Abstract
The aim of this study was to optimize the culture conditions of Fusarium solani KCCM90040 on cereal grain for the production of neoN-methylsansalvamide, a novel low-molecular-weight cyclic pentadepsipeptide exhibiting cytotoxic and multidrug resistance reversal effects. From the analysis of variance results using response surface methodology, temperature, initial moisture content, and growth time were shown to be important parameters for the production of neoN-methylsansalvamide on cereal grain. A model was established in the present study to describe the relationship between environmental conditions and the production of neoN-methylsansalvamide on rice, the selected cereal grain. The optimal culture conditions were determined at 25.79 °C with the initial moisture content of 40.79%, and 16.19 days of growth time. This report will give important information concerning the optimization of environmental conditions using statistic methodology for the production of a new cyclic pentadepsipeptide from fungi.
Graphical Abstract
Optimization of culture conditions of Fusarium solani for the production of neoN-methylsansalvamide
Various reviews of peptides from fungi have revealed that Fusarium strains found in fungi-infected plants produce several cyclic depsipeptides such as beauvericin,Citation1) enniatins,Citation2,3) zygosporamide,Citation4) and neosansalvamide analogsCitation5,6) via the non-ribosomal peptide synthesis system.Citation7,8) These cyclic depsipetides exhibit numerous pharmaceutical benefits such as cytotoxic,Citation9,10) antifungal,Citation11) and antibioticCitation1) effects as well as calcium channel antagonism.Citation12) Another cytotoxic cyclic pentadepsipeptides, sansalvamide analogs, were produced by Fusarium strains.Citation13,14) Sansalvamide A was first reported from the marine micro-organism Halodule wrightii,Citation13) and it consists of four hydrophobic amino acids [phenylalanine (l-Phe), two leucines (l-Leu), and one valine (l-Val)] and one hydroxy acid [leucic acid (O-Leu)]. Interestingly, some Fusarium strains isolated from green algae produced its N-methylated analog, N-methylsansalvamide, which is composed of a hydroxy acid (O-Leu) and four amino acids [l-Phe, l-Leu, N-methyl-leucine, and l-Val].Citation14) This N-methylated sansalvamide exhibited in vitro cytotoxicity in a National Cancer Institute human tumor cell line screen (EC50 = 8.3 μmol).Citation14) Sansalvamide showed cytototoxic potency against HCT116 (colon carcinoma), COLO205 (colon), and SK-MEL-2 (melanoma) cancer cell lines with EC50 values of 9.8, 3.5, and 5.9 μg/mL, respectively.Citation13) Sansalvamide and its analogs, which were produced by organic synthesis, caused marked time- and concentration-dependent inhibition of DNA synthesis and cell proliferation of human pancreatic cancer cell lines.Citation15–17) Furthermore, sansalvamide and its analogs showed a strong cytotoxic effect against multidrug resistance (MDR) human colon cancer cell lines.Citation18) These anticancer effects of sansalvamide analogs can be attributed to their inhibition of topoisomerase I, and their cytotoxicity may be mediated by this mechanism.Citation17,19) Sansalvamide analogs exhibited cytotoxic activity against human cancer cell lines, suggesting that they could be valuable therapeutic agents.Citation17,18)
Our research group isolated several Fusarium strains producing different cyclic depsipeptides including enniatins H, I, and MK1688 with beauvericin.Citation20) Interestingly, one isolate (Fusarium solani KCCM90040) produced a novel cyclic pentadepsipeptide, neoN-methylsansalvamide, which exhibited in vitro cytotoxic effects on human cancer cell lines.Citation5) NeoN-methylsansalvamide consists of five subunits linked in the following order: (S)-2-hydroxy-4-methylpantanoic acid, N-methyl-l-leucine, l-valine, l-leucine, and l-phenylalanine. It shows very similar cyclic peptide structure with N-methylated sansalvamide which consisted of same components such as four hydrophobic amino acids [phenylalanine (l-Phe), two leucines (l-Leu), and one valine (l-Val)] and one hydroxy acid [leucic acid (O-Leu), with different peptide sequences. Interestingly, neoN-methylsansalvamide exhibited a strong synergistic cytotoxic effect with paclitaxel on the human cancer lines MES-SA and HCT15 and their MDR sub-lines, MES-SA/DX5 and HCT15/CL05.Citation21)
In general, efficiency of secondary metabolite production from fungus is influenced by multiple parameters such as temperature, growth time, nutrition; their effects may be either independent or interactive.Citation22) Therefore, the production of neoN-methylsansalvamide can be effected by environmental conditions. In our preliminary study, F. solani KCCM90040 produced neoN-methylsansalvamide only on solid culture media of cereals, and the optimal culture conditions for production of this cyclic pentadepsipeptide have not yet been studied. Therefore, in this study, the effects of various environmental conditions on the production patterns of neoN-methylsansalvamide were investigated in order to get the optimized culture conditions using response surface methodology (RSM) for further application of this cyclic depsipeptide in various fields.
Materials and methods
Chemicals
The purified neoN-methylsansalvamide produced by F. solani KCCM90040 isolated from potato in our previous studyCitation5) was lyophilized and used as the standard in the current study. Water, acetonitrile, and methanol for analysis by high-performance liquid chromatography (HPLC) and liquid chromatography–tandem mass spectrometry (LC–MS) were obtained from J. T. Baker (Phillipsburg, NJ, USA). All other chemicals were of analytical grade from Sigma-Aldrich (St. Louis, MO, USA)
Selection of cereal substrate
Fifty grams of 6 different cereals (rice, barley, wheat, rye, maize, and Indian millet kernels) were autoclaved for 30 min at 121 °C, and sterilized water was added to produce a moisture content of about 40% in a 500-mL Erlenmeyer flask. After cooling, cereals were inoculated with spores of F. solani KCCM90040 (approximately 105 spores) then culture was cultivated at 25 °C and the level of neoN-methylsansalvamide was measured at 1, 2, 3, and 4 weeks. The culture was extracted with acetonitrile : methanol : water = 16 : 3 : 1 (v/v/v),Citation22) and the extract was defatted twice with 25 mL of n-heptane. After the bottom layer was evaporated to dryness, the residue was dissolved in 50 mL of methanol : water = 55 : 45 (v/v) and extracted twice with 25 mL of dichloromethane (CH2Cl2). The CH2Cl2 phase was evaporated to dryness, and the residue was dissolved in HPLC-grade methanol for further analysis.
Optimization of culture conditions by using RSM
After selecting the optimal cereal substrate, F. solani KCCM90040 was incubated at 4 different temperatures (15, 25, 35, and 45 °C), 5 different initial moisture contents (10, 20, 30, 40, and 50%), and 4 different growth times (1, 2, 3, and 4 weeks) to test the effect of culture conditions on the production of neoN-methylsansalvamide. After determination of the range of culture conditions, RSM was applied to further optimize the culture conditions. Design Expert (version 7; Minneapolis, MN, USA) software was used to generate the experimental designs for statistical analysis and application of regression models. Central composite design (CCD) with a quadratic model was employed. The 3 independent variables were temperature (X1 = 15–35 °C), moisture content (X2 = 30–50%), and growth time (X2 = 10–20 days), each with 3 levels: −1, 0, and +1. There were 20 combinations of variables, including five replicates of the center point, each assigned with the coded value of 0. A quadratic polynomial regression model was assumed for predicting the Y variable (Y = amount of produced neoN-methylsansalvamide). The model proposed for the Y response fitted the following equation:
The first- and second-order coefficients of the polynomial equations were generated by regression analysis.
Analysis of neoN-methylsansalvamide
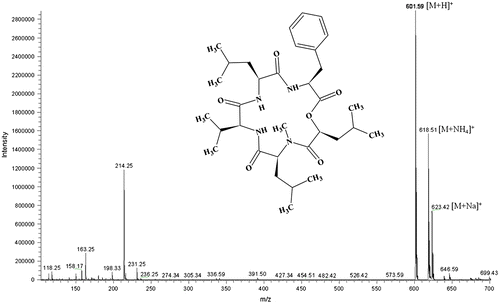
A Fusarium strain producing a novel cytotoxic cyclic pentadepsipeptide was isolated as described previously,Citation20) and this strain was deposited in the KCCM (Korean Culture Center of Microorganisms) as number 90040. NeoN-methylsansalvamide was analyzed according to the method of Moretti et al. (1994) with minor modifications.Citation23) A Shiseido pack C18 column (0.46 × 25 cm; i.d., 5 μm Shiseido, Tokyo, Japan) was used for the analysis with an acetonitrile : water solution (70:30, v/v) as the mobile phase at a flow rate of 1 mL/min for 40 min at 210 nm. The structural confirmation of produced neoN-methylsansalvamide was carried out using a MS/LTQ Velos system (Thermo Fisher Scientific, Bremen, Germany) according to our previous reportCitation20) with minor modifications. The extracts were dissolved in methanol, and then 10 μL of sample was injected into the reverse-phase C18 column (2.1 × 100 mm; i.d., 1.9 μm, Thermo Fisher Scientific). The mobile phase was acetonitrile (0.1% formic acid) : water (0.1% formic acid) = 70:30, v/v) at a flow rate of 0.5 mL/min. The mass spectrometer was initially programmed to perform full scans at m/z = 100–700 to observe the protonated molecular ion signal of neoN-methylsansalvamide produced in each culture condition. With the LC–MS operation conditions described above, the peak of neoN-methylsansalvamide was identified at a retention time of about 13.4 min. The mass spectrum of neoN-methylsansalvamide exhibited a protonated molecular ion at 601.59 m/z, with ammonium and sodium adducts at 618.51 and 623.42 m/z, respectively (Fig. ). The concentration of this cytotoxic cyclic pentadepsipeptide on the cereal substrate culture of F. solani KCCM90040 was calculated by comparing the peak areas of the sample with that of the neoN-methylsansalvamide standard. The squared correlation coefficient (R2) for the five-point calibration curve for neoN-methylsansalvamide was 0.9985. The limits of detection and quantification of neoN-methylsansalvamide were 0.15 and 0.50 mg/kg, respectively. All samples were analyzed in triplicate.
Statistical analysis
Statistical significances were determined by analysis of variance (ANOVA). The fit of the model was evaluated by the determination coefficients (R2 and adjusted R2) and a test for lack of fit, which involved comparing the mean square (MS) lack of fit to the MS pure experimental error from the ANOVA. The statistical significance of all terms was determined based on the F value at a probability (p) of 0.05.
Results and discussion
Selection of cereal substrate
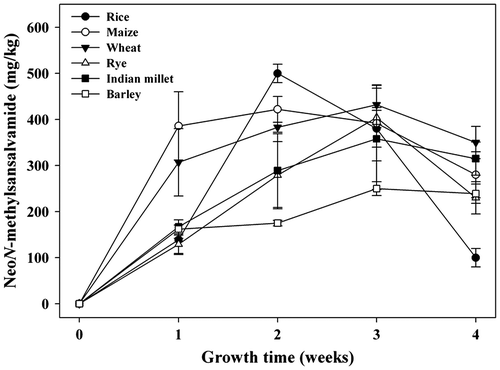
In this experiment, six different cereal substrates including rice, barley, maize, wheat, rye, and Indian millet were studied for the optimal production of neoN-methylsansalvamide. Fig. shows the pattern of neoN-methylsansalvamide production on cereal substrates by F. solani KCCM90040. NeoN-methylsansalvamide yield was the maximum at the second week after inoculation on rice (mean of 500 ± 20 mg/kg). When F. solani KCCM90040 was cultivated on maize, wheat, and rye, neoN-methylsansalvamide levels were 422 ± 28, 432 ± 43, and 404 ± 22 mg/kg after same culture time, respectively. The production of neoN-methylsansalvamide on rice was about 1.5- and twofold higher than Indian millet (358 ± 110 mg/kg) and barley (250 ± 15 mg/kg), respectively.
Various cereal substrates have been applied to optimize the production of secondary metabolites from Fusarium strains. The production of the bioactive cyclic hexadepsipeptide beauvericin from F. proliferatum was optimized on a solid medium of maize, and fusaproliferin could be produced at a maximum from F. proliferatum on the same maize.Citation23) Even though maize was frequently used for the production of several mycotoxins from Fusarium strains, other grains were also employed to test the production ability of the mycotoxin. Kokkonen et al.Citation24) also claimed the production of beauvericin and T-2 toxin by F. langsethiae and F. sporotrichioides on rice-flour and cereal-flour medium. In addition, Song et al.Citation25) reported that F. oxysporum KFCC11363P coproduced beauvericin and enniatin analogs (H, I, and MK1688) at a maximum amount on rice.
Kokkonen et al. reported that both F. langsethiae and F. sporotrichioides produced beauvericin more predominant on rice-flour medium compared to cereal-flour medium.Citation24) Consistent with these results, the maximum production of beauvericin and enniatins H, I, and MK1688 was observed on solid medium of rice among the five different cereals (rice, barley, maize, wheat, and Indian millet).Citation25)
Effects of culture conditions on response variables
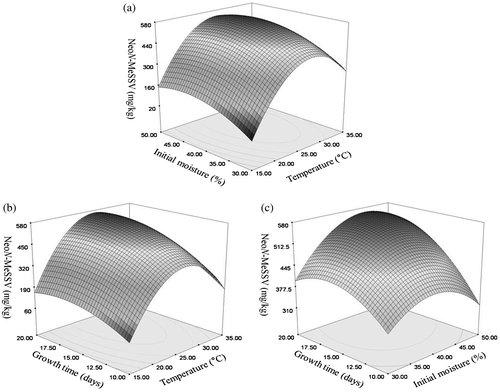
The influence of temperature on neoN-methylsansalvamide formation on the selected cereal medium is presented in Fig. (a). The maximum amount of neoN-methylsansalvamide was observed at 25 °C with production yields of 500 ± 50 mg/kg. However, the production decreased rapidly when F. solani KCCM90040 was cultivated at 35 °C (Fig. (a)). The optimal culture temperature for F. proliferatum, F. langsethiae, and F. sporotrichioides to produce the cyclic hexadepsipeptide beauvericin was 25 °C.Citation23,24) For F. subglutinans ITEM-1434, the highest amount of beauvericin was produced on rice at 25 °C.Citation22) Generally, the optimum temperature for producing various secondary metabolites (e.g. fusaproliferin, enniatins, and beauvericin) from Fusarium strains has been shown to be 20–30 °C.Citation20,22,23,26–28) Consistent with these data, results from this study indicate that an optimum temperature for the maximal production of neoN-methylsansalvamide was about 25 °C.
Fig. 3. Effect of environmental conditions on the production of neoN-methylsansalvamide. (a) Temperature. (b) Initial moisture content. (c) Growth time.
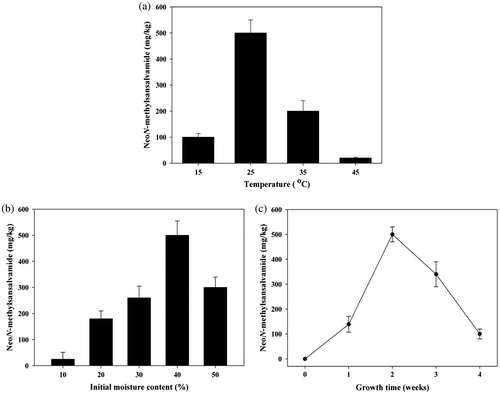
The effect of initial moisture content on the production of neoN-methylsansalvamide is shown in Fig. (b). The production of neoN-methylsansalvamide increased rapidly at 20% (w/w) of initial moisture content and increased gradually until it reached the highest level at 40% of initial moisture content. Song et al.Citation20) have reported that the maximum production of enniatins H, I, and MK1688 and beauvericin by F. oxysporum KFCC11363P was observed at 40% moisture content on rice. Dilkin et al. suggested an optimized moisture content of 42% when describing the effects of moisture content on the production of fumonisins from F. moniliforme.Citation29)
The maximum amount of neoN-methylsansalvamide (500 ± 30 mg/kg) was observed at 25 °C and 40% (w/w) moisture content the second week after inoculation (Fig. (c)). The production of neoN-methylsansalvamide increased rapidly during the first and second week and began to decrease in the third and fourth week (Fig. (c)). Song et al.Citation25) have reported that the maximum amount of enniatins H, I, and MK1688 and beauvericin from F. oxysporum was observed the second week after inoculation on the solid medium of rice. In our previous report,Citation30) the maximum production yields of enniatins H, I, and MK1688 by F. oxysporum KFCC11363P were 185.4, 349.1, and 541.1 mg/L, respectively, in submerged cultivation. In this experiment, the optimal growth time for producing neoN-methylsansalvamide was about two weeks after cultivation.
Based on the above results, the range of parameters, i.e. temperature, initial moisture content, and growth time, for application in RSM were determined to be 15–35 °C, 30–50%, and 10–20 days, respectively.
Model fitting
The principles of RSM were applied to model neoN-methylsansalvamide production by F. solani KCCM90040 as a function of temperature, initial moisture content, and growth time on the solid medium of rice to increase the amount of biologically bioactive compounds. The amount of neoN-methylsansalvamide in every experimental design generated by RSM is shown in Table . The coefficient of the polynomial equation, based on the level of neoN-methylsansalvamide generated from the experimental results, is presented with the statistical significance in Table . All linear and quadratic terms of culture conditions were found to exert highly significant (p < 0.05) effects on the production of neoN-methylsansalvamide. However, the interaction of temperature and initial moisture content had no significant effect on neoN-methylsansalvamide production with a p value of 0.7803. The fits of the quadratic polynomial model were evaluated by R2 and adjusted R2, which indicate the fraction of variation of the response described by the model, and lack of fit from ANOVA (Table ). The values of R2 and adjusted R2 for neoN-methylsansalvamide were 0.97787 and 0.957952, respectively, and the model had no lack of fit at the 95% level of significance. In addition, the probability for the regression observed was <0.0001, and these data indicate that the proposed model was significant. From the ANOVA results, it was indicated that temperature, initial moisture content, and growth time are critical parameters for the production of cyclic pentadepsipeptides on solid medium. The proposed model in the present study describing the relationship between culture conditions and the production of neoN-methylsansalvamide from F. solani KCCM90040 on solid medium of rice was successfully established.
Table 1. CCD arrangement and response.
Table 2. Regression coefficients of the quadratic polynomials as functions of the conditions for the amount of neoN-methylsansalvamide.
Table 3. ANOVA table.
Optimization of culture conditions by RSM
The optimal conditions (temperature, initial moisture content, and growth time) for the maximum amount of neoN-methylsansalvamide produced from F. solani KCCM90040 were determined by the model equation. The response surface plots for the amount of a novel cyclic pentadepsipeptide produced as a function of the growth conditions are shown in Fig. . The optimal conditions for temperature, initial moisture content, and growth time were 25.79 °C, 40.79%, and 16.19 days, respectively. The model predicted that the largest amount of neoN-methylsansalvamide would be 613.68 mg/kg if F. solani KCCM90040 was cultivated under the optimized conditions. In the actual experiment, when cultured under the optimized conditions as indicated by the model equation, F. solani KCCM90040 produced about 650 mg/kg of neoN-methylsansalvamide indicating close agreement between the observed response and the amount predicted by the model. In contrast, Belofsky et al.Citation13) reported that sansalvamide was produced at about 38 mg/L by marine Fusarium strains in a seawater-based medium, and the N-methyl analog of sansalvamide. N-methylsansalvamide was produced at about 3.1 mg/L by the Fusarium strain CNL-619 in a seawater-based medium.Citation14) These results indicate that sansalvamide analogs were better produced on the solid media of cereal than in submerged cultures.
Conclusion
MDR is one of major obstacles to the successful chemotherapy of human cancer. Therefore, a number of biochemical, pharmacological, and clinical strategies have been devised to overcome it. This study is the first to report the optimization of culture conditions for the largest production of a novel cyclic pentadepsipeptide, neoN-methylsansalvamide exhibiting cytotoxic and in vitro MDR reversal effects from a Fusarium strain. In the present work, we focused on the effects of variables on the production of neoN-methylsansalvamide and consequently, the optimum culture conditions on cereal substrates using RSM were achieved. These findings will assist in formulating solid culture media of cereal substrates that are suitable for producing neoN-methylsansalvamide from Fusarium strains and for further application in pharmacological and clinical strategies.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A2A2A01003635). This research also supported by the Chung-Ang University Research Scholarship Grants in 2012.
Notes
Abbreviations: ANOVA, analysis of variance; CCD, Central composite design; CH2Cl2, dichloromethane; HPLC, high-performance liquid chromatography; LC-MS, liquid chromatography-tandem mass spectrometry; L-Leu, leucines; L-Phe, phenylalanine; L-Val, valine; NCI, National Cancer Institute; N-MeLeu, N-methyl-leucine; O-Leu, leucic acid; R2, squared correlation coefficient; RSM, response surface methodology.
References
- Uhlig S, Torp M, Heier BT. Beauvericin and enniatins A, A1, B and B1 in Norwegian grain: a survey. Food Chem. 2006;94:193–201.10.1016/j.foodchem.2004.11.004
- Oueslati S, Meca G, Mliki A, Ghorbel A, Mañes J. Determination of Fusarium mycotoxins enniatins, beauvericin and fusaproliferin in cereals and derived products from Tunisia. Food Control. 2011;22:1373–1377.10.1016/j.foodcont.2011.02.015
- Evans BS, Robinson SJ, Kelleher NL. Surveys of non-ribosomal peptide and polyketide assembly lines in fungi and prospects for their analysis in vitro and in vivo. Fungal Genet. Biol. 2011;48:49–61.10.1016/j.fgb.2010.06.012
- Oh DC, Jensen PR, Fenical W. Zygosporamide, a cytotoxic cyclic depsipeptide from the marine-derived fungus Zygosporium masonii. Tetrahedron Lett. 2006;47:8625–8628.10.1016/j.tetlet.2006.08.113
- Lee HS, Lee C. Structural analysis of a new cytotoxic demethylated analogue of neoN-methylsansalvamide with different peptide sequence produced by Fusarium solani isolated from potato. J. Agric. Food Chem. 2012;60:4342–4347.
- Song HH, Lee HS, Lee C. A new cytotoxic cyclic pentadepsipeptide, neo N-methylsansalvamide produced by Fusarium solani KCCM90040, isolated from potato. Food Chem. 2011;126:472–478.10.1016/j.foodchem.2010.11.023
- Lee C, Gorisch H, Kleinkauf H, Zocher R. A highly specific d-hydroxyisovalerate dehydrogenase from the enniatin producer Fusarium sambucinum. J. Biol. Chem. 1992;267:11741–11744.
- Meca G, Ritieni A, Mañes J. Reduction in vitro of the minor Fusarium mycotoxin beauvericin employing different strains of probiotic bacteria. Food Control. 2012;28:435–440.10.1016/j.foodcont.2012.04.002
- Hyun U, Lee DH, Lee C, Shin CG. Apoptosis induced by enniatins H and MK1688 isolated from Fusarium oxysporum FB1501. Toxicon. 2008;53:7–8.
- Lee HS, Song HH, Jeong JH, Shin CG, Choi SU, Lee C. Cytotoxicities of enniatins H, I, and MK1688 from Fusarium oxysporum KFCC 11363P. Toxicon. 2008;51:1178–1185.10.1016/j.toxicon.2008.02.002
- Fukuda T, Arai M, Tomoda H, Omura S, Tomoda H, Omura S. New beauvericins, potentiators of antifungal miconazole activity, produced by Beauveria sp. FKI-1366: II. Structure elucidation. J. Antibiot. 2004;57:117–124.10.7164/antibiotics.57.117
- Nakamura T, Ono A, Tanzawa K, Hamano K, Ochi R. Voltage- and use-dependent block of L-type calcium current by leualacin, a peptide isolated from Hapsidospora irregularis. J. Mol. Cell. Cardiol. 1992;24:107.10.1016/0022-2828(92)90347-3
- Belofsky GN, Jensen PR, Fenical W. Sansalvamide: a new cytotoxic cyclic depsipeptide produced by a marine fungus of the genus Fusarium. Tetrahedron Lett. 1999;40:2913–2916.10.1016/S0040-4039(99)00393-7
- Cueto M, Jensen PR, Fenical W. N-Methylsansalvamide, a cytotoxic cyclic depsipeptide from a marine fungus of the genus Fusarium. Phytochemistry. 2000;55:223–226.10.1016/S0031-9422(00)00280-6
- Heiferman MJ, Salabat MR, Ujiki MB, Strouch MJ, Cheon EC, Silverman RB, Bentrem DJ. Sansalvamide induces pancreatic cancer growth arrest through changes in the cell cycle. Anticancer Res. 2010;30:73–78.
- Pan PS, McGuire KL, McAlpine SR. Identification of sansalvamide a analog potent against pancreatic cancer cell lines. Bioorg. Med. Chem. Lett. 2007;17:5072–5077.10.1016/j.bmcl.2007.07.025
- Ujiki MB, Milam B, Ding XZ, Roginsky AB, Salabat MR, et al. A novel peptide sansalvamide analogue inhibits pancreatic cancer cell growth through G0/G1 cell-cycle arrest. Biochem. Biophys. Res. Commun. 2006;340:1224–1228.10.1016/j.bbrc.2005.12.131
- Otrubova K, Lushington G, Vander Velde D, McGuire KL, McAlpine SR. Comprehensive study of sansalvamide A derivatives and their structure-activity relationships against drug-resistant colon cancer cell lines. J. Med. Chem. 2008;51:530–544.
- Sellers RP, Alexander LD, Johnson VA, Lin CC, Savage J, et al. Design and synthesis of Hsp90 inhibitors: exploring the SAR of sansalvamide A derivatives. Bioorg. Med. Chem. 2010;18:6822–6856.10.1016/j.bmc.2010.07.042
- Song HH, Lee HS, Jeong JH, Park HS, Lee C. Diversity in beauvericin and enniatins H, I, and MK1688 by Fusarium oxysporum isolated from potato. Int. J. Food Microbiol. 2008;122:296–301.10.1016/j.ijfoodmicro.2008.01.009
- Lee HS, Phat C, Choi SU, Lee C. Synergistic effect of a novel cyclic pentadepsipeptide, neoN-methylsansalvamide, and paclitaxel on human multidrug resistance cancer cell lines. Anti-Cancer Drugs. 2013;24:455–460.10.1097/CAD.0b013e32835f060d
- Geraldo MRF, Tessmann DJ, Kemmelmeier C. Production of mycotoxins by Fusarium graminearum isolated from small cereals (wheat, triticale and barley) affected with scab disease in Southern Brazil. Braz. J. Microbiol. 2006;37:58–63.
- Moretti A, Logrieco A, Bottalico A, Ritieni A. Production of beauvericin by Fusarium proliferatum from maize in Italy. Mycotoxin Res. 1994;10:73–78.10.1007/BF03192255
- Kokkonen M, Jestoi M, Laitila A. Mycotoxins production of Fusarium langsethiae and Fusarium sporotrichioides on cereal-based substrates. Mycotoxin Res. 2011;28:25–35.
- Song HH, Lee HS, Lee GP, Ha SD, Lee C. Structural analysis of enniatin H, I, and MK1688 and beauvericin by liquid chromatography–tandem mass spectrometry (LC–MS/MS) and their production by Fusarium oxysporum KFCC 11363P. Food Addit. Contam. 2009;26:518–526.10.1080/02652030802562904
- Popovski S, Celar FA. The impact of environmental factors on the infection of cereals with Fusarium species and mycotoxin production—a review. Acta Agriculturae Slovenica. 2013;101:105–116.
- Gupta S, Krasnoff SB, Underwood NL, Renwick JAA, Roberts DW. Isolation of beauvericin as an insect toxin from Fusarium semitectum and Fusarium moniliforme var. subglutinans. Mycopathologia. 1991;115:185–189.10.1007/BF00462223
- Kriek NPL, Marasas WFO, Steyn PS, Van Rensburg SJ, Steyn M. Toxicity of a moniliformin-producing strain of Fusarium moniliforme var. subglutinans isolated from maize. Food Cosmet. Toxicol. 1977;15:579–587.10.1016/0015-6264(77)90073-6
- Dilkin P, Mallmann CA, Almeida CAA, Stefanon EB, Fontana FZ, Milbradt EL. Production of fumonisins by strains of Fusarium monoliforme according to temperature, moisture and growth period. Braz. J. Microbiol. 2002;33:111–118.
- Lee HS, Kang JW, Kim BH, Park SG, Lee C. Statistical optimization of culture conditions for the production of enniatins H, I, and MK1688 by Fusarium oxysporum KFCC 11363P. J. Biosci. Bioeng. 2011;111:279–285.10.1016/j.jbiosc.2010.10.013