Abstract
To verify the effects of hiba essential oil in restrained stressed rats, we analyzed physiological variables and psychophysiological behavior. Stressed-HEO rats inhaled hiba essential oil aroma after restraint period. The quantities of food and water intake and the excretion amount of stressed rats were smaller than those of non-stressed control rats. Body weights of stressed rats decreased compared with those of control rats. These physiological variables of stress-HEO rats significantly recovered compared with those of stressed rats (P < 0.001). Stress-related anxiety was assessed using the elevated plus-maze test. Entry times into the open arms of stressed rats were less than those of control rats (P < 0.05). In contrast, the suppression of entry times into the open arms of stressed rats was restored by the inhalation of hiba oil. The results suggest that hiba essential oil inhalation reduced stress-induced growth inhibition and stress-related anxiety.
Graphical Abstract
Inhalation of hiba essential oil recovered behavioral responses in stressed rats, indicating a decrease in the stress level.
Animals receive many types of stress stimuli (stressors) and show various responses to stressors, such as avoidance behavior and clinical conditions characterized by widespread pain. The mechanisms by which stressors adversely affect patients have been generally considered to be at the central nervous system level. Stressors are initially detected by the sensory system, and information from the sensory system is transmitted to the brain. Subsequently, the release of corticosteroid and noradrenaline, which are caused by the activity of hypophysis and sympathetic nerves, respectively, changes the physiological and behavioral state.
Recently, lipid-soluble extracts from plants, i.e. essential oils, have attracted the attention of health care providers as substances that reduce stress responses. Plant-derived essential oils have traditionally been used in the treatment of some illnesses. In fact, the abilities of essential oils to restore emotional well-being and to reduce depression, anxiety, and stress have been revealed by human studies.Citation1–4) For example, exposure to lavender oil alleviated cancer pain and associated anxiety,Citation4) and lavender and rosemary essential oils enhanced free radical-scavenging activity and decreased stress hormones such as cortisol.Citation1) The effects of essential oils extracted from plants, such as lavender,Citation5) rose,Citation6–8) lemon,Citation9,10), and orange,Citation11) have also been reported using animal models. Fukada et al. (2012) revealed that the inhalation of rose essential oil significantly inhibits an increase in stress hormones in restraint stressed rats.Citation7) The study by Carvalho-Freitas and Costa (2002) on the treatment of mice with orange oil also revealed that this oil could be useful as an antianxiety agent.Citation11) Moreover, studies on rats have reported the neuroprotective effect of mastic tree essential oil in protecting the brain tissue against ischemia/reperfusion injury and that of lavender oil in alleviating oxidative damage in the brain tissue.Citation12,13) These observations provide some evidence for the use of essential oils as therapeutic agents.
Hiba (Thujopsis dolabrata) is a coniferous tree species endemic to Japan and is used as building materials for temples and shrines. The strong antimicrobial activity and healing effects of hiba essential oil are well known and can be attributed to thujopsene (C15H24), which is a major component of aroma, and hinokitiol (C10H12O2), which is a major component with antimicrobial activity, contained in the oil.Citation14) However, to the best of our knowledge, the efficacy of hiba essential oil has not been evaluated scientifically using animal models, although hiba essential oil potentially has efficient stress reduction or relaxation properties similar to other essential oils. The aim of the present study was to evaluate the effect of hiba essential oil on stress reduction using an irritable bowel syndrome (IBS) rat model. This rat model was developed as a method for assessing the symptoms of IBS in our laboratory.Citation15)
IBS is exacerbated by stressful life events.Citation16,17) The symptoms of IBS related to gastrointestinal dysfunction include abdominal cramping with pain and concurrent abnormal bowel habits, such as episodes of diarrhea, constipation, or both. IBS is most likely a multifactorial biopsychosocial disorder in which physiological, psychological, behavioral, and environmental factors all contribute to the clinical expression of the disorder. The data of the present study may possibly lead to the development of a simple method for reduction of the symptoms of IBS using aroma.
Materials and methods
Animals
Fischer 344 (F344) male rats (n = 36) were used in the experiments. Animals at 4 weeks of age were purchased from CLEA Japan, and a pair of rats was maintained in a standard rodent cage until 14 weeks of age. Twenty-four rats were subjected to restraint stress as described below and were called stressed rats. Half of stressed rats inhaled hiba essential oil after the restraint stress period (see below) and were called stress-HEO rats. Other 12 rats that were not subjected to stress were called control rats. Rats were housed and provided with food and water ad libitum in their cages at 25 °C in a light–dark–controlled room (lights on at 7:00–19:00). All the experiments were performed in accordance with the guidelines of the Physiological Society of Japan and were approved by the Animal Care and Use Committee of Iwate University.
Restraint stress and inhalation of hiba essential oil
The forepaws and hindpaws of stressed rats aged 5–14 weeks were lightly bound with rubber bands three times per week to induce IBS development. At 16:00 on Monday, Wednesday, and Friday, the rats with bound paws were placed in an acrylic cage (30- × 55- × 10-cm high) placed on a Kimtowel for 10 min (restraint period). Stress-HEO rats inhaled hiba essential oil in a 10-l capped container. A filter paper impregnated with 50-μl hiba essential oil was placed in the container, and stress-HEO rats were maintained in the container for 30 min after the restraint stress period. The concentration of hiba essential oil in the container was at a concentration that was appropriate for use in humans. Stressed rats were also maintained in a container without the oil. Control rats were placed in an acrylic cage without restraint and maintained in a container without oil with the same time schedule as that used for stress-HEO rats.
Measurements of changes in physiological variables
To estimate changes in physiological variables, the quantities of food and water intake and the excretion amount of rats in each cage were measured on Monday, Wednesday, and Friday. The values of the three measurements were summed to determine the weekly values. Stressed rats excreted soft feces during the restraint period. The excretion amount included the amount of soft feces during this period. The body weight was measured every Friday.
Elevated plus-maze test
The elevated plus-maze consists of two open arms (10 × 50 cm) and two enclosed arms (10 × 50 × 40 cm high) opposite to each other and 50 cm from the floor. At the beginning of the test, the rat was placed in the center of the elevated plus-maze and allowed to explore freely for 10 min. The test was performed in a quiet and dimly lit room from 16:00 to 17:30. The maze was cleaned after each rat was tested. Behavioral parameters, such as entry times into the open arms (number of visits to the open arms) and total moving distance (total locomotion distance), were recorded into a personal computer using a digital video camera (Handycam, Sony, Japan) for offline analysis. The data were analyzed using analysis software (Smart Junior, Panlab, Spain).
Anatomical analysis
When rats reached 14 weeks of age, they were euthanized with an overdose of pentobarbital sodium (1–2 ml/rat i.p.) (Kyoritsu Seiyaku Co., Tokyo, Japan). The adrenal glands, thymus, and gastrointestinal tract were then removed carefully by an expert in anatomy. Weights of the adrenal glands and thymus were measured using a precision balance (HR-202i, A&D Co., Tokyo, Japan), and the condition of the intestines was observed meticulously.
Statistical analyses
The differences in the time courses of the quantities of food and water intake, excretion amount, and body weight among stressed, stress-HEO, and control rats were statistically examined using two-way ANOVA. Ratios of weights of the adrenal glands and thymus relative to the body weight were used for statistical analyses. To calculate these ratios, weights of the adrenal glands and thymus of each rat were divided by the body weight of each rat at 14 weeks of age. The significant differences in weights of the adrenal glands and thymus between stressed and control rats and between stressed and stress-HEO rats were analyzed using the t-test. The differences in behavioral parameters were also analyzed using the t-test. Based on the results of the behavioral experiment, the data from two stressed rats were excluded because of abnormal values for the number of visits to the open arms (threefold greater than the average value of control rats). Values are presented as mean ± SD.
Results
Changes in physiological variables during the stress period
Stressed rats showed a significant decrease in the quantities of food and water intake and the excretion amount after 6 weeks of age compared with those of control rats [food: F(1,9) = 48.35, P < 0.001; water: F(1,9) = 73.21, P < 0.001; excretion: F(1,9) = 50.08, P < 0.001] (Fig. (A–C)). The body weight of stressed rats was less than that of control rats [F(1,9) = 32.41, P < 0.001] (Fig. (D)). These results indicate that mild restraint of the paws caused physiological stress.Citation15)
Fig. 1. Changes in the physiological variables.
Note: Changes in the quantities of food intake (A) and water intake (B), excretion amount (C), and body weight (D) during the experimental period are shown. All parameters of stressed rats were less than those of both control rats and stress-HEO rats during the experiment [horizontal hatched bars, food: F(2,18) = 40.47, P < 0.001; water: F(2,18) = 58.74, P < 0.001; excretion: F(2,18) = 39.41, P < 0.001; weight: F(2,18) = 31.50, P < 0.001]. Open and closed circles indicate the values of control (n = 12) and stressed rats (n = 10), respectively. Closed squares indicate the values of stress-HEO rats (n = 12). Error bars indicate SD.
![Fig. 1. Changes in the physiological variables.Note: Changes in the quantities of food intake (A) and water intake (B), excretion amount (C), and body weight (D) during the experimental period are shown. All parameters of stressed rats were less than those of both control rats and stress-HEO rats during the experiment [horizontal hatched bars, food: F(2,18) = 40.47, P < 0.001; water: F(2,18) = 58.74, P < 0.001; excretion: F(2,18) = 39.41, P < 0.001; weight: F(2,18) = 31.50, P < 0.001]. Open and closed circles indicate the values of control (n = 12) and stressed rats (n = 10), respectively. Closed squares indicate the values of stress-HEO rats (n = 12). Error bars indicate SD.](/cms/asset/8f9c9e6d-c25f-4b57-ac5c-512eb6c46d23/tbbb_a_918492_f0001_b.gif)
In contrast, the inhalation of hiba essential oil by stressed rats improved all physiological variables. The quantities of food and water intake and the excretion amount of stress-HEO rats showed greater values than those of stressed rats [food: F(1,9) = 36.78, P < 0.001; water: F(1,9) = 86.88, P < 0.001; excretion: F(1,9) = 14.72, P < 0.01] (Fig. (A–C)). The body weight of stress-HEO rats was also greater than that of stressed rats [F(1,9) = 52.36, P < 0.001] (Fig. (D)). These results suggest that the inhalation of hiba essential oil by stressed rats caused a decrease in the stress level compared with that of stressed rats not treated with the oil.
Anatomical findings
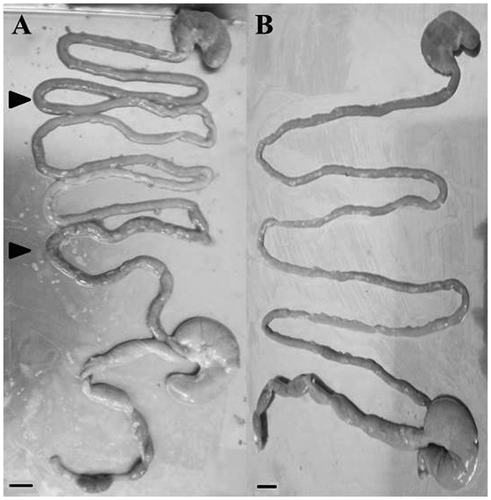
The ratio of the weight of the adrenal glands relative to the body weight of stressed rats was greater than that of control rats (P < 0.01) (Fig. (A)). Although the weight of the thymus of stressed rats was significantly less than that of control rats (0.18 ± 0.02 g in stressed rats, 0.22 ± 0.03 g in control rats, P < 0.05), no significant difference was observed in the ratio of the weight of the thymus between stressed and control rats (Fig. (B)). Intestinal bleeding was observed in 50% of stressed rats (Fig. (A)), whereas it was not observed in control rats.
Fig. 2. Ratios of weights of the adrenal glands and thymus relative to the body weight.
Note: (A) Ratios of weights of the adrenal gland of stressed and stress-HEO rats were greater than those of control rats (*P < 0.05, †P < 0.01). No significant difference was observed in the ratio of the weight of the adrenal glands between stress and stress-HEO rats. (B) No significant difference was observed in the ratio of the weight of the thymus among control, stressed, and stress-HEO rats. White, black, and gray columns indicate the percents of control (n = 12), stressed (n = 10), and stress-HEO rats (14 weeks of age, n = 12), respectively. Error bars indicate SD.
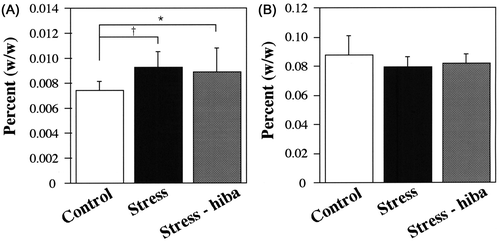
Fig. 3. Representative images of gastrointestinal tracts (14 weeks of age).
Note: (A) Gastrointestinal tracts from stressed rats. (B) Gastrointestinal tracts from stress-HEO rats. Intestinal bleeding was observed in stressed rats (arrowhead) but not in stress-HEO rats. Scale bar = 1 cm.
The ratio of the weight of the adrenal glands relative to the body weight of stress-HEO rats was also greater than that of control rats (P < 0.05) (Fig. (A)). Thus, no significant difference was observed in the ratio of the weight of the adrenal glands between stress-HEO and stressed rats. The weight of the thymus of stress-HEO rats was significantly greater than that of stressed rats (0.20 ± 0.02 g in stress-HEO rats, 0.18 ± 0.02 g in stressed rats, P < 0.01). However, no significant difference was observed in the ratio of the weight of the thymus between stress-HEO and stressed rats (Fig. (B)). Intestinal bleeding was observed in one stress-HEO rat (~8%) (Fig. (B)).
Behavioral observations in the elevated plus-maze test
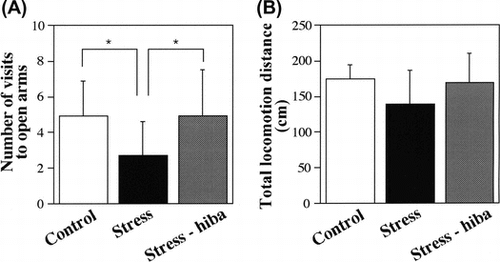
Rats placed in the center of the plus-maze apparatus began to explore the four arms. The number of visits to the open arms by stressed rats during the 10-min period was smaller than that by control rats (2.7 ± 1.9 in stressed rats, 4.9 ± 1.9 in control rats, P < 0.05) (Fig. (A)). In contrast, the number of visits to the open arms by stress-HEO rats was greater than that by stressed rats (2.7 ± 1.9 in stressed rats, 4.9 ± 2.6 in stress-HEO rats, P < 0.05) (Fig. (A)). This indicated that the number of entry times into the open arms was recovered in stress-HEO rats compared with control rats.
Fig. 4. Behavioral analysis in the elevated plus-maze test.
Note: (A) The number of visits to the open arms during the 10-min period. The number of visits to the open arms by stressed rats was smaller than that of control rats (P < 0.05), and the number of visits to the open arms by stress-HEO rats was recovered to the value of control rats (P < 0.05). (B) The total locomotion distance during the recording period (10 min). No significant difference was observed in the total locomotion distance among stressed, stress-HEO, and control rats. Note that the inhalation of hiba oil by stressed rats may have caused a decrease in the stress level compared with that of stressed rats not treated with the oil. White, black, and gray columns indicate the percents of control (n = 12), stressed (n = 10), and stress-HEO rats (n = 12), respectively. Error bars indicate SD.
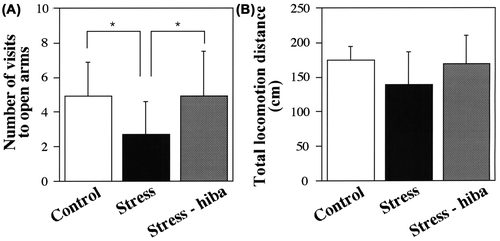
No significant difference was observed in the total locomotion distance among stressed, stress-HEO, and control rats (174.1 ± 20.2 cm in control rats, 138.6 ± 48.1 cm in stressed rats, 169.2 ± 40.7 cm in stress-HEO rats), although it was slightly shorter in stressed rats than in control and stress-HEO rats (Fig. (B)). These results indicate that the inhalation of hiba essential oil recovered behavioral responses in stressed rats.
Discussion
Chronic stress leads to decrease in the body weight and changes in physiological variables because of unbalanced homeostasis between the sympathetic and parasympathetic nervous systems. The unbalanced homeostasis during the stress period causes a decrease in the rate of secretion of mucus and induces intestinal bleeding.Citation18) When animals receive stimuli from stressors, the pituitary gland secretes adrenocorticotrophic hormone, which induces hyperplasia of the adrenal glands and atrophy of the thymus.Citation18,19) In the present study, physiological variables, such as the quantities of food and water intake, excretion amount, and body weight, of stressed rats were less than those of control rats (Fig. ). Weights of the adrenal glands and thymus also changed during the stress period (Fig. ). These results indicate that mild restraint of the paws caused a change in hormonal secretion chronically and then caused chronic stress responses, such as the symptoms of IBS, due to the repeated applications of stimuli.Citation15)
Sarro et al. (2014) reported that early life trauma can lead to long-term changes in the genetic profile of the amygdala that could contribute largely to the emergence of anxiety-like behaviors in adulthood with concomitant changes in genes associated with neurotransmission.Citation20) Social defeat stress also alters the expression levels of some proteins in specific brain areas and leads to oxidative stress-induced anxiety depression-like behaviors.Citation21) The combination of atenolol with alprazolam/escitalopram has the potential to be highly efficacious in treating anxiety and depressive disorders as well as oxidative stress.Citation22) These observations strongly indicate an interaction between stress and anxiety-like behavior. It is well known that the elevated plus-maze test is an excellent and robust test of anxiety-like behavior in rats. During the plus-maze test, rats are required to explore a new environment that includes two pairs of arms suspended at a certain level above the floor. Thus, this enables the study of anxiety in rodents. Because rats are afraid of open spaces, the decision to choose between the open and closed arms indicates their level of drive to explore open spaces, which is decreased by stress. In the present study, we showed that the symptoms of IBS in stressed rats affected the results of behavioral analysis (Fig. ). Stressed rats showed a reduced number of visits to the open arms in the plus-maze, while spontaneous locomotion was not significantly affected, indicating an increase in anxiety. The results indicate that mild restraint of the paws caused anxiety-like behavior due to the symptoms of IBS in rats.
We revealed that certain physiological and behavioral parameters of stress were decreased in stress-HEO rats. All physiological variables, i.e. quantities of food and water intake, excretion amount, and body weight, of stress-HEO rats were improved (Fig. ), although these did not completely recover to the levels of control rats. Lavender oil increases food intake and body weight by activating the parasympathetic nervous system,Citation23) whereas grapefruit oil decreases them by stimulating the sympathetic nervous system.Citation24) Hiba essential oil inhalation may exert physiological effects via inhibition of sympathetic nervous system activity, which causes stress responses. Moreover, the number of visits to the open arms by stress-HEO rats was almost the same as that by control rats, and the duration of spontaneous locomotion was not changed significantly (Fig. ). This indicated a reduction in anxiety-like effects in stress-HEO rats. These observations suggest that the inhalation of hiba oil by stress-HEO rats caused a decrease in the stress level and anxiety-like behavior compared with those of stressed rats. Hiba oil contains approximately 50% thujopsene,Citation14) which is a lipid-soluble compound and the major component of aroma. Although we could not identify the substance with stress-relieving effects in the present experiment, it is possible that thujopsene is the major component that relieved the stress level of the IBS rat model.
A similar efficacy in behavioral responses to aroma has been obtained with many essential oils. The inhalation of rose oil produced an anxiolytic-like effect, which led to an increase in the number of visits to and time spent in the open arms of the elevated plus-maze.Citation6) Orange oil also increased the time spent in the open arms of the elevated plus-maze.Citation11) Olfactory stimuli such as rose, orange, and hiba essential oils may possibly be absorbed into the blood via the membrane of the nose or lung and trigger a certain reaction in the brain and/or skin, with consequent reductions in stress-induced activation of the hypothalamo–pituitary–adrenocortical axis and skin barrier disruption.Citation7)
In the present study, we could not obtain recovery of ratios of weights of the adrenal glands and thymus relative to the body weight of stress-HEO rats (Fig. ). The reason for this is unclear, but we propose two possibilities. One is that the repeated applications of binding stimuli to the rat paws caused digestive dysfunction and may have led to a change in balance between the sympathetic and parasympathetic nervous systems,Citation15) leading to no change in functions of the adrenal glands and thymus. The other possibility is that the inhalation of hiba essential oil only was insufficient to eliminate the effect of stimuli from the stressor. In fact, physiological variables of stress-HEO rats did not recover to the levels of control rats (Fig. ). In conclusion, the inhalation of hiba essential oil relieved the stress level, although it was difficult to completely eliminate the effect of stimuli from the stressor. In this study, we could not sufficiently elucidate the mechanism of the efficacy of the oils. However, hiba essential oil contains useful substances that reduce the chronic stress level, i.e. the symptoms of IBS, in animals.
Acknowledgments
We are grateful to Asuka Nishino, Aki Kikuchi, Ayana Nakamura, and Tsubasa Suzuki for their helpful assistance in this experiment. This work was partially supported by the grant-in-aid for scientific research received by T. M. from the Japan Society for the Promotion of Science.
Notes
Abbreviations: stress-HEO rats, stressed rats inhaled hiba essential oil after the restraint stress period; IBS, irritable bowel syndrome.
References
- Atsumi T, Tonosaki K. Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Res. 2007;150:89–96.10.1016/j.psychres.2005.12.012
- Edge J. A pilot study addressing the effect of aromatherapy massage on mood, anxiety and relaxation in adult mental health. Complement. Ther. Nurs. Midwifery. 2003;9:90–97.10.1016/S1353-6117(02)00104-X
- Kyle G. Evaluating the effectiveness of aromatherapy in reducing levels of anxiety in palliative care patients: results of a pilot study. Complement. Ther. Clin. Pract. 2006;12:148–155.10.1016/j.ctcp.2005.11.003
- Louis M, Kowalski SD. Use of aromatherapy with hospice patients to decrease pain, anxiety, and depression and to promote an increased sense of well-being. Am. J. Hospice. Palliat. Care. 2002;19:381–386.
- Umezu T. Behavioral effects of plant-derived essential oils in the geller type conflict test in mice. Jpn. J. Pharmacol. 2000;83:150–153.10.1254/jjp.83.150
- Almeida RN, Motta SC, de Brito Faturi C, Catallani B, Leite JR. Anxiolytic-like effects of rose oil inhalation on the elevated plus-maze test in rats. Pharmacol. Biochem. Behav. 2004;77:361–364.10.1016/j.pbb.2003.11.004
- Fukada M, Kano E, Miyoshi M, Komaki R, Watanabe T. Effect of “rose essential oil” inhalation on stress-induced skin-barrier disruption in rats and humans. Chem. Senses. 2012;37:347–356.10.1093/chemse/bjr108
- Umezu T. Anticonflict effects of plant-derived essential oils. Pharmacol. Biochem. Behav. 1999;64:35–40.10.1016/S0091-3057(99)00115-X
- Buchbauer G, Jirovetz L, Jager W, Plank C, Dietrich H. Fragrance compounds and essential oils with sedative effects upon inhalation. J. Pharm. Sci. 1993;82:660–664.10.1002/(ISSN)1520-6017
- Ceccarelli I, Lariviere WR, Fiorenzani P, Sacerdote P, Aloisi AM. Effects of long-term exposure of lemon essential oil odor on behavioral, hormonal and neuronal parameters in male and female rats. Brain Res. 2004;1001:78–86.10.1016/j.brainres.2003.10.063
- Carvalho-Freitas MI, Costa M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium. Biol. Pharm. Bull. 2002;25:1629–1633.10.1248/bpb.25.1629
- Quartu M, Serra MP, Boi M, Pillolla G, Melis T, Poddighe L, Del Fiacco M, Falconieri D, Carta G, Murru E, Cordeddu L, Piras A, Collu M, Banni S. Effect of acute administration of Pistacia lentiscus L. essential oil on rat cerebral cortex following transient bilateral common carotid artery occlusion. Lipids Health Dis. 2012;11:1–8.
- Hancianu M, Cioanca O, Mihasan M, Hritcu L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine. 2013;20:446–452.10.1016/j.phymed.2012.12.005
- Okabe T, Ono H, Kodate S. Tree extraction ingredient “Aomori hiba oil” from Aomori hiba wood –Development of the antibacterial insecticide technique by the mist dispersion of the nanohiba oil–. J. Jpn. Assoc. Odor Environ. (in Japanese). 2012;43:128–137.
- Matsuura T, Takimura R, Yamaguchi M, Ichinose M. Estimation of restraint stress in rats using salivary amylase activity. J. Physiol. Sci. 2012;62:421–427.10.1007/s12576-012-0219-6
- Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, Schmulson M, Mayer EA. Use of aromatherapy with hospice patients to decrease pain, anxiety, and depression and to promote an increased sense of well-being. Am. J. Gastroenterol. 1999;94:1320–1326.10.1111/ajg.1999.94.issue-5
- Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52.10.1016/0016-5085(95)90267-8
- Selye H. Forty years of stress research: principal remaining problems and misconceptions. Can. Med. Assoc. J. 1976;115:53–56.
- Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381.10.1038/nrendo.2009.106
- Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258:147–161.10.1016/j.neuroscience.2013.10.064
- Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86.10.1016/j.brainres.2013.09.033
- Shahzad N, Ahmad J, Khan W, Al-Ghamdi SS, Ain MR, Ibrahim IA, Akhtar M, Khanam R. Interactions of atenolol with alprazolam/escitalopram on anxiety, depression and oxidative stress. Pharmacol. Biochem. Behav. 2014;117:79–84.10.1016/j.pbb.2013.12.015
- Shen J, Niijima A, Tanida M, Horii Y, Maeda K, Nagai K. Olfactory stimulation with scent of lavender oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci. Lett. 2005;383:188–193.10.1016/j.neulet.2005.04.010
- Shen J, Niijima A, Tanida M, Horii Y, Maeda K, Nagai K. Olfactory stimulation with scent of grapefruit oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci. Lett. 2005;380:289–294.10.1016/j.neulet.2005.01.058
