Abstract
To identify the molecular target of diallyl trisulfide (DATS) in human leukemic cell line U937, we examined modification of thiol group(s) of cellular proteins by the redox 2D PAGE. A unique protein spot appeared by DATS treatment was identified to be heat shock protein 27 (HSP27). Hsp27 is suggested to be one of the molecular target of DATS in U937.
Garlic (Allium sativum L.) is a commonly available vegetable, and it has long history as a spice and the alternative medicine. Several lines of evidences obtained by the epidemiological studies have proven the anticancer effect of garlic.Citation1) It has been also reported that garlic-derived organosulfur compounds can suppress proliferation of cultured cancer cells through the induction of cell cycle arrest with/without apoptosis.Citation2–7) Our previous studies indicated that diallyl trisulfide (DATS) induces cell cycle arrest at the mitotic phase with disruption of microtubule, and thus hampers spindle formation in human colon cancer cells.Citation4,8) DATS inhibits the tubulin polymerization through S-allyl modification of Cys12 and Cys354 in β-tubulin molecule. These results suggest that DATS attacks cysteine residue of the target protein(s), and induces cell cycle arrest followed by cell death. However, it is still unknown whether DATS forms intra- or intermolecular disulfide bond in the cellular protein. Here we examined the disulfide bond formation with DATS using redox 2D-PAGE, which sequentially resolves proteins under both non-reducing and reducing conditions.
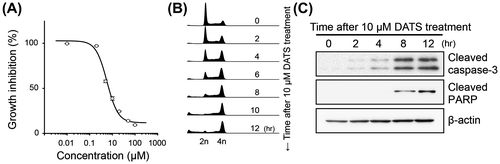
We first examined the effect of DATS on the growth of human monocytic leukemia U937 cells. U937 cells (obtained from Health Science Research Resources Bank, Osaka, Japan) were grown and maintained in RPMI-1640 medium (Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Biowest, Nuaillé, France) at 37 °C in 95% air and 5% CO2. DATS was synthesized and purified (more than 99% purity) as we descried before.Citation4) Stock solution of DATS was prepared in dimethyl sulfoxide (DMSO) at a concentration of 5 mM and then added to fresh medium to give a final concentration of 10 μM. Same amount of DMSO was also added to vehicle control culture medium to give a final concentration of 0.2%. The cells were treated with DATS for 24 h and counted numbers by a hemocytometer. The cell growth was significantly suppressed by DATS in a concentration dependent manner (Fig. (A)). The IC50 value was calculated to be 5.8 μM. The cell cycle distribution of the U937 cells was measured by flow cytometry. The cells (~5 × 105 cells) were fixed with ice-cold 70% ethanol at −20 °C for 18 h. The fixed cells were then treated with 500 μg/mL RNase A (Sigma-Aldrich, St. Louis, MO, USA) followed by staining with 25 μg/mL propidium iodide (Sigma-Aldrich) and analyzed by using a flow cytometer FACSCalibur (BD Biosciences, San Jose, CA, USA) and FlowJo software (TreeStar Inc., Ashland, OR, USA). The population of cells at G1 phase was decreased and that at G2/M phase was reciprocally increased with 10 μM DATS in a time-dependent manner (Fig. (B)). Sub-G1 DNA was also increased by DATS treatment (2.7% at 0 h, 39.5% at 12 h). The expression levels of apoptosis-related proteins were detected by Western blotting using caspase-3, cleaved caspase-3, and cleaved poly(ADP-ribose) polymerase (PARP) antibodies, those were purchased from Cell Signaling Technology (Danvers, MA, USA). Monoclonal β-actin antibody was from Sigma-Aldrich. In accordance with the increase of sub-G1 DNA, the cells, those were treated with 10 μM DATS, represented increased levels of cleaved caspase-3 and cleaved PARP after 4–8 h (Fig. (C)). These results suggest that DATS induces the cell cycle arrest at G2/M phase and the apoptosis in U937 cells.
Fig. 1. The effect of DATS on cell growth, cell cycle and activation of the proteins regulating apoptosis in U937.
Notes: U937 cells were seeded at a cellular density of 2 × 105 cells/mL and cultured for 24 h. Then the cells were incubated with various concentrations of DATS for 24 h. (A) The cell number was counted by hemocytometer and expressed as a percentage to the vehicle-treated control. (B) Cell cycle distribution analyzed by a flow cytometer. (C) Cleavage of caspase-3 and PARP detected by Western blotting.
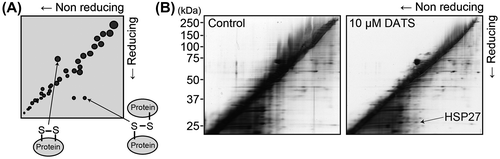
We next tried to identify the protein(s) that forms either intra- or intermolecular disulfide bond by DATS treatment by method of redox 2D-PAGE.Citation9) The redox 2D-PAGE is a diagonal gel electrophoresis technique (Fig. (A)). Proteins are firstly electrophoresed under non-reducing condition and secondly under reducing condition. The proteins with intermolecular disulfide bonds show slower electrophoretic mobility under non-reducing condition than reducing condition, therefore they appear as spots to the right side of the diagonal band line. On the other hand, the proteins with intramolecular disulfide bond have a faster electrophoretic mobility under non-reducing condition, then these were slowly moved under reducing condition, locating their spots at the left side of diagonal line. The total cellular protein samples (40 μg) were subjected to non-reducing electrophoresis in the first dimension. After the electrophoresis, the gel lanes were cut and immersed in SDS sample buffer containing 100 mM DTT for 20 min at room temperature. The gel slices were further immersed in SDS sample buffer containing 100 mM iodoacetamide (IAA) for 10 min. Each gel strip was then applied to another gel, and electrophoresis was performed in the second dimension. The gels were stained by the Pierce Silver Stain for Mass Spectrometry (Thermo Fisher Scientific, Rockford, IL, USA). A spot with 27 kDa was detected in the right of the diagonal line in the cells treated for 10 min with 10 μM DATS (Fig. (B), the arrowhead). The spot was cut from the gels, reduced in 10 mM DTT, 25 mM NH4HCO3 solution for 60 min at 56 °C, and then alkylated in 55 mM IAA, 25 mM NH4HCO3 solution for 45 min at room temperature. Then the gel was incubated with 10 μg/mL trypsin solution (Promega, Madison, WI, USA) for overnight at 37 °C. After the incubation, the digested sample was analyzed by liquid chromatography tandem mass spectrometry by using a MAGIC C18 column (0.15 mm × 50 mm; Michrom Bioresources, Auburn, CA, USA). The peptides were separated over a 20 min period with a linear gradient 5–65% in terms of solvent B going from solvent A (2% (v/v) acetonitrile, 0.1% formic acid) to solvent B (90% (v/v) acetonitrile, 0.1% formic acid) with a flow rate of 0.8 μL/min. The tryptic peptide samples were analyzed by a LCQ Deca XP ion trap mass spectrometer (ThermoFinnigan, San Jose, CA). The tandem mass spectrometry data obtained were analyzed by using SEQUEST, a computer program that allows the correlation of experimental data with theoretical spectra generated from known protein sequences. The 27 kDa protein spot was, thereby, identified to be heat shock protein 27 (HSP27).
Fig. 2. Redox 2D-PAGE analysis of U937 treated with DATS.
Notes: (A) Schematic outline of the redox 2D-PAGE. (B) Typical redox 2D-PAGE analysis of U937 treated with DATS. U937 cells were seeded at a cellular density of 2 × 105 cells/mL and cultured for 24 h. Then the cells were treated with 0.2% DMSO as vehicle control (Left panel) or with 10 μM DATS (Right panel) for 10 min and the cell lysates were subjected to the redox 2D-PAGE. The specific spot at approximately 27 kDa was identified to be HSP27.
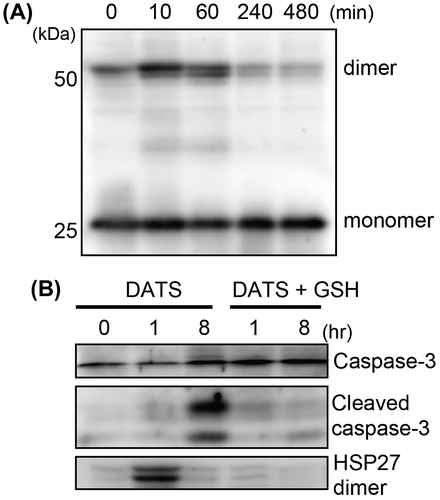
HSP27 has a single cysteine residue at position 137 that can be disulfide cross-linked in homodimer. We further tried to confirm whether a homodimer of HSP27 is formed by DATS treatment. The DATS-treated cell lysate was resolved by non-reducing SDS-PAGE, and HSP27 protein was detected by Western blotting. HSP27 antibody was purchased from Cell Signaling Technology. HSP27 homodimer was clearly observed in the cells treated with 10 μM DATS for 10 and 60 min, but not observed at 240 and 480 min after the treatment (Fig. (A)). These data suggest that DATS rapidly reacts with cysteine residue in HSP27 molecule and this disulfide bond formation is transient or reversible. To examine if this modification in cysteine residue with DATS is responsible for the induction of apoptosis, the cells were pretreated with GSH and then treated with DATS. In the cells treated with DATS, HSP27 dimer formation (1 h) as well as cleaved caspase-3 (8 h) was observed. Pretreatment of the cells with GSH competitively inhibited either the dimer formation of HSP27 or the following cleavage of caspase-3 (Fig. (B)).
Fig. 3. Formation of HSP27 dimer and cleaved caspase-3 in DATS-treated U937.
Notes: U937 cells were treated with 10 μM DATS for the times indicated in the panels. (A) HSP27 dimer formation was evaluated by non-reduced SDS-PAGE followed by Western blotting. (B) HSP27 dimer formation and the cleavage of caspase-3 were detected by Western blotting. U937 cells were also pretreated with GSH (1 mM) for 30 min prior to the DATS treatment (DATS + GSH).
HSP27, a molecular chaperone, is a member of the small heat shock protein family. HSP27 regulates apoptosis through the interaction with key components of the apoptotic signaling pathway.Citation10) Choi et al. reported that zerumbone, a cytotoxic compound isolated from Zingiber zerumbet Smith, induced HSP27 dimerization resulted in a sensitizing effect to tumors after the treatment with radiation.Citation11) DATS also induced HSP27 dimer formation as well as apoptosis in U937 leukemic cell. Further studies are needed to clarify the detailed mechanism between HSP27 dimer formation and the induction of apoptosis.
In summary, we demonstrated for the first time that garlic-derived DATS works to form a HSP27 homodimer. Hsp27 is suggested to be one of the molecular target of DATS in U937 leukemic cell.
Acknowledgement
This work was supported by grants from Nihon University (to T.S. and T. H.), the programs Grants-in-Aid for Scientific Research (B) (#25292077 to T.S.) and (C) (#23780145 to T.H.) from the Japan Society for the Promotion of Science (JSPS).
Notes
Abbreviations: DATS, diallyl trisulfide; DMSO, dimethyl sulfoxide; HSP27, heat shock protein 27; IAA, iodoacetamide; PARP, poly(ADP-ribose) polymerase.
References
- Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141:80–89.10.1053/j.gastro.2011.03.057
- Busch C, Jacob C, Anwar A, Burkholz T, Aicha Ba L, Cerella C, Diederich M, Brandt W, Wessjohann L, Montenarh M. Diallylpolysulfides induce growth arrest and apoptosis. Int. J. Oncol. 2010;36:743–749.
- Chen M, Li B, Zhao X, Zuo H, He X, Li Z, Liu X, Chen L. Effect of diallyl trisulfide derivatives on the induction of apoptosis in human prostate cancer PC-3 cells. Mol. Cell. Biochem. 2012;363:75–84.10.1007/s11010-011-1159-9
- Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki T, Ariga T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of β-tubulin. J. Biol. Chem. 2005;280:41487–41493.10.1074/jbc.M507127200
- Shankar S, Chen Q, Ganapathy S, Singh KP, Srivastava RK. Diallyl trisulfide increases the effectiveness of TRAIL and inhibits prostate cancer growth in an orthotopic model: molecular mechanisms. Mol. Cancer Ther. 2008;7:2328–2338.10.1158/1535-7163.MCT-08-0216
- Wang HC, Yang JH, Hsieh SC, Sheen LY. Allyl sulfides inhibit cell growth of skin cancer cells through induction of DNA damage mediated G2/M arrest and apoptosis. J. Agric. Food Chem. 2010;58:7096–7103.10.1021/jf100613x
- Xiao D, Zeng Y, Singh SV. Diallyl trisulfide-induced apoptosis in human cancer cells is linked to checkpoint kinase 1-mediated mitotic arrest. Mol. Carcinog. 2009;48:1018–1029.10.1002/mc.v48:11
- Hosono T, Hosono-Fukao T, Inada K, Tanaka R, Yamada H, Iitsuka Y, Seki T, Hasegawa I, Ariga T. Alkenyl group is responsible for the disruption of microtubule network formation in human colon cancer cell line HT-29 cells. Carcinogenesis. 2008;29:1400–1406.10.1093/carcin/bgn124
- Cumming RC, Andon NL, Haynes PA, Park M, Fischer WH, Schubert D. Protein disulfide bond formation in the cytoplasm during oxidative stress. J. Biol. Chem. 2004;279:21749–21758.10.1074/jbc.M312267200
- Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70.10.1023/A:1021601103096
- Choi SH, Lee YJ, Seo WD, Lee HJ, Nam JW, Lee YJ, Kim J, Seo EK, Lee YS. Altered cross-linking of HSP27 by zerumbone as a novel strategy for overcoming HSP27-mediated radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:1196–1205.10.1016/j.ijrobp.2010.10.025
