kanekiyo K, Lee J-B, Hayashi K, Takenaka H, Hayakawa Y, Endo S, Hayashi T. J. Nat. Prod. 2005;68:1037–1041.10.1021/np050056c
- Jaki B, Orjala J, Sticher O. J. Nat. Prod. 1999;62:502–503.10.1021/np980444x
- Jaki B, Orjala J, Heilmann J, Linden A, Vogler B, Sticher O. J. Nat. Prod. 2000;63:339–343.10.1021/np9903090
- Jaki B, Heilmann J, Sticher O. J. Nat. Prod. 2000;63:1283–1285.10.1021/np000033s
- Kobayashi A, Kawazu K, Kanzaki H, Inawaka K, Japan Kokai Tokkyo Koho, 1994–116159, ( Apr. 26, 1994).
- Kobayashi A, Kajiyama S, Inawaka K, Kanzaki H, Kawazu K. Z. Naturforsch. C. Biosci. 1994;49:464–470.
- Kajiyama S, Kanzaki H, Kawazu K, Kobayashi A. Tetrahedron Lett. 1998;39:3737–3740.10.1016/S0040-4039(98)00573-5
- Watanabe F, Miyamoto E, Fujita T, Tanioka Y, Nakano Y. Biosci. Biotechnol. Biochem. 2006;70:3066–3068.10.1271/bbb.60395
- Okajima MK, Miyazato S, Kaneko T. Langmuir. 2009;25:8526–8531.10.1021/la8036956
- Koidzumi G. Shokubutsugaku Zasshi. 1919;33:263–264. Japanese.
- Harada N. Bull Niimi Coll. 2007;28:202–215. Japanese.
- Hirose H, Hirano M, Hirose H, Yamagishi T, editors. Illustration of the Japanese fresh-water algae. Tokyo: Uchida Rokakuho; 1977. p. 31–99. Japanese.
- Komatsuzaki M. In: Shigaken hoshokai, Shigaken hoshokai, editors. Shigaken tennenkinenbutsu chohsahohkoku (in Japanese). Vol. 1. Shiga; 1924. p. 31.
- Sugawa T. Toyama no seibutsu. 2006;45:33–35. Japanese.
- Findlay JA, Patil AD. J. Nat. Prod. 1984;47:815–818.10.1021/np50035a010
- Juttner F. J. Phycol. 2001;37:744–755.10.1046/j.1529-8817.2001.00130.x
- Shirasaka N, Umehara T, Fukuda Y, Yoshizumi H, Shimizu S. Mycoscience. 2005;46:329–333.10.1007/S10267-005-0255-7
- Klenk E, Steinbach H. Hoppe-Seyler’s Z. physiol. Chem. 1959;316:31–44.10.1515/bchm2.1959.316.1.31
- Jantzen E, Andreas H, Morgenstern K, Roth W. Fette, Seifen, Anstrichmittel. 1961;63:685–688.10.1002/(ISSN)1521-4133
- Gellerman JL, Schlenk H. Experientia. 1963;19:522–523.10.1007/BF02150889
- Kabara J, Swieczkowski DM, Conley AJ, Truant JP. Antimicrob. Agents Chemother. 1972;2:23–28.10.1128/AAC.2.1.23
- Wille JJ, Kydonieus A. Skin Pharmacol. Physiol. 2003;16:176–187.10.1159/000069757
- Drake DR, Brogden KA, Dawson DV, Wertz PW. J. Lipid Res. 2008;49:4–11.10.1194/jlr.R700016-JLR200
- Greenway DLA, Dyke KGH. J. Gen. Microbiol. 1979;115:233–245.10.1099/00221287-115-1-233
- Desbois AP, Smith VJ. Appl. Microbiol. Biotechnol. 2010;85:1629–1642.10.1007/s00253-009-2355-3
