Abstract
We previously reported bacteriostatic action of nukacin ISK-1 against Bacillus subtilis JCM 1465T. Here, we found its bactericidal activity against Micrococcus luteus DSM 1790 and Staphylococcus simulans 22, showing decrease in cell viability, cell lysis, and dissipation of the membrane potential. Moreover, leakage of small molecules such as K+, suggested the formation of small-sized or specific K+-conducting-pores by nukacin ISK-1.
In the search to find strong alternatives to fight against continuously emerging antibiotic-resistant bacteria, lantibiotics have long been considered as potential candidates because of their stability, safety, and high potency at nanomolar concentrations. Lantibiotics are a post-translationally modified class I group of bacteriocins, which are ribosomally synthesized antimicrobial peptides.Citation1) Nisin is the most well-known representative of the lantibiotic group and has already been used as a commercial food preservative and found to be effective for medical applications.Citation2,3) Lantibiotics are classified into two types: A and B, based on their structural topology; type A is further sub-classified into type-A(I) and type-A(II).Citation4) As the structure differs, the complex mode of action of lantibiotics from different groups also varies greatly.Citation5) Nisin, a 34-amino-acids long, flexible peptide, is a type A(I) lantibiotic, which acts by binding to lipid II, thereby inhibiting cell wall biosynthesis. Nisin exposure also results in the formation of 2- to 2.5-nm-sized pores on the cell membrane leading to cell death, which is characterized as bactericidal activity.Citation6) Nukacin ISK-1 produced by Staphylococcus warneri ISK-1, on the other hand, is a 27-amino-acid peptide with a linear N-terminal and globular C-terminal region, which is a type A(II) lantibiotic.Citation7) Earlier, it was reported that nukacin ISK-1 binds to lipid II resulting in the arrest of cell wall biosynthesis,Citation8) and in Bacillus subtilis JCM 1465T, it only stops cell growth without any lytic or pore-forming activity,Citation9) a clear indication of only bacteriostatic activity. In this study, we demonstrated that nukacin ISK-1 also showed bactericidal activity against some indicator strains. To the best of our knowledge, this is the first report of a type-A(II) lantibiotic having both bactericidal and bacteriostatic modes of action.
In this work, nukacin ISK-1was purified by a standard purification protocol,Citation10) and nisin was purified from commercial nisin A (Sigma-Aldrich, St. Louis, MO) using reverse-phase-HPLC. Seven different indicator strains were used to assess their susceptibility against the peptides in microtiter plates. The minimum inhibitory concentrations (MICs) of both peptides were determined by measuring the minimum concentration required for growth inhibition in liquid mediumCitation11) (Table ). To determine the bacteriostatic or bactericidal nature, turbidity (optical density: OD) was measured and the colony-forming unit (CFU) was determined. CFU was counted by the spread plate method after plating the cells on suitable agar in 10-fold serial dilutions. In some indicator strains such as B. subtilis and Lactobacillus sakei, nukacin ISK-1 did not decrease the OD or CFU count, but rather halted the growth compared to the untreated control cells indicating its bacteriostatic activity (Table ). In two indicator strains, Staphylococcus simulans 22 and Micrococcus luteus DSM 1790, the viability of exponentially growing cultures was sharply decreased at a similar level to nisin (Fig. (A)). The OD was also lowered collaterally indicating the bactericidal activity of the peptides against these two indicator strains. Although there was a slight decrease in the viability count of Streptococcus bovis JCM 5802T by nukacin ISK-1 (Table ), it was not significant compared to that in S. simulans or M. luteus (not shown). A further detailed mechanism of action study was then carried out in these two indicator strains using 5X MIC of peptides and 10X MIC for transmission electron microscopy (TEM) experiment. Since L. sakei JCM 1157T showed no decrease in the OD or CFU either in current or previous work,Citation9) it was used in further experiments as a control against which nukacin ISK-1 showed bacteriostatic activity.
Table 1. Susceptibility of different indicator strains to nukacin ISK-1 and nisin A.
Fig. 1. Antibacterial activity of nukacin ISK-1.
CFU values (A) immediately after peptide addition (white bars) and 24 h after peptide addition (black bars) in S. simulans and M. luteus are shown. Membrane depolarization (B), ATP release (C), and K+ leakage (nisin is considered 100% release) (D) were measured on S. simulans, M. luteus, and L. sakei cells with no peptide (gray bars), nukacin ISK-1 (white bars), and nisin (black bars) treatment. About 5X MIC of peptides were used for all experiments. The values shown are the means ± standard errors (error bars) for the three independent experiments.
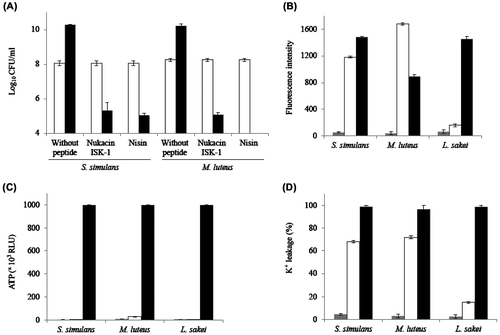
Since nukacin ISK-1 showed bactericidal activity against two indicator strains, we predicted that nukacin ISK-1 may influence the membrane integrity by membrane potential dissipation and pore formation. Therefore, we performed membrane depolarization assay by observing the fluorescence emitted by 3,3′-dipropyl thiadicarbocyanine iodide (DiS-C3) (Molecular Probes, Eugene, OR). Nukacin ISK-1 dissipated the membrane potential of S. simulans and M. luteus similar to the positive control, nisin (Fig. (B)). The inability of nukacin ISK-1 to dissipate the membrane potential of B. subtilisCitation9) and L. sakei (Fig. (B)) further indicates the necessity of cell membrane depolarization for bactericidal activity of the peptide. Previously, daptomycin, a lipopeptide antibiotic, showed a clear dose-dependent positive correlation between bactericidal activity and membrane depolarization.Citation12) In case of nisin, membrane integrity is also influenced by pore formation. Assuming that nukacin ISK-1 is also able to form pores, we performed an ATP leakage assay using a Lucifer-HS ATP detection kit (Kikkoman, Tokyo, Japan). Unlike nisin, nukacin ISK-1 did not release ATP from any indicator strains (Fig. (C)) suggesting that it cannot make pores large enough to translocate relatively large solutes like ATP under the conditions tested. Subsequently, we performed a K+ leakage assay using potassium-binding benzofuranisophthalate-AM (Molecular Probes) to observe the release of K+ ions, which have a smaller molecular size than ATP. Nukacin ISK-1 released K+ ions from S. simulans and M. luteus (Fig. (D)), suggesting the formation of a small-sized, transient pore similar to that caused by the type A(II) lantibiotic, SA-FF22Citation13) or a specific potassium ion-conducting pore similar to those reported by bacteriocin, enterocin PCitation14) and type A(I) lantibiotic, bovicin HC5.Citation15) Furthermore, a slight efflux by nukacin ISK-1-treated cells of L. sakei (Fig. (D)) and no efflux in B. subtilisCitation9) confirmed its selective activity. Pore-forming activity has been observed in another bactericidal type A(II) lantibiotic SA-FF22; however, until now, there has not been a report of a type A(II) lantibiotic having both bactericidal and bacteriostatic activities like nukacin ISK-1.
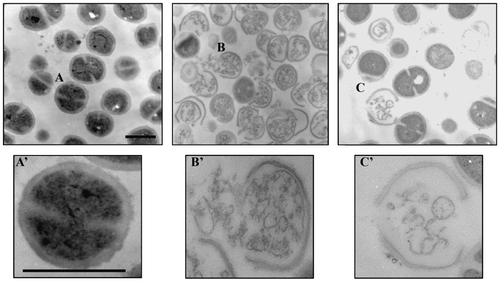
To visualize the cellular changes after nukacin ISK-1 treatment, TEM was performed using M. luteus cells treated with nukacin ISK-1 or nisin for 2 h.Citation16) In B. subtilis, nukacin ISK-1 treatment resulted in reduction in cell wall thickness without any abnormal changes that indicated cell death.Citation9) On the other hand, severe cell damage characterized by thinning and breakage of the cell wall resulting in the release of intracellular contents were found in nisin- and nukacin ISK-1-treated M. luteus cells compared with untreated control cells (Fig. ). Numbers of abnormal cells, however, were more evident in nisin-treated cells indicating the strong and quick action of nisin in the indicator cells. Since the cells were processed after 2 h of peptide treatment, it was expected that a longer incubation of cells with nukacin ISK-1 would increase the number of abnormal cells.
Fig. 2. Transmission electron micrographs of M. luteus cells.
Micrographs of the cells treated without peptide (A), with 10X MIC of nisin (B), and nukacin ISK-1 (C) for 2 h. Enlarged cross-sections of the cells without peptide (A’), with nisin (B’), and nukacin ISK-1 (C’). Bars in panels A and A’: 500 nm.
From the above results we conclude that nukacin ISK-1 has bactericidal activity against M. luteus and S. simulans where the peptide binds to lipid II followed by alteration of cell membrane integrity due to dissipation of membrane potential and formation of small-sized or specific potassium ion-conducting pores, which leads to cell death. Two other type A(I) lantibiotics, gallidermin and epidermin, have shown a dual mode of action to exhibit pore formation and inhibit cell wall synthesis, and they make a pore at the cytoplasmic membrane of selective bacterial species.Citation11) Christ et al.Citation17) reported that the dual activity of gallidermin is attributed to the difference in bacterial membrane lipid composition. Bactericidal and bacteriostatic modes of action of nukacin ISK-1 against the indicator strains may be due to the specific characteristics in bacterial species such as membrane lipid composition, membrane thickness, and/or the presence of unknown specific resistance mechanisms. Further, detailed studies are needed to shed light on the molecular mechanism controlling the specificity of activity.
Acknowledgment
Urmi Roy acknowledges a MEXT, Japan fellowship.
Funding
This work was partially supported by JSPS KAKENHI [grant number 23380050], and grants from the Japan Science Society, the Novartis Foundation (Japan) for the Promotion of Science, the Novozymes Japan Research Fund, and the Nagase Science and Technology Foundation.
References
- Cotter PD, Ross RP, Hill C. Bacteriocins- a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013;11:95–105.
- Delves-Broughton J, Blackburn P, Evans RJ, Hugenholtz J. Applications of the bacteriocin, nisin. Antonie van Leeuwenhoek. 1996;69:193–202.10.1007/BF00399424
- Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1:295–305.10.1002/cam4.35
- Jung G. Lantibiotics: a survey. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden: ESCOM; 1991. p. 1–34.
- Willey JM, van der Donk WA. Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol. 2007;61:477–501.10.1146/annurev.micro.61.080706.093501
- Wiedemann I, Benz R, Sahl H-G. Lipid II-mediated pore formation by the peptide antibiotic nisin: a black lipid membrane study. J. Bacteriol. 2004;186:3259–3261.10.1128/JB.186.10.3259-3261.2004
- Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJ, Rebuffat S, Ross RP, Sahl HG, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Süssmuth RD, Tagg JR, Tang GL, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013;30:108–160.10.1039/c2np20085f
- Islam MR, Nishie M, Nagao J, Zendo T, Keller S, Nakayama J, Kohda D, Sahl H-G, Sonomoto K. Ring A of nukacin ISK-1: a lipid II-binding motif for type-A(II) lantibiotic. J. Am. Chem. Soc. 2012;134:3687–3690.10.1021/ja300007h
- Asaduzzaman SM, Nagao J, Iida H, Zendo T, Nakayama J, Sonomoto K. Nukacin ISK-1, a bacteriostatic lantibiotic. Antimicrob. Agents Chemother. 2009;53:3595–3598.10.1128/AAC.01623-08
- Aso Y, Okuda K, Nagao J, Kamenasa Y, Phuong NTB, Koga H, Shioya K, Sashihara T, Nakayama J, Sonomoto K. A novel type of immunity protein, NukH, for the lantibiotic nukacin ISK-1 produced by Staphylococcus warneri ISK-1. Biosci. Biotechnol. Biochem. 2005;69:1403–1410.10.1271/bbb.69.1403
- Bonelli RR, Schneider T, Sahl H-G, Wiedemann I. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 2006;50:1449–1457.10.1128/AAC.50.4.1449-1457.2006
- Silverman JA, Perlmutter NG, Shapiro HM. Correction of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003;47:2538–2544.10.1128/AAC.47.8.2538-2544.2003
- Jack R, Benz R, Tagg J, Sahl H-G. The mode of action of SA-FF22, a lantibiotic isolated from Streptococcus pyogenes strain FF22. Eur. J. Biochem. 1994;219:699–705.10.1111/ejb.1994.219.issue-1-2
- Herranz C, Cintas LM, Hernandez PE, Moll GN, Driessen AJM. Enterocin P causes potassium ion efflux from Enterococcus faecium T136 cells. Antimicrob. Agents Chemother. 2001;45:901–904.10.1128/AAC.45.3.901-904.2001
- Mantovani HC, Russel JB. Bovicin HC5, a lantibiotic produced by Streptococcus bovis HC5, catalyzes the efflux of intracellular potassium but not ATP. Antimicrob. Agents Chemother. 2008;52:2247–2249.10.1128/AAC.00109-08
- Iida H, Wang L, Nishii K, Ookuma A, Shibata Y. Identification of rab12 as a secretory granule-associated small GTP-binding protein in atrial myocytes. Circ. Res. 1996;78:343–347.10.1161/01.RES.78.2.343
- Christ K, Al-Kaddah S, Wiedemann I, Rattay B, Sahl H-G, Bendas G. Membrance lipids determine the antibiotic activity of the lantibiotic gallidermin. J. Membr. Biol. 2008;226:9–16.10.1007/s00232-008-9134-4
