Abstract
Rubus coreanus Miquel (RCM) is used to promote prostate health and has been shown to have anti-oxidant and anti-carcinogenic activities. However, the effects and mechanisms of RCM on prostate cancer metastasis remain unclear. PC-3 and DU 145 cells were treated with ethanol or water extract of unripe or ripe RCM and examined for cell invasion, migration, and matrix metalloproteinases (MMPs) activity and expression. Phosphoinositide 3-kinase (PI3K) and Akt activities were examined. Unripe RCM extracts exerted significant inhibitory effects on cell migration, invasion, and MMPs activities. A significant reduction in MMPs activities by unripe RCM ethanol extract treatment (UE) was associated with reduction of MMPs expression and induction of tissue inhibitors of metalloproteinases (TIMPs) expression. Furthermore, PI3K/Akt activity was diminished by UE treatment. In this study, we demonstrated that UE decreased metastatic potential of prostate cancer cells by reducing MMPs expression through the suppression of PI3K/Akt phosphorylation, thereby decreasing MMP activity and enhancing TIMPs expression.
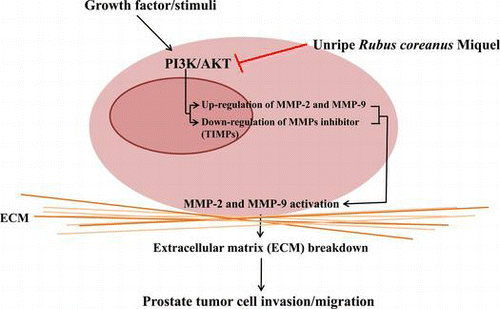
Graphical Abstract
Unripe Rubus coreanus Miquel decreased metastatic potential of prostate cancer cells by reducing MMPs activity through the suppression of PI3K/Akt phosphorylation.
Prostate cancer is the second leading cause of cancer-related deaths among men worldwide.Citation1) A recent report indicated that the incidence of prostate cancer in South Korea has increased by approximately 13.2% since 1999.Citation2) The sudden increase in the incidence of prostate cancer is suggested to be associated with extended lifespan and dietary changes that include consumption of westernized foods high in calories and animal fats and low in dietary fiber.Citation3) Benign prostate tumors can be removed by surgery, radiation, or chemotherapy, but metastasized prostate cancer is difficult to eliminate.Citation4) Thus, blocking the metastasis of prostate cancer cells is an effective way to improve the survival rate of patients with prostate cancer.
Rubus coreanus Miquel (RCM) is used as a traditional herbal medicine for impotence, spermatorrhoea, enuresis, and asthma treatment.Citation5) RCM is a rich source of antioxidants and contains many antioxidant compounds such as niga-ichigoside F1, gallic acid, 2,3-(S)-HHDP-D-glucopyranose, sanguin, protocatecuic acid, caffeic acid, courmaric acid, rosemarinic acid, anthocyanin, pelargonidin 3-glucoside, vanillic acid, syringic acid, and salicylic acid.Citation6–9) These RCM constituents have been shown to exert diverse functional effects, including anti-osteoporosis, anti-inflammation, and anti-carcinogenesis.Citation10,11)
Anti-carcinogenic functions of RCM are suggested to act partly by inducing DNA damage,Citation12) activating apoptosis,Citation13) and reducing angiogenesis.Citation14) Sanguiin H-6 extracted from RCM has been shown to decrease angiogenesis in endothelial cells via the inhibition of vascular endothelial growth factor receptor–ligand binding.Citation14) The anti-ulcer activity of RCM anthocyanins has also been associated with reduced matrix metalloproteinase (MMP)-2 expression levels.Citation15) In several previous studies, RCM was able to successfully reduce the proliferation of prostate cancer cells and induce apoptosis in both in vitro and animal models.Citation16,17) These anti-tumor effects appear to be androgen-independent, and are stronger when unripe RCM is used than ripe RCM.Citation16,17) Even though several studies have reported anti-tumor effects of RCM, there is no clear evidence for an anti-metastatic role of RCM in prostate cancer and little is understood about the possible mechanisms underlying such effects. In the present study, we aimed to investigate whether RCM has anti-metastatic potential in malignant prostate cancer cells and hypothesized that RCM could hinder tumor invasion and migration by inducing changes in the activity and/or expression levels of MMPs and tissue inhibitors of metalloproteinases (TIMPs).
Materials and methods
Cell culture
Human prostate cancers, PC-3 and DU 145, cells were purchased from the Korea Cell Line Bank (Seoul, Korea). Cells were cultured at 37 °C in Rosewell Park Memorial Institute 1640 (RPMI 1640; WelGENE, Korea) containing 10% fetal bovine serum (FBS; Gibco, USA) and 1% penicillin streptomycin (P/S; WelGENE, Korea) under a humidified 5% CO2 atmosphere.
Preparation of RCM extracts
Ripe and unripe fruits of RCM were harvested in the Gochang province of South Korea and freeze-dried. Four different extracts were prepared from the fruits: (i) 50% ethanol extracts of unripe RCM (UE), (ii) aqueous extracts of unripe RCM (UH), (iii) 50% ethanol extracts of ripe RCM (RE), and (iv) aqueous extracts of ripe RCM (RH). Freeze-dried unripe or ripe RCM were prepared by removing seed, pulverized, and extracted in solutions of 30 grams of berries per liter of distilled water or 50% ethanol by heating at 45 °C for 1.5 h. Supernatants were collected and frozen overnight at −80 °C and dried with a lyophilizer. RCM was dissolved in 0.4% (v/v) dimethyl sulfoxide at a final concentration of 100 mg/mL. Stock solutions of the RCM were aliquoted and kept at −20 °C for storage.
Cell viability assay
PC-3 and DU 145 cells (4 × 104) were plated into a 48-well plate, and 100 and 200 μg/mL of RCM extracts were added to the cultured cells for 24 h. Cell viability was assessed by adding 1 mg/mL of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to each well and incubated for 2 h at 37 °C. After removal of the medium, isopropyl alcohol was added to dissolve purple formazan crystals that were formed by the live cells. The absorbance of the colored solution was measured at 450 nm using a microplate reader (Spectra MAX 340; Molecular Devices).
Wound healing migration assay
PC-3 and DU 145 cells were seeded at a density of 1 × 106 cells in 60 mm-dishes and grown to approximately 90–95% confluence after 24 h. The medium was removed, and the cell monolayer was wounded by scraping the cells with a pipette tip to create a denuded zone of constant width. Debris was removed and cells were cultured with fresh medium containing 100 μg/mL of RCM extracts for 24, 48, and 72 h time points. Cell migration was observed by light microscopy and photographed. The wound area was measured using the program Image J (provide by NCBI). The percentage of wound closure was calculated by the following equation:
where Tt time after wounding, and T0 time immediately after wounding.
Matrigel invasion assay
Polycarbonate filters (8-μm pore) were pre-coated with matrigel. PC-3 and DU 145 cells were seeded into the upper chamber of a Boyden matrigel with 5 × 104 cells/well in serum-free medium containing 100 μg/mL of RCM extracts, and the complete medium containing 5% FBS was added to the lower chamber as a chemo-attractant. After incubating the cells for 40 h, the non-migrated cells in the upper chamber were wiped with a cotton swab. Cells that invaded the lower surface of the membrane were fixed and stained with 0.2% crystal violet. The invaded cells on the lower surface of the membrane filter were scored using random field light microscopy.
Gelatin zymography
The activities of MMP-2 and MMP-9 in PC-3 and DU 145 cells were measured following treatment with RCM extracts. Cells (4 × 104 cells/well) plated in 48-well plates were incubated in serum-free medium with 100 μg/mL of RCM extracts for 24 h, after which the conditioned medium was collected. Samples were mixed with loading buffer (0.125 M Tris–HCl [pH 6.8], 4% SDS, 20% glycerol, and 0.01% bromophenol blue) and electrophoresed at 100 V for 2 h at 4 °C on 8% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE) containing 10% gelatin. After electrophoresis, gels were washed in 2.5% Triton X-100 in dH2O twice for a total of 40 min at room temperature to remove SDS, followed by incubation at 37 °C in reaction buffer (1 M Tris–HCl [pH 7.5], 1 M CaCl2, 5 M NaCl, 0.2 mM ZnCl2, 25% Triton X-100, and 0.2% NaN3). After 24 h, the gels were stained with Comassie Brilliant Blue R-250 solution (10% acetic acid, and 40% methanol) for 1 h and destained with a destaining solution (10% acetic acid, 40% methanol, and 50% dH2O) until bands could be clearly visualized. The quantification of band density was performed using the NIH Image J software.
Western blot analysis
PC-3 and DU 145 cells were treated with 100 or 200 μg/mL of UE for 24 h then resuspended with RIPA lysis buffer containing cocktail protease inhibitors and phosphatase inhibitors (Sigma, USA). The cell extractions were centrifuged at 10,000 × g for 30 min at 4 °C, and the supernatants were collected as cell lysates. The cell lysates were quantified using a Bio-Rad protein assay kit (Hercules, CA, USA) with bovine serum albumin as a standard. The abundance of MMP-2, MMP-9, TIMP-1, TIMP-2, β-actin (Santa Cruz Biochem, USA), p-phosphoinositide 3-kinase (PI3K), and p-Akt (Cell Signaling Technology, USA) were determined by SDS-PAGE and western blotting as previously described.Citation18)
RNA preparation and RT-PCR analysis
PC-3 and DU 145 cells were incubated with various concentrations of UE for 24 h. Total RNA was prepared using RNeasy mini kits (QIAGEN, Inc., Valencia, CA, USA) according to the manufacturer’s protocol and stored at −80 °C before use. Total RNA was reverse-transcribed with Superscript First strand synthesis systems kits (Invitrogen, Carlsbad, CA, USA) to obtain cDNA. All PCR reactions using a thermal cycler (GenePro) were subsequently carried out in a 20 μL volume containing Takara Ex Taq mixture (Takara, Japan), 100 pmol of 5′ and 3′ primers, and cDNA. The following PCR primers were used: MMP-2: 5′-CTCCACTGGATGGAGGAAAA-3′ (forward) and 5′-CTTTCCAGCAGACACCATCA-3′ (reverse); MMP-9: 5′-GGCTAGGTGACCTATGACATCC-3′ (forward) and 5′-ACTCCTCCCTTTCCTCCAGA-3′ (reverse); TIMP-1: 5′-AAGGCTCTGAAAAGGGCTTC-3′ (forward) and 5′-GAAAGATGGGAGTGGGAACA-3′ (reverse); TIMP-2: 5′-CAGAAAAAGCTGGGTCTTGC-3′ (forward) and 5′-AGTGTCCTGGAGGCTGAGAA-3′ (reverse); GAPDH: 5′-ACATCATCCCTGCCTCTACTGG-3′ (forward) and 5′-AGTGGGTGTCGCTGTTGAAGTC-3′ (reverse). The reaction conditions were as follows: denaturation at 94 °C for 30 s; annealing at 58 °C (MMP-2, TIMP-1 and TIMP-2), 60 °C (MMP-9), or 62 °C (GAPDH) for 30 s; and extension at 72 °C for 30 s. Varying cycles of PCR (GAPDH, 28 cycles; MMP-2, 38 cycles; MMP-9, 37 cycles; TIMP-1 and TIMP-2, 27 cycles) were carried out to determine linear ranges of PCR products. PCR products were electrophoresed on a 1% agarose gel and visualized under UV after ethidium bromide staining.
Statistical analysis
Experiments were repeated at least three times, and results were expressed as the mean ± standard error. All data were statistically analyzed using one-way analysis of variance, followed by Duncan’s multiple range test. In all cases, results were considered significant if p value was less than 0.05. All statistical tests were performed using the statistical analysis software (SPSS package V20.0, Chicago, IL, USA).
Results
Effects of RCM on prostate cancer cell viability
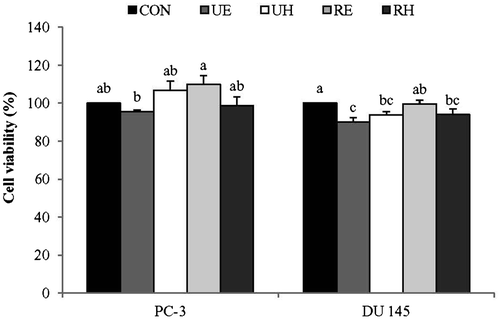
No significant differences in cell viability were observed for UE, UH, RE, or RH treated PC-3 cells compared to the control (Fig. ). These data suggest that ripe and unripe RCM extracts do not have significant effects on PC-3 cell viability at a concentration of 100 μg/mL regardless of the solvents used for extraction. On the other hand, there was approximate 10% reduction in DU145 cell viability after UE, UH, or RH treatment but not after RE treatment in comparison to the control (Fig. ). Thus, 100 μg/mL of RCM extracts appear to either exert no significant effects or about a slight reduction in the viability of metastatic prostate cell lines of PC-3 and DU145.
Fig. 1. Effects of R. coreanus Miquel (RCM) Extracts on Cell Viability in PC-3 and DU 145.
Notes: PC-3 and DU 145 were treated with UE, UH, RE, and RH (100 μg/mL) for 24 h, at which time cell viability was measured using an MTT assay. Values not sharing the same letter were significantly different (p < 0.05). (CON: control, UE: unripe R. coreanus Miquel ethanol extract, UH: unripe R. coreanus Miquel water extract, RE: ripe R. coreanus Miquel ethanol extract, and RH: ripe R. coreanus Miquel water extract).
Effects of RCM on prostate cancer cell migration and invasion
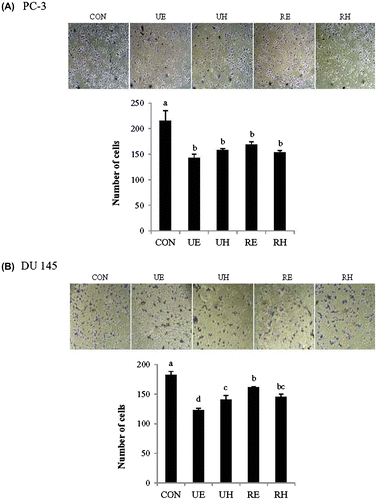
In order to assess the effects of RCM extracts on prostate cancer cell migration, we determined whether RCM extracts affect the migration of PC-3 and DU 145 tumor cells using an in vitro wound-healing assay and a matrigel invasion assay. First, the migration rate of the control cells was compared to those of the RCM extract-treated cells in an in vitro wound-healing experiment after a wound was introduced to mono-layered cells. The control cells were able to recover 80% of the wound area within 3 days in both PC-3 and DU 145 cells (Fig. (A) and (B)). However, most RCM extracts, except for RE, showed a significant delay in covering the denuded area compared to the control. UE, UH, and RH inhibited about 70% of DU 145 cell migration and 50–60% of PC-3 cell migration. RE only showed a 16–20% reduction in both cell lines compared to the control at the end of the experiment (Fig. (A) and (B)). For the matrigel invasion assay, the number of prostate cancer cells from the Boyden upper chamber, containing either RCM extracts or the control, that invaded the lower chamber containing 5% FBS-containing media was compared among the groups. PC-3 and DU 145 cells treated with any of the RCM extracts showed a significant reduction of invasion compared to the control (Fig. (A) and (B)). However, the degree of reduction in cell invasion was the greatest upon UE treatment in both PC-3 and DU 145 cells. Taken together, these results indicate that RCM extracts diminished the migration and invasion of prostate cancer cells.
Fig. 2. Time-dependent Effects of RCM Extracts on Migration in PC-3 and DU 145.
Notes: PC-3 (A) and DU 145 cells (B) were grown to confluency in 60-mm cell culture dishes, and then a scratch was made through the cell layer. After washing with PBS, cells were treated with UE, UH, RE, and RH (100 μg/mL) for 24, 48, and 72 h. The percentage of cell migration at each time point was measured using the wound healing migration assay. Values not sharing the same letter were significantly different (p < 0.05). (CON: control, UE: unripe R. coreanus Miquel ethanol extract, UH: unripe R. coreanus Miquel water extract, RE: ripe R. coreanus Miquel ethanol extract, and RH: ripe R. coreanus Miquel water extract).
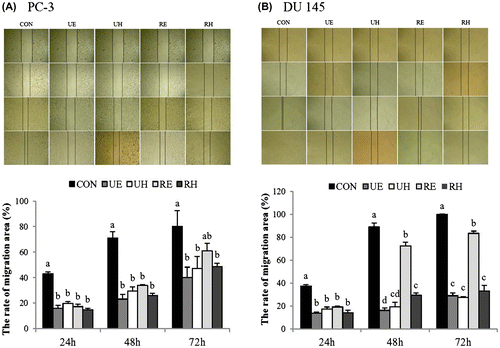
Fig. 3. Effects of RCM Extracts on Cell Invasion in PC-3 and DU 145.
Notes: PC-3 (A) and DU 145 (B) cells were treated with UE, UH, RE, and RH (100 μg/mL) for 40 h in a Boyden chamber. Invasion was quantified by counting the number of cells that invaded the underside of the porous polycarbonate membrane under microscopy and represent the average of 3 independent experiments. Values not sharing the same letter were significantly different (p < 0.05). (CON: control, UE: unripe R. coreanus Miquel ethanol extract, UH: unripe R. coreanus Miquel water extract, RE: ripe R. coreanus Miquel ethanol extract, and RH: ripe R. coreanus Miquel water extract).
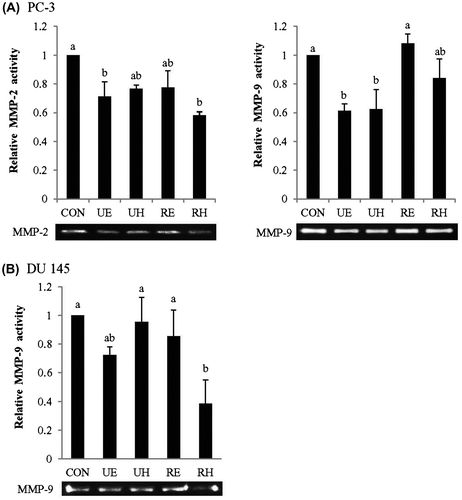
Effects of RCM on the activity of MMPs
We investigated whether the reduced migration and invasion of PC-3 and DU 145 cells by RCM extracts were associated with diminished activity of MMPs. After 24 h incubation with 100 μg/mL of RCM extracts, the activity of MMPs was examined in PC-3 and DU 145 cells by zymography (Fig. (A) and (B)). UE and RH elicited a significant reduction of MMP-2 activity and UE and UH lowered MMP-9 activity compared to the control in PC-3 cells (Fig. (A)). Treatment of PC-3 cells with UE alone demonstrated a significant reduction in the activities of both MMP-2 and MMP-9 (Fig. (A)). In DU 145 cells, MMP-9 activity was lowered by UE and RH but UE was less effective than RH with no statistical significance compared to the control (Fig. (B)). MMP-2 activity was too weak to detect its activity in DU 145 cells. Overall, 100 μg/mL of UE are the most effective in lowering both MMP-2 and MMP-9 activities in PC-3 cells among RCM extracts whereas 100 μg/mL of RH or possibly UE are effective in lowering MMP-9 activity in DU 145 cells. Hence, we chose to use two different doses of UE (100 μg/mL and 200 μg/mL) for the remaining experiments to investigate the mechanism of their effects on prostate cancer cell metastasis in both PC-3 and DU 145 cells.
Fig. 4. Effects of RCM Extracts on MMP-2 and MMP-9 Activities in PC-3 and DU 145 Cells.
Notes: PC-3 (A) and DU 145 (B) were seeded in 48-well plates at a density of 4 × 104 cells/mL. Cells were treated with UE, UH, RE, and RH (100 μg/mL) for 24 h. Medium was collected, and the activities of MMP-2 and MMP-9 were measured by gelatin zymography. Relative activities of MMP-2 and MMP-9 were measured by zymography. Values not sharing the same letter were significantly different (p < 0.05). (CON: control, UE: unripe R. coreanus Miquel ethanol extract, UH: unripe R. coreanus Miquel water extract, RE: ripe R. coreanus Miquel ethanol extract, and RH: ripe R. coreanus Miquel water extract).
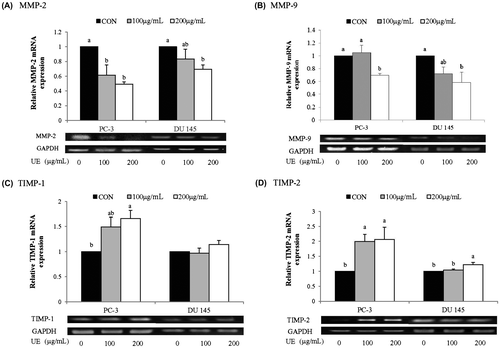
Effects of UE on MMP and TIMP expression
The reduction of MMP activity by UE could be due to lowered expression levels of MMPs and/or increased expression levels of TIMPs. Thus, we measured mRNA and protein levels of MMP-2 and MMP-9, as well as TIMP-1 and TIMP-2, in UE-treated prostate cancer cells. 200 μg/mL of UE had no significant effect on PC-3 cell viability but reduced about 40% of DU 145 cell viability (data not shown). Both PC-3 and DU 145 cells demonstrated a tendency of a reduction in the protein and mRNA levels of MMP-2 and MMP-9 with as low as 100 μg/mL of UE (Figs. (A) and (B) and (A) and (B)) except MMP-9 mRNA in 100 μg/mL of UE treated PC-3 cells. A higher dose of UE (200 μg/mL) significantly down-regulated the expression of MMP-2 and MMP-9 in both cell lines at both mRNA and protein levels. The reduced expression levels of the precursor form of MMP-2 by UE were related to a decrease in the active form of MMP-2 in UE-treated cells (Fig. (A)). Furthermore, a significant up-regulation of TIMP-1 and TIMP-2 expression were detected from a concentration of 100 μg/mL UE in PC-3 cells whereas TIMP-2 alone was slightly up-regulated in DU 145 cells treated with 200 μg/mL of UE. Therefore, it appears that UE treatment resulted in a reduction of MMP-2 and MMP-9 activity through a reduction in the expression of MMPs and an induction in the expression of TIMPs.
Fig. 5. Effects of RCM Extracts on MMP-2, MMP-9, TIMP-1, and TIMP-2 Protein Expression in PC-3 and DU 145.
Notes: PC-3 and DU 145 were treated with UE (100 and 200 μg/mL) for 24 h. Relative levels of MMP-2 (A), MMP-9 (B), TIMP-1 (C), and TIMP-2 (D) protein expression were measured by western blotting. Values not sharing the same letter were significantly different (p < 0.05). (CON: control and UE: unripe R. coreanus Miquel ethanol extract).
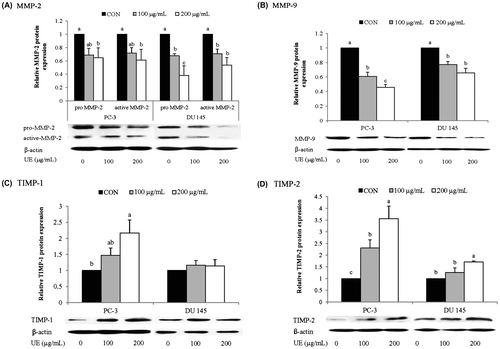
Fig. 6. Effects of RCM Extracts on MMP-2, MMP-9, TIMP-1, and TIMP-2 mRNA Expression in PC-3 and DU 145.
Note: PC-3 and DU 145 were treated with UE (100 and 200 μg/mL) for 8 h. Relative levels of MMP-2 (A), MMP-9 (B), TIMP-1 (C), and TIMP-2 (D) mRNA expression were measured by RT-PCR. Values not sharing the same letter were significantly different (p < 0.05). (CON: control and UE: unripe R. coreanus Miquel ethanol extract).
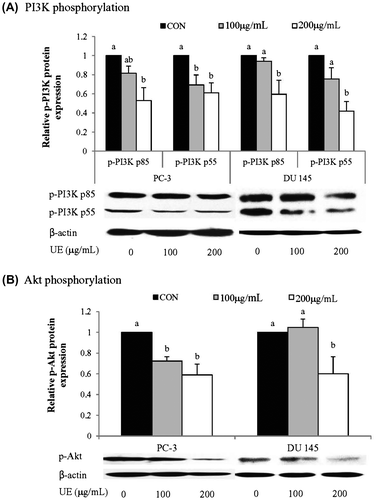
Inhibitory effects of UE on PI3K and Akt phosphorylation
To determine whether the effects of UE on the metastatic potential of prostate cancer cells are associated with the regulation of PI3K/Akt signaling pathway, we assessed the phosphorylation status of PI3K/Akt following UE treatment (Fig. (A)). UE treatment led to a significant decrease of p-PI3K and p-Akt in a dose-dependent manner in PC-3 cells (Fig. (A) and (B)). On the other hand, DU 145 cells decreased the levels of p-PI3K and p-Akt only with 200 μg/mL of UE (Fig. (A) and (B)). These results indicate that UE inhibits the PI3K/Akt signaling pathway in PC-3 cells with greater degree than in DU 145 cells. UE-mediated down-regulation of MMPs and up-regulation of TIMPs might involve the inhibition of the PI3K/Akt pathway in prostate cancer cells.
Fig. 7. Effects of RCM Extracts on p-PI3K and p-Akt activity in PC-3 and DU 145.
Notes: PC-3 and DU 145 were treated with UE (100 and 200 μg/mL) for 8 h. Relative p-PI3K (A) and p-Akt (B) activity were measured by western blotting. Values not sharing the same letter were significantly different (p < 0.05). (CON: control and UE: unripe R. coreanus Miquel ethanol extract).
Discussion
We have demonstrated that RCM is capable of exerting anti-metastatic effects by reducing the expression and activity of MMPs along with inhibition of the PI3K/Akt signaling pathway. In general, unripe ethanol extracts appear to be the most effective among RCM extracts in their ability to hinder the metastatic potential of prostate cancer cells based on the strongest inhibition of cell migration and invasion assay results compared to other extracts. The types and/or amounts of chemical compounds found in RCM can change over the course of maturation.Citation9,19,20) For instance, unripe RCM has more protocatechuic acid, cinnamic acid, ferulic acid, vanillic acid, and epicatechine than does ripe RCM.Citation9,19,20) Therefore, it could be possible that the methods used here (ethanol extraction) were biased toward the extraction of active constituents abundant in unripe RCM and that these compounds are most responsible for the strong anti-metastatic effects observed in prostate cancer cells following UE treatment.
In order to understand the mechanisms of the anti-metastatic action of RCM, changes in the expression levels of MMPs and TIMPs were examined in UE-treated cells. MMPs and TIMPs are important regulators of metastasis in cancer cells, and they operate by regulating the cleavage of the extracellular matrix, thereby facilitating the migration of metastatic cells.Citation21) There are 17 different kinds of MMPs, among which MMP-2 and MMP-9 are considered critical in the regulation of tumor invasion and metastasis.Citation22–24) It has been shown that the up-regulation of MMP-9 leads to tumor invasion and metastasis in hepatoma cells.Citation25) Conversely, knocking down MMP-2 and MMP-9 genes was found to decrease tumor invasion, migration, metastasis, and angiogenesis in glioblastoma and prostate cancer cells.Citation26–29) TIMPs inhibit the activity of MMP-2 and MMP-9.Citation30) Elevated expression levels of TIMPs result in the suppression of MMP-2 activity and reduces tumor metastasis,Citation31) an effect that has been noted in various in vitro and in vivo cancer models, including stomach, pancreatic, and ovarian cancers. Citation32) Therefore, the relative expression levels of MMPs and TIMPs are considered crucial for the determination of cell migration and invasion. In our study, the UE-mediated down-regulation of MMPs and up-regulation of TIMPs led to a drastic reduction in the expression of MMPs relative to the expression levels of TIMPs. These changes induced by UE appear to lead to a marked reduction in the migration and invasion of prostate cancer cells, as shown in our in vitro wound healing and matrigel invasion assays.
The importance of PI3K/Akt signaling in cell migration and invasion has been implicated by its role in the regulation of MMP and TIMP gene expression.Citation33,34) Upon activation of PI3K/Akt by neuronal growth factors, an increase in MMP-2 gene expression and a decrease in TIMP-2 gene expression were observed, accompanied by AP-2 transcriptional activation.Citation35) In addition, in liver cancer cells, radiation-triggered PI3K/Akt signaling was reported to increase MMP-9 expression through an increase in transcriptional activity of NF-κB.Citation36) Furthermore, fibronectin, a major component of the extracellular matrix was able to activate MAPK and PI3K/Akt signals, resulting in an increase in the secretion of MMP-9 in ovarian cancer cells.Citation37) Therefore, the activation of PI3K/Akt signals likely up-regulate the expression of MMPs and mediate the metastasis of cancer cells. Our study demonstrating that UE treatment inactivated the PI3K/Akt signaling pathway may suggest that UE effects on the expression of MMPs and TIMPs in prostate cancer cells could be mediated by aborting PI3K/Akt signals. Overall the UE effects in the regulation of MMP activity and MMP and TIMP expression along with activation of PI3K signals was more conspicuous in PC-3 cells than DU 145 cells, implicating that DU 145 cells might involve other regulatory mechanism as well.
According to previous reports, gallic acid, fisetin, and silibinin suppressed the migration of cancer cells by inhibiting PI3K/Akt activation and expression of MMP-2 and MMP-9.Citation38–41) Various phenolic compounds, including cyanidin, indole, gallic acid, and silibinin, also reduced metastasis by suppression of MMP-2 and MMP-9 expression.Citation6,38,42–47) Therefore, down-regulation of the PI3K/Akt signaling pathway by UE might be attributable to high levels of phenolic components.
High phenolic compounds containing RCM extracts significantly reduced reactive oxygen species (ROS) formation compared to control (data not shown). These anti-oxidant activities of RCM constituents could contribute to its anti-metastatic effects. ROS are demonstrated to increase the metastatic potential of cancer cells, in part through activating PI3K/Akt signals, along with inducing changes in the expression levels of MMPs and TIMPs.Citation48–50) ROS production is generally elevated in rapidly growing cancer cells due to enhanced glucose metabolism.Citation51) ROS activate PI3K/Akt signals and increase the expression of genes involved in cancer cell migration and neovascular formation.Citation33,34,52) Thus, treatment with antioxidants appears to suppress cancer cell metastasis by eliminating ROS, which leads to the subsequent decrease of PI3K/Akt signals.Citation40)
Quercetin, kaempferol, and myricetin, which act as ROS scavengers, suppressed the production of ROS in cancer cells and lowered PI3K/Akt and MAPK/Erk signals.Citation53) These changes are thought to result in the prevention of growth and metastasis of colon and prostate cancer cells.Citation53) Other studies also demonstrated that antioxidant activities reduced MMP expression and tumor invasion.Citation54–56) Based on these reports, it is likely that antioxidants in UE could greatly suppress PI3K/Akt signaling and prevent prostate cancer cell metastasis.
RCM is widely used for the prevention of prostate-related diseases, including prostate cancer in Korea. Its unripe ethanol extracts may be more effective in the prevention of the migration and invasion of prostate cancer cells. Although further studies are warranted, the mechanisms underlying the effects of UE as an anti-metastatic agent are likely involved in the scavenging of ROS, inhibition of the PI3K/Akt signaling pathway, suppression of MMP expression, and activation of TIMP expression. Our data provide supporting evidence for the use of UE as a supplement for the prevention of prostate cancer metastasis.
Author contributions
Yesl Kim contributed to analyze data and write the manuscript. Seung Min Lee and Jung-Hyun Kim, as co-corresponding authors, equally contributed to edit the data and to prepare the manuscript.
bbb-130858-File012.docx
Download MS Word (10.3 KB)Acknowledgments
We thank Sunbok Lee for technical assistance.
Funding
This research was financially supported by the National Research Foundation of Korea (Project No. 2009-0068227).
References
- Ferlay J, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2. IARC CancerBase No. 10. Lyon: International Agency for Research on Cancer; 2010.
- Ministry of Health and Welfare, Korea Central Cancer Registry, and National Cancer Center. Cancer registration statistics program. National Cancer Center: Seoul; 2012. p. 33.
- Kim SO. Effect of indole-3-carbinol on inhibition of MMP activy via MAPK signaling pathway in human prostate cancer cell line, PC3 cells. Korean J Nutr. 2008;41:224–231.
- Ha HK, Yun CJ, Lee SS, Shin DG, Lee W, Lee ZZ, Chung MK. Survival rates and related factors in men with hormone-refractory prostate cancer. Korean J. Urol. 2009;50:649–655.10.4111/kju.2009.50.7.649
- Heo J. Donguibogam. Seoul: Yeogang; 1994. p. 946–947.
- Choi YH, Kim SO. Inhibition of cell invasion by indole-3-carbinol in OVCAR-3 human ovarian cancer cells. J. Life Sci. 2011;21:923–931.10.5352/JLS.2011.21.7.923
- Lee J, Dossett M, Finn CE. Rubus fruit phenolic research: the good, the bad, and the confusing. Food Chem. 2012;130:785–796.10.1016/j.foodchem.2011.08.022
- Pang K, Kim M, Lee M. Hydrozable tannins from the fruits of Rubus coreanum. Korean J Pharmacogn. 1996;27:366–370.
- Kim HS, Park SJ, Hyun SH, Yang SO, Lee J, Auh JH, Kim JH, Cho SM, Marriott PJ, Choi HK. Biochemical monitoring of black raspberry (Rubus coreanus Miquel) fruits according to maturation state by 1H NMR using multiple solvent systems. Food Res. Int. 2011;44:1077–1988.
- Ju HK, Cho EJ, Jang MH, Lee YY, Hong SS, Park JH, Kwon SW. Characterization of increased phenolic compounds from fermented Bokbunja (Rubus coreanus Miq.) and related antioxidant activity. J. Pharm. Biomed. Anal. 2009;49:820–827.10.1016/j.jpba.2008.12.024
- Choung MG, Lim JD. Antioxidant, anticancer and immune activation of anthocyanin fraction from Rubus coreanus Miquel fruits (Bokbunja). Korean J. Med. Crop Sci. 2012;20:259–269.10.7783/KJMCS.2012.20.4.259
- Jeon SK, Lee JW, Lee IS. Effect of antioxidant activity and induction of DNA damage on human gastric cancer cell by Rubus coreanus Miquel. J. Life Sci. 2007;17:1723–1728.10.5352/JLS.2007.17.12.1723
- Jung J, Son MY, Jung S, Nam P, Sung JS, Lee SJ, Lee KG. Antioxidant properties of Korean black raspberry wines and their apoptotic effects on cancer cells. J. Sci. Food Agric. 2009;89:970–977.10.1002/jsfa.v89:6
- Lee SJ, Lee HK. Sanguiin H-6 blocks endothelial cell growth through inhibition of VEGF binding to VEGF receptor. Arch. Pharm. Res. 2005;28:1270–1274.10.1007/BF02978211
- Kim SJ, Lee HJ, Kim BS, Lee D, Lee SJ, Yoo SH, Chang HI. Antiulcer activity of anthocyanins from Rubus coreanus via association with regulation of the activity of matrix metalloproteinase-2. J Agric. Food Chem. 2011;59:11786–11793.10.1021/jf104192a
- Kim Y, Kim J, Lee SM, Lee HA, Park S, Kim Y, Kim JH. Chemopreventive effects of Rubus coreanus Miquel on prostate cancer. Biosci. Biotechnol. Biochem. 2012;76:737–744.10.1271/bbb.110857
- Baek EY, Lee SM, Lee J, Park E, Kim Y, Jung IK, Kim JH. Effect of Rubus coreanus Miquel on prostate tumour growth. J. Funct. Foods. 2013;5:1478–1486.10.1016/j.jff.2013.06.005
- Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J. Biol. Chem. 2006;281:30678–30683.10.1074/jbc.C600120200
- Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000;48:140–146.10.1021/jf9908345
- Kim JM, Shin M. Characteristics of Rubus coreanus Miquel fruits at different ripening stages. Korean J Food Sci. Technol. 2011;43:341–347.
- John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001;7:14–23.10.1007/BF03032599
- Liabakk NB, Talbot I, Smith RA, Wilkinson K, Balkwill F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996;56:190–196.
- Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin. Exp. Metastasis. 2005;22:237–246.10.1007/s10585-005-8115-6
- Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229.
- Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, Zhang LJ. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol. Res. 2009;39:177–186.10.1111/hep.2009.39.issue-2
- Kargiotis O, Chetty C, Gondi CS, Tsung AJ, Dinh DH, Gujrati M, Lakka SS, Kyritsis AP, Rao JS. Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27:4830–4840.10.1038/onc.2008.122
- Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689.10.1038/sj.onc.1207616
- Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. Down-regulation of urokinase plasminogen activator and matrix metalloproteinases and up-regulation of their inhibitors by a novel nutrient mixture in human prostate cancer cell lines PC-3 and DU-145. Oncol. Rep. 2011;26:1407–1413.
- Escaff S, Fernández JM, González LO, Suárez A, González-Reyes S, González JM, Vizoso FJ. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br. J. Cancer. 2010;102:922–929.10.1038/sj.bjc.6605569
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839.10.1161/01.RES.0000070112.80711.3D
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67.10.1016/j.cell.2010.03.015
- Bloomston M, Shafii A, Zervos EE, Rosemurgy AS. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J. Surg. Res. 2002;102:39.10.1006/jsre.2001.6318
- Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 2008;78:356–365.10.1093/cvr/cvm111
- Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via αVβ3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int. J. Cancer. 2010;127:1081–1095.10.1002/ijc.v127:5
- Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J. Biol. Chem. 2007;282:30485–30496.10.1074/jbc.M701081200
- Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-κB signal transduction pathway. Oncogene. 2006;25:7009–7018.10.1038/sj.onc.1209706
- Thant AA, Nawa A, Kikkawa F, Ichigotani Y, Zhang Y, Sein TT, Amin AR, Hamaguchi M. Fibronectin activates matrix metalloproteinase-9 secretion via the MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin. Exp. Metastasis. 2000;18:423–428.10.1023/A:1010921730952
- Liu KC, Huang AC, Wu PP, Lin HY, Chueh FS, Yang JS, Lu CC, Chiang JH, Meng M, Chung JG. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and -9 signaling pathways. Oncol. Rep. 2011;26:177–184.
- Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012;38:76–87.10.1016/j.ctrv.2011.03.001
- Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell. Biochem. 2010;333:169–180.10.1007/s11010-009-0217-z
- Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem. Biol. Interact. 2005;156:141–150.10.1016/j.cbi.2005.08.005
- Lee SJ, Hong S, Yoo SH, Kim GW. Cyanidin-3-O-sambubioside from Acanthopanax sessiliflorus fruit inhibits metastasis by downregulating MMP-9 in breast cancer cells MDA-MB-231. Planta Med. 2013;79:1636–1640.
- Chae SC. Inhibitory effect of Naringenin on MMP-2, -9 activity and expression in HT-1080 cells. J Toxicol. Environ. Toxicol. 2009;24:63–70.
- Chu KS, Kim WK, Kang N. Effect of cyanidin on cell motility and invasion in MDA-MB-231 human breast cancer cells. Korean J Nutr. 2008;41:711–717.
- Lee SJ, Nam H, Kim MM, Jang HJ, Park JA, Kim BW, Chung KT. In vitro inhibitory effect of aged black garlic extract with antioxidant activity on MMP-2 and MMP-9 related to metastasis. J Life Sci. 2010;20:760–767.
- Shin DY, Yoon MK, Choi YW, Gweon HC, Kim JI, Choi TH, Choi YH. Effects of aged black garlic extracts on the tight junction permeability and cell invasion in human gastric cancer cells. J. Life Sci. 2010;20:528–534.10.5352/JLS.2010.20.4.528
- Wu K, Zeng J, Zhu G, Zhang L, Zhang D, Li L, Fan J, Wang X, He D. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol. Sin. 2009;30:1162–1168.10.1038/aps.2009.94
- Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, Shi X, Jiang BH. Arsenite induces HIF-1α and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol. Cell. Biochem. 2004;255:33–45.10.1023/B:MCBI.0000007259.65742.16
- Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO rep. 2002;3:420–425.10.1093/embo-reports/kvf094
- Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T. Transforming growth factor-beta1-dependent urokinase up-regulation and promotion of invasion are involved in Src-MAPK-dependent signaling in human ovarian cancer cells. J. Biol. Chem. 2004;279:8567–8576.10.1074/jbc.M309131200
- Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705.
- Shukla S, MacLennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int. J. Cancer. 2007;121:1424–1432.10.1002/(ISSN)1097-0215
- Tseng HL, Li CJ, Huang LH, Chen CY, Tsai CH, Lin CN, Hsu HY. Quercetin 3-O-methyl ether protects FL83B cells from copper induced oxidative stress through the PI3K/Akt and MAPK/Erk pathway. Toxicol. Appl. Pharmacol. 2012;264:104–113.10.1016/j.taap.2012.07.022
- Wang J, Hao H, Yao L, Zhang X, Zhao S, Ling EA, Hao A, Li G. Melatonin suppresses migration and invasion via inhibition of oxidative stress pathway in glioma cells. J. Pineal Res. 2012;53:180–187.10.1111/jpi.2012.53.issue-2
- Kim YS, Sull JW, Sung HJ. Suppressing effect of resveratrol on the migration and invasion of human metastatic lung and cervical cancer cells. Mol. Biol. Rep. 2012;39:8709–8716.10.1007/s11033-012-1728-3
- Chung JG, Peng HY, Chu YC, Hsieh YM, Wang SD, Chou ST. Anti-invasion and apoptosis induction of chlorella (Chlorella sorokiniana) in Hep G2 human hepatocellular carcinoma cells. J. Funct. Foods. 2012;4:302–310.10.1016/j.jff.2011.12.008
