Abstract
Bisphenol A (BPA) is considered to be an endocrine disruptor, but the mechanisms by which it disrupts endocrine functions are poorly understood. Here, we have shown that BPA binds both estrogen receptor (ER)-α and ER-beta (ER-β) using a fluorescence polarization competitive binding assay. In addition, we found that BPA induced cell proliferation by modulating cell cycle-related genes in the MCF-7 human mammary cancer cell line. Moreover, using a BG1 luciferase ER transactivation assay, we found that BPA has estrogenic activity. Modulating the MAPK pathway by using an ERK inhibitor (PD98059) or a JNK inhibitor (SP600125) had no effect on the ability of BPA to induce estrogenic activity. However, the antiestrogen, ICI 182,780, and the p38 inhibitor, PD 169316 successfully blocked BPA-induced estrogenic activity. Our findings suggest that BPA mimics ER-dependent estrogenic activity by targeting proteins that regulate the cell cycle and p38 MAPK.
Graphical Abstract
Bisphenol A exerts estrogen receptor–dependent estrogenic activity by targeting proteins that regulate the cell cycle and p38 MAPK.
Endocrine disruptors are exogenous materials that interfere with the normal function of the endocrine system. They mimic or block hormone action by binding to hormone receptors such as the estrogen, androgen, and thyroid receptors, respectively, or togetherCitation1−Citation2) and also disrupt the synthesis, movement, metabolism, and secretion of naturally occurring hormones.Citation3) In addition to these hazardous effects, endocrine disruptors might cause cancer, which is associated with endocrine disturbances.Citation4)
Bisphenol A (BPA) is utilized in the manufacture of polycarbonate plastics and epoxy resins.Citation5−Citation6) Polycarbonate plastics are widely used in the manufacture of several products, ranging from containers for edible products to mechanical devices such as water and infant bottles, compact discs, impact-resistant safety equipment, and medical devices. Although BPA is considered to be an endocrine disruptor, it is still used in wide-ranging applications in various areas.Citation7) In 1936, it was first observed that BPA acts as an estrogenic compound when it is injected into ovariectomized adult rats.Citation8) Subsequently, several studies demonstrated that BPA has weak estrogenic activity.Citation9–11) However, little is known about the mechanism by which it exerts its estrogenic activity.
The estrogen receptor (ER) pathway is known to regulate cell proliferation-related target genes under the influence of cell cycle-related genes.Citation12) The MAPK family members, ERK, JNK, and p38 MAPK, control cell cycle progression.Citation13) Recent studies suggest that the ER pathway and related MAPK family members play an important role in regulating cancer cell proliferation.Citation14) Studies have also examined the role of MAPK activityCitation7,15,Citation16) in the ability of BPA to disrupt endocrine function, but these mechanisms are still poorly understood.
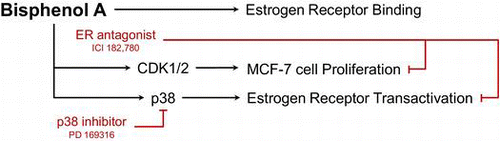
In this study, we showed that BPA binds to ER-α and ER-β using a fluorescence polarization competitive binding assay. We also confirmed that the proliferative effect of BPA on a MCF-7 human mammary cancer cell line was due to the modulation of the cell cycle-related genes, CDK1 and CDK2. BPA-induced cell proliferation was inhibited by treatment with an ER antagonist ICI 182,780. In addition, using a transcription assay, we found that BPA had estrogenic activity. We also found that while the ERK inhibitor PD98059 and the JNK inhibitor SP600125 had no effect on BPA estrogenic activity, the p38 inhibitor PD 169316 successfully blocked BPA estrogenic activity. Our findings suggest that BPA is related to ER-dependent estrogenic activity and p38 MAPK.
Materials and methods
Human ER-α and ER-β fluorescence polarization competitive binding assay
BPA binding affinities of ER-α and ER-β were evaluated using a fluorescence probe according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Competitive binding assays were performed in 96-well black plates (PerkinElmer, Wellesley, MA) in triplicate. BPA (Sigma-Aldrich, St. Louis, MO) was prepared by performing 10-fold serial dilutions sequentially with a screening buffer (100 mM potassium phosphate (pH7.4), 100 μg/mL BGG, 0.02% NaN3). The concentration of the solvent did not exceed 1% ethanol for this assay. After that mix BPA and Fluormone™ ES2 with human recombinant ER-α and ER-β, respectively, the plates were shaking incubated in the dark at room temperature for 2 h. The fluorescence polarization values were measured in each well by using 480 nm excitation and 535 nm emission interference filters from Envision (PerkinElmer, Wellesley, MA). Relative binding affinity (RBA; relative to E2) values were calculated as follows: RBA (%) = (IC50 of E2)/(IC50 of test chemical) × 100.
Cell proliferation assay
MCF-7 cells were purchased from the American Type Culture Collection. The cells (1 × 104 cells/well) were grown in 96-well culture plates (Nunc, Roskilde, Denmark) in phenol red-free RPMI 1640 supplemented with 5% charcoal-stripped fetal bovine serum (FBS; PAA, Linz, Austria), penicillin (100 U/mL), streptomycin (100 μg/mL), 6 ng/mL insulin, 4 mM l-glutamine, and 1% nonessential amino acids. All the cell culture agents used, except for the charcoal-stripped FBS, were obtained from Invitrogen (Carlsbad, CA). After 24 h, the cells were incubated with BPA for 72 h. An MTT assay was used to assess cell growth according to the manufacturer’s instructions (Sigma, St. Louis, MO). Assays were quantitated by measuring the absorbance at 570 nm on a microplate spectrophotometer (EL808; Bio-Tek Instruments, Winooski, VT).
BG1 Luc ER transactivation test
Stably transfected BG1Luc4E2 cells were kindly provided by Dr. Michael Denison, U.C. Davis. The cells were cultured in RPMI 1640 supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL). For the test, the cells were cultured in phenol red-free DMEM media supplemented with 5% charcoal-stripped FBS and antibiotics. The luciferase activities were determined in accordance with OECD TG457.Citation17) Briefly, The cells (4 × 104 cells/well) were seeded in 96-well white culture plates. After 24 h, the cells were incubated with reference chemicals and serially diluted test chemicals for 24 h, harvested, and assayed for luciferase activity using a commercial kit (Promega, Madison, WI). All the MAPK inhibitors were purchased from Calbiochem, La Jolla, CA. The activities were measured using a luminometer (MicroBeta2™ LumiJET/Microplate scintillation & luminescence counter; PerkinElmer, Wellesley, MA).
Western blot analysis
Western blot analysis was performed as previously described.Citation18) Antibodies against CDK1, cyclin D, cyclin E, p15, pp38Thr180/Tyr182, and p38 were purchased from Cell Signaling Technology, Danvers, MA. Antibodies against CDK2, CDK4, CDK6, cyclin A, cyclin B, p21, and p27 were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. The antibody against β-actin was purchased from Sigma-Aldrich, St. Louis, MO.
Statistical analysis
All data have been expressed in terms of mean ± SEM. Comparison of mean values among experimental groups was performed with one-way ANOVA followed by the Newman–Keuls Multiple Comparison post hoc test using GraphPad Prism. P < 0.05 was considered statistically significant.
Results
BPA binds both ER-α and ER-β
To evaluate the estrogenic activity of BPA, we first tested whether BPA physically binds to an ER by using a fluorescence polarization competitive binding assay. Both the reference chemical (17β-estradiol) and BPA were tested with full-length human recombinant ER-α and ER-β proteins. The binding assay showed that the binding affinity of BPA to ER-α was very similar to that of ER-β (Fig. (A) and (B)). The ER-α IC50 values were 2.51 × 10−9 M and 5.98 × 10−6 M for 17β-estradiol (E2) and BPA, respectively, while the ER-β IC50 values were 4.43 × 10−9 M and 6.53 × 10−6 M for E2 and BPA, respectively (Table ). The relative binding affinities (RBAs) of BPA were approximately 2500 and 1500-fold lesser than those of E2 for ER-α and ER-β, respectively.
Fig. 1. Competitive binding curves of the reference chemical E2 and the test chemical BPA to ER-α and ER-β.
Note: (A) Competitive binding against ERα and (B) competitive binding against ERβ.
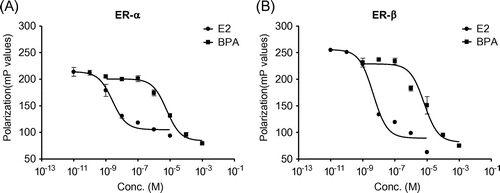
Table 1. IC50 and RBA values for human estrogen receptor-α and human estrogen receptor-β in the fluorescence polarization competitive binding assay.
BPA induces proliferation by targeting cell cycle regulatory genes
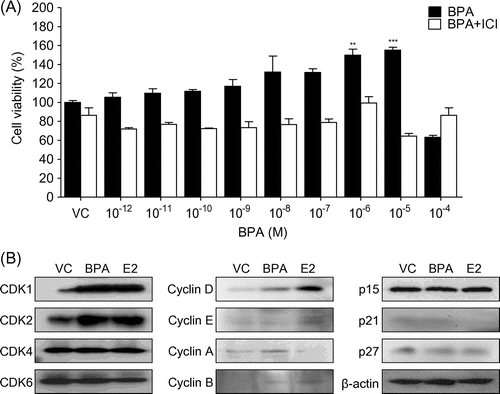
To ascertain whether BPA has proliferative activity in MCF-7 cells, we performed MTT assays. We found that the viable cell population dose-dependently increased in relation to concentrations of BPA up to 10−5 M (Fig. (A)), and the EC50 value of BPA on MCF-7 cell proliferation was 2.6 × 10−7 M. However, 10−4 M BPA reduced the viable cell population. To determine whether BPA induced cell proliferation through an ER-dependent mechanism, we used the ER inhibitor ICI 182,780. When cells were cotreated with BPA and ICI 182,780, the proliferative effect of BPA on MCF-7 cells was completely blocked. In addition, western blot analysis showed that cell cycle expression of cyclin D, cyclin A, CDK1, and CDK2 was upregulated by BPA, whereas p27 expression was slightly downregulated (Fig. (B)). Thus, our results demonstrate that BPA induces ER-dependent cell proliferation by targeting cell cycle regulatory genes in MCF-7 cells.
Fig. 2. BPA induces proliferation by targeting cell cycle regulatory genes.
Note: The MCF-7 cells were incubated with BPA with or without ICI 182,780 at the indicated concentrations for 72 h prior to MTT assays (A). EtOH was used as the vehicle control. Cell viability is expressed as a relative value to that of the vehicle treated cells, which is set to 100%. The figures show the mean ± SEM (n = 3). **P < 0.01, ***P < 0.005. (B) The cells were treated with BPA (10−5 M) or E2 (10−8 M) for 24 h prior to western blot analysis.
BPA elevates ER transcriptional activation in BG1Luc4E2 cells
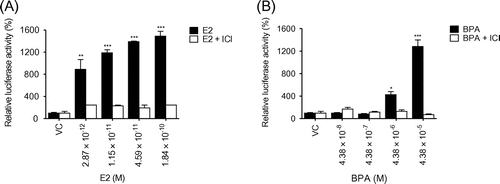
To assess the effects of BPA on ER transcriptional activity, we performed a luciferase assay using stably transfected BG1Luc4E2 cells containing an estrogen response element in the pGudLuc7.0 reporter gene constructs.Citation19) Our results showed that luciferase activity markedly increased in cells treated with both the reference chemical E2 and BPA in a dose-dependent manner (Fig. (A) and (B)). The EC50 values of E2 and BPA on BG1 Luc ER transactivation were 9 × 10−11 M and 3.1 × 10−6 M, respectively. This effect was restrained by treatment with the ER inhibitor ICI 182,780. Therefore, BPA elevates ER-mediated transcriptional activation of luciferase in BG1Luc4E2 cells.
Fig. 3. BPA elevates ER transcriptional activation in BG1Luc4E2 cells.
Note: The stably transfected BG1Luc4E2 cells were treated with E2 (A) or BPA (B) at the indicated concentrations in the absence or presence of ICI 182,780 for 24 h. After removal of the media, the luciferase solution was added (Steady-Glo) and the luciferase activities were measured using Luminometer (MicroBeta2, Perkin Elmer). Luciferase activity is expressed as a relative value to that of the vehicle (DMSO)-treated cells which is set to 100%. The figures show the mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.005.
BPA mimics estrogenic activity through p38 MAPK
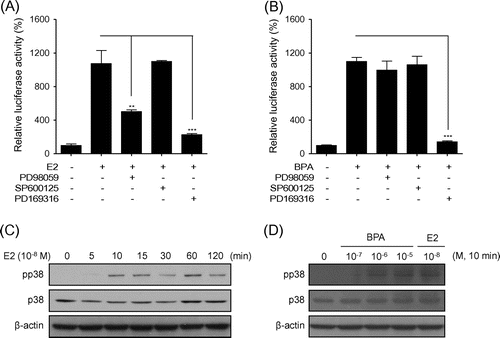
Because E2 is a well-known activator of MAPK,Citation20) we used MAPK inhibitors to assess the role of MAPK in the ER pathway in BG1Luc4E2 cells. The p38 inhibitor completely suppressed the luciferase activity elevated by E2, whereas the ERK inhibitor only had a partially inhibitory effect. In contrast, the JNK inhibitor had no effect (Fig. (A)). The ERK inhibitors did not have an effect on the luciferase activity elevated by BPA; however, the p38 inhibitor was able to suppress BPA signaling (Fig. (B)). These data suggest that p38 activation is crucial for ER-mediated transcriptional activation. Western blot analysis showed that both E2 and BPA increased the phosphorylation levels of p38 (Fig. (C) and (D)). Our data show that BPA mimics estrogenic activity through p38 MAPK.
Fig. 4. BPA mimics estrogenic activity through p38 MAPK.
Note: The BG1Luc4E2 cells were treated with (A) E2 (1.84 × 10−10 M), (B) BPA (4.38 × 10−5 M), and/or the indicated inhibitors. The luciferase activity is expressed as a relative value compared to that of the vehicle-treated cells, which is set to 100%. The data were expressed as the mean ± SEM (n = 3). **P < 0.01, ***P < 0.005. PD98059, ERK inhibitor; SP600125, JNK inhibitor; PD159316, p38 kinase inhibitor. (C) Western blot analysis was performed using crude extracts that were prepared from the cells treated with E2 at 10−8 M for the indicated times. (D) The cells were treated with BPA at the indicated concentrations and with 10−8 M E2 at 10 min prior to western blot analysis.
Discussion
ERs exist in two forms, that is, ER-α and ER-β, which are encoded by separate genes.Citation21) ER-α and ER-β play distinct and overlapping biological functions that are dependent on the tissue and cell type.Citation22) Hormone-activated ER-α and ER-β form homodimers or heterodimers,Citation22) but the effect of endocrine disruptors on ER heterodimers are poorly understood. In this study, we have shown that BPA can physically bind not only ER-α but also ER-β with similar patterns of binding affinity. Therefore, our findings suggest that BPA is a useful chemical for future investigation of the effects of endocrine disruptors on ER homodimers and heterodimers.
ER signaling can be categorized into a genomic pathway and a non-genomic pathway. These two pathways are key factors in the regulation of estrogen target genes. Citation23) In the genomic pathway, ER interacts directly with DNA and other transcription factors can be involved in ER-DNA binding.Citation24) The non-genomic pathway is induced by intracellular calcium mobilization due to estrogen exposureCitation25) or rapid activation of cAMP productionCitation26) and various protein kinases.Citation27) The ER non-genomic pathway is induced by estrogen and ER phosphorylation. Activation of the ER non-genomic pathway can also be induced by a cell cycle-dependent increase in the activation of p38 MAPK in breast cancer cells.Citation28) However, the effects of BPA on the non-genomic pathway have not clearly been investigated. In this study, we observed that CDK1/2 increased as well as influenced the activity of p38 kinase following treatment with BPA. These findings will be useful in future investigations on the ER non-genomic pathway especially with respect to oncogenic studies.
The BG1 Luc ER transactivation test used in this study had been adopted in OECD test guideline 457 (TG457) in 2012.Citation17) Using this protocol, we not only screened the estrogenic activity of BPA but also performed the test for a candidate substance through inhibitors that can confirm MAPK signaling. Thus, if the ER non-genomic pathway can be confirmed in an endocrine disruptor study, we could get a better understanding of test chemicals.
Recently, the OECD recommended an alternative to animal testing in endocrine disruptor research due to animal welfare consideration, and an in vitro assay which can effectively replace animal experiment was introduced and practiced by many researchers, following OECD guidelines.Citation29) However, one of the known limitations in in vitro assays is that there is no operative method to resolve the deficiency in the metabolism of test chemicals. Therefore, further mechanistic study on the estrogenic activity of BPA metabolites is required.
In summary, the present study demonstrates that BPA exerts its estrogenic effects by modulating CDK1/2 and p38 MAP kinase activation. Our results provide a method for future research on ER signaling.
Conflict of interest
The authors have no conflicts of interest to declare.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.921557
Acknowledgment
The authors are grateful to Dr Michael Denison, UC Davis for providing the BG1Luc4E2 cells. They also thank to Ryeo-Eun Go for providing binding assay data. This research was supported by a grant (12161MFDS741) from the Ministry of Food and Drug Safety awarded in 2012.
Funding
This research was supported by Ministry of Food and Drug Safety [grant number 12161MFDS741], awarded in 2012.
Notes
Abbreviations: BPA, Bisphenol A; FBS, fetal bovine serum; ER, estrogen receptor; E2, 17β-estradiol; RBA, relative binding affinity.
References
- Bonefeld-Jørgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Toxicology. 2001;158:141–153.10.1016/S0300-483X(00)00368-1
- Jung KK, Kim SY, Kim TG, Kang JH, Kang SY, Cho JY, Kim SH. Arch. Pharmacal Res. 2007;30:616–623.10.1007/BF02977657
- Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, Baetcke KP, Hoffmann JL, Morrow MS, Rodier DJ, Schaeffer JE, Touart LW, Zeeman MG, Patel YM. Environ. Health Perspect. 1998;106:11–56.10.1289/ehp.98106s111
- Soto AM, Sonnenschein C. Nat. Rev. Endocrinol. 2010;6:363–370.10.1038/nrendo.2010.87
- Burridge E. Eur. Chem. News. 2003;17:14–20.
- http:www.bisphenol-a.org (2013).
- Kim JY, Han EH, Kim HG, Oh KN, Kim SK, Lee KY, Jeong HG. Toxicol. Lett. 2010;193:200–208.10.1016/j.toxlet.2010.01.011
- Dodds EC, Lawson W. Nature. 1936;137:996–996.10.1038/137996a0
- Grignard E, Lapenna S, Bremer S. Toxicol. Vitro. 2012;26:727–731.10.1016/j.tiv.2012.03.013
- Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. Environ. Health Perspect. 1995;103(Suppl 7):113–122.10.1289/ehp.95103s7113
- Miller D, Wheals BB, Beresford N, Sumpter JP. Environ. Health Perspect. 2001;109:133–138.
- Lee SJ, McEwen BS. Annu. Rev. Pharmacol. Toxicol. 2001;41:569–591.10.1146/annurev.pharmtox.41.1.569
- MacCorkle RA, Tan TH. Cell Biochem. Biophys. 2005;43:451–462.10.1385/CBB:43:3:451
- Wagner EF, Nebreda ÁR. Nat. Rev. Cancer. 2009;9:537–549.10.1038/nrc2694
- Canesi L, Lorusso LC, Ciacci C, Betti M, Zampini M, Gallo G. Gen. Comp. Endocrinol. 2004;138:58–69.
- Dong S, Terasaka S, Kiyama R. Environ. Pollut. 2011;159:212–218.10.1016/j.envpol.2010.09.004
- OECD (2012), Test No. 457: BG1Luc Estrogen Receptor Transactivation Test Method for Identifying Estrogen Receptor Agonists and Antagonists, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing. doi: 10.1787/9789264185395-en
- Park EJ, Kim BJ, Kim SH, Kim SY, Sung TS, Chae HG, Kim SJ, Kim J, Park HH, So I, Jeon JH. Biol. Pharm. Bull. 2009;32:1790–1794.10.1248/bpb.32.1790
- Rogers JM, Denison MS. In Vitro Mol. Toxicol. 2000;13:67–82.
- Björnström L, Sjöberg M. Mol. Endocrinol. 2005;19:833–842.10.1210/me.2004-0486
- Leclercq G. J. Steroid Biochem. Mol. Biol. 2002;80:259–272.10.1016/S0960-0760(02)00026-2
- Shanle EK, Xu W. Chem. Res. Toxicol. 2011;24:6–19.10.1021/tx100231n
- Vasudevan N, Pfaff DW. Front. Neuroendocrinol. 2008;29:238–257.10.1016/j.yfrne.2007.08.003
- Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. J. Steroid Biochem. Mol. Biol. 2000;74:279–285.10.1016/S0960-0760(00)00104-7
- Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. FASEB J. 1998;12:613–619.
- Zhang D, Trudeau VL. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006;144:306–315.10.1016/j.cbpa.2006.01.025
- Wehling M. Annu. Rev. Physiol. 1997;59:365–393.10.1146/annurev.physiol.59.1.365
- Bhatt S, Xiao Z, Meng Z, Katzenellenbogen BS. Mol. Cell Biol. 2012;32:1928–1943.10.1128/MCB.06561-11
- Lee HK, Kim TS, Kim CY, Kang IH, Kim MG, Jung KK, Kim HS, Han SY, Yoon HJ, Rhee GS. J. Toxicol. Sci. 2012;37:431–437.10.2131/jts.37.431
