Abstract
Peroxisome proliferator activated receptor α (PPARα) ligands, fibrates used to control hyperlipidemia. We demonstrated CYP2B induction by clofibric acid (CFA) however, the mechanism was not clear. In this study, HepG2 cells transfected with expression plasmid of mouse constitutive androstane receptor (CAR) or PPARα were treated with CFA, phenobarbital (PB) or TCPOBOP. Luciferase assays showed that CFA increased CYP2B1 transcription to the same level as PB, or TCPOBOP in HepG2 transfected with mouse CAR But failed to induce it in PPARα transfected cells. CYP2B expressions were increased with PB or CFA in Wistar female rats (having normal levels of CAR) but not in Wistar Kyoto female rats (having low levels of CAR). The induction of CYP2B by PB or CFA was comparable to nuclear CAR levels. CAR nuclear translocation was induced by CFA in both rat strains. This indicates that fibrates can activate CAR and that fibrates-insulin sensitization effect may occur through CAR, while hypolipidemic effect may operate through PPARα.
Graphical Abstract
A transcriptional proof of the mechanism through which clofibric acid induce CYP2B through constitutive androstane receptor (CAR) not through PPARα
Fibrates are used in the treatment of dyslipidemia in patients with and without type 2 diabetes.Citation1) These compounds are known to be ligands of peroxisome proliferator-activated receptors α (PPARα)Citation2) through which they can induce increases in lipoprotein lipase, acyl-coenzyme A synthetase, and fatty acid transport protein, apolipoprotein A-I and A-II, as well as reductions in apolipoprotein C-III and cholesterol ester transfer protein activity in hepatocyte.Citation3) The involvement of PPAR in cell proliferation and tumor promotion in rodents is well documented in studies using PPAR knockout mice.Citation4,5) Following exposure to peroxisome proliferators, rodents exhibit biological and biochemical responses such as peroxisome proliferation, increased microsomal fatty acid oxidation, increased hepatocytes hydrogen peroxide formation, hepatomegaly, hyperplasia, and subsequent neoplasia.Citation6) This effect seems to occur mainly in the hepatocytes as PPAR was demonstrated to express mainly in the hepatocytes 8–20-fold higher than endothelial cells and Kupffer cells in rats.Citation7) The human liver appears to be insensitive to the carcinogenic properties of peroxisome proliferators, as PPAR is expressed in the human liver at low levels compared to the levels seen in the rodent liver.Citation6,8,9) Consequently, the long-term use of PPARα ligands, including fibrates, as hypolipidemic agents in patients with Type 2 diabetes results in a lower incidence of PPARα-dependent hyperplasia. Moreover, the ability of fibrates to activate constitutive androstane receptor (CAR) still need more elucidations. The constitutive active/androstane receptor (CAR) is a member of the orphan nuclear receptor subfamily. CAR is activated by a variety of xenobiotics and mediates the upregulation of several phase I and II drug-metabolizing enzymes, such as CYP450s, glutathione S-transferases, and uridine diphosphate-glucuronosyltransferases in mice.Citation10) CAR also contributes to the metabolism of endogenous compounds such as bilirubin, and steroid hormones in response to exogenous agents.Citation11) Human CAR is expressed primarily in the liver and small intestine, and at lower levels in heart, muscle, kidney, and lung tissue.Citation12) In mice, CAR is activated by phenobarbital (PB) and a group of structurally diverse agents described as “phenobarbital-like”.Citation13) PB facilitates the translocation of CAR from the cytoplasm to the nucleus where it forms a heterodimer with the retinoid X receptor (RXR). Subsequently, this can bind to the PB-responsive element located within the promoter region of CAR target genes, leading to changes in their expression.Citation14) Long-term treatment with CAR activators causes liver tumors in miceCitation15) and CAR was found to be essential for mice liver tumorigenesis in response to chronic treatment with PB and PB-like chemicals.Citation16) We have previously stated that clofibric acid is able to induce CYP2B expression in the rat liver.Citation17) However, the mechanism behind this induction was not investigated. Different fibrate derivatives have been used for a long time to control hyperlipidemia in patients with or without type 2 diabetes.Citation1) Therefore, it is important to investigate the ability of fibrates to activate CAR. In the present study, we investigated whether the induction of CYP2B expression by clofibric acid occurs through PPARα or CAR.
Materials and methods
Materials
Bovine serum albumin (BSA), rabbit horseradish peroxidase-labeled anti-goat IgG, and PB were purchased from Sigma Chemical Co. (St Louis, MO, USA). Diaminobenzidine tetrahydrochloride was obtained from Kanto Chemical Co. (Tokyo Japan), and clofibric acid (2-(p-chlorophenoxy)-2-methylpropionic acid) of 97% purity was from Pfaltz & Bauer, Inc. (NY, USA). Polyclonal goat anti-rat CYP2B1 antibody was purchased from Daiichi Pure Chemical Co., Ltd (Tokyo, Japan). Goat horseradish peroxidase-labeled anti-rabbit and goat anti-rat CAR and PPARα antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Other chemicals and solvents were of analytical grade. A Full-length human PPARα expression plasmid from human liver cDNA libraries (BD Biosciences Co., MA, USA) was a generous gift from Professor K. Kimura, Laboratory of Biochemistry, Graduate School of Veterinary Medicine, Hokkaido University. The mouse CAR (mCAR) expression plasmid [pCR3-mCAR (full length mCAR)] and the rat CYP2B reporter NR1 [5 Tandem NR1(CAR response element) and TK minimum promoter], were a generous gift from Dr. Masahiko Negishi, Laboratory of Reproductive and Developmental Toxicology, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, North Carolina.
Animals
A 20 female Wistar and 20 female Wistar Kyoto rats aged seven weeks were obtained from Charlze River, Nakagawa, Yokohama, Japan. In the first part of the experiment, five rats from each strain were intraperitoneally injected with PB (80 mg/kg) and rats were sacrificed 24 h later. In the second part of the experiment, 15 rats from either strain were divided into three groups of five. The first group in each strain was used as a control, the second group was treated with clofibric acid (CFA), and the third group was treated with PB. Rats were housed at 24 ± 1 °C with a 12 h light/12 h dark cycle, and given laboratory food (MR stock, NOSA N Co., Yokohama, Japan) and water ad libitum. All experiments using animals were performed under the supervision and approval of the Institutional Animal Care and Use Committee of Hokkaido University. The CFA-treated group was orally administered CFA (300 mg/kg) at 8 am for three consecutive days, while the other groups were administered the same volume of corn oil as a vehicle control. On the third day at 8 am, the third group received PB (80 mg/kg) by intraperitoneal injection, while all other groups were injected with the same volume of saline. Twenty-four hours later, rats were sacrificed with carbon dioxide, and the livers were removed and perfused with ice-cold buffer I (50 mM Tris-HCl, pH 7.4; 25 mM KCl; 5 mM MgCl2; 0.25 mM sucrose; 0.1 mM PMSF). The microsomal fractions from half of the livers were prepared according to the method of Omura and Sato,Citation18) with slight modifications. Liver samples were minced and homogenized using a Teflon homogenizer in three times their volume of ice-cold 0.1 M potassium phosphate buffer (KPB), pH 7.4. Homogenized samples were centrifuged at 9000 g at 4 °C for 20 min. The supernatant fraction was centrifuged at 105,000 g at 4 °C for 70 min to obtain a mitochondrion-free microsomal pellet. The washed microsomes were then suspended in 0.1 M KPB, pH 7.4, divided into 1.5 mL tubes, snap-frozen in liquid nitrogen, and kept at −80 °C until use. Microsomal protein concentrations were determined according to the method of Lowry et al.Citation19) using BSA as the standard.
Nuclear extraction
Nuclei were isolated from the second half of the liver samples according to the method of Carey et al.Citation20) with slight modifications. Briefly, samples were homogenized in ice-cold buffer I (50 mM Tris-HCl, pH 7.4; 25 mM KCl; 5 mM MgCl2; 0.25 mM sucrose; 0.1 mM PMSF) using a Teflon homogenizer. The resulting homogenate was centrifuged at 600 g for 10 min. Pellets were then homogenized in twice their volume of buffer I. The homogenates were layered onto buffer I containing 2.3 M sucrose (ratio 4:1), and centrifuged at 12,000 g for 40 min. The middle layer fraction containing the nuclei was washed with buffer I and re-suspended in nuclear lysis buffer (20 mM HEPES, pH 7.8; 1.5 mM MgCl2; 420 mM NaCl; 0.2 mM EDTA pH 8; 1 mM DTT; 0.5 mM PMSF; 25% glycerol) and kept on ice for 30 min, after which it was centrifuged at 12,000 g at 4 °C for 2 min. The resulting supernatant containing the nuclear extract was snap-frozen in liquid nitrogen and kept at −80 °C until use. Nuclear protein concentrations were determined according to the method of Lowry et al.Citation19) using BSA as the standard.
Luciferase activity assay
HepG2 cells obtained from the American Type Culture Collection (Manassas, VA, USA) were grown in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin) at 37 °C in a humidified atmosphere of 5% CO2 in air. Cells were seeded at 70% confluence on 96 well collagen-coated plates. Twenty-four hours later, cells were transfected with pCR3-mCAR expression vector or pDEST-h PPARα full-length hPPARα expression vector, using 100 ng of vector per well. Cells were also transfected with the empty pDEST vector containing equal DNA loading as a control. In addition, co-transfection with NR1 reporter plasmid (50 ng/well) and PRL40 vector (5 ng/well) was performed using Trans-It (Mirus, Madison, WI, USA), according to the instruction manual, for 16 h. The media were then replaced with fresh medium containing DMSO (control), 750 μM PB, 2 mM CFA, or 250 nM TCPOBOP for 24 h. Cells were lysed, and firefly and Renilla luciferase activities were measured from six independent transfections using the Dual-Luciferase Reporter Assay System (Promega). Transfection data were expressed as the induction folds of firefly to Renilla luciferase activities relative to the empty vector or vehicle control.
Western blotting
Aliquots of liver microsomal protein (12 μg) or nuclear protein (30 μg) from treated and control rats were applied to 10% sodium dodecyl-sulfate (SDS) polyacrylamide gels and separated by electrophoresis using a Protean 2 mini 1-D cell (BioRad, Hercules, CA, USA). The proteins were transferred electrophoretically to nitrocellulose membranes, blocked with 5% skim milk in phosphate-buffered saline (PBS) containing 1% Tween 20 for 2 h at room temperature, and probed with the appropriate antibody in PBS containing 1% Tween 20 on a shaker for 2 h at room temperature. Horseradish peroxidase-labeled anti-goat IgG or anti-rabbit IgG was used as secondary antibodies. Immunoreactive protein bands were revealed either by oxidation of 0.025% 3,3-diaminobenzidine tetrahydrochloride with 0.0075% hydrogen peroxide catalyzed by peroxidase in 50 mM Tris-HCl (pH 7.6), or by detection with the ECL-Plus chemiluminescence kit (Amersham Life Science, Cleveland, OH, USA). Intensities of the immunoreactive bands were analyzed densitometrically using the public domain NIH Image program (www.rbs.info.nih.gov/nih-image/).
RNA extraction
Total RNA was isolated from 50 mg liver tissues using Tri Reagent (Sigma Chemical Co.; St. Louis, MO, USA). Briefly, liver tissue samples were homogenized in 1 mL Tri Reagent, then 0.3 mL chloroform was added to the sample. The mixture was then shaken for 30 s and centrifuged at 4 °C and 12,000 g for 20 min. The supernatant layers were transferred to a new set of tubes, and equal volumes of isopropanol were added to the samples, shaken for 15 s, and centrifuged at 4 °C and 12,000 g for 15 min. The RNA pellets were washed with 70% ethanol. RNA was dissolved in deionized, diethylpyrocarbonate (DEPC) water. The integrity of the prepared RNA was checked by agarose electrophoresis, and concentrations were further checked by measuring the optical density at 260 nm. The optical density of all RNA samples was 1.7–1.9 based on the 260/280 ratio.
RT-PCR
cDNA was synthesized as follows: A mixture of 5 μg total RNA and 0.5 ng oligo dT primer, in a total volume of 24 μL sterilized ultra-pure water, was incubated at 70 °C for 10 min and then removed from the thermal cycler. The mixture was made up to 40 mL in 8 U RT-buffer (5X), 2 μL 10 mM dNTP, 2 μL DEPC water, and 2 μL reverse transcriptase (Toyobo Co., Ltd, Osaka, Japan). The mixture was then re-incubated in the thermal cycler at 42 °C for 1 h and at 90 °C for 10 min to prepare the cDNA. Semi-quantitative PCR was performed as follows: 1 μL aliquots of the synthesized cDNA were added to 19 μL of a mixture containing sterilized ultra-pure water, 2 μL PCR buffer (10X), 2 μL dNTP (2.5 mM), 0.3 μL sense and 0.3 μL anti-sense specific primers (100 pM), and 0.1 μL EX-Taq polymerase (Takara, Kyoto, Japan). Amplification was initiated by denaturation with one cycle at 94°C for 4 min followed by an appropriate number of cycles, each cycle consisted of denaturation at 94°C for 1 min, annealing at the appropriate temperature for 1 min, extension at 72°C for 1 min, then the final cycle is followed by a final incubation for 7 min at 72°C, using a DNA thermal cycler (BioRad). Amplified PCR products were subjected to electrophoresis using 1.0–1.5% agarose gels. Bands were stained with ethidium bromide and visualized by ultraviolet illumination. Photographic images were converted into computer files with an Epson color-image scanner in combination with Adobe PhotoShop 6.0 software. Intensities of the bands were densitometrically analyzed on a Macintosh computer using the public domain NIH image program. The primer sequences and annealing temperature for each PCR reaction are shown in Table .
Table 1. Primers used in the amplification of genes by semi-quantitative PCR.
Real-time quantitative PCR
Real-time quantitative PCR for CAR and G3PDH mRNA levels was performed using an ABI PRISM 7700 and the SYBR green PCR kit (Giagen, Inc., CA, USA). The reaction mixture (final volume 10 uL) for PCR was prepared with final concentration of 1X Master Mix reagents, 150nM of each primer, 300 ng cDNA in 1 uL RNase-free water for CAR, and G3PDH the mixture was completed to the final volume by RNase-free water. The reaction was performed for 40 cycles; initial activation at 95 °C for 15 min, denaturation at 95 °C, annealing at 60 °C for 1 in, extension at 72 °C for 30 s. The measurements of each CAR and G3PDH were performed in duplicate and repeated three times. The expression of CAR was normalized to the expression of G3PDH and was calculated relative to that of Wistar rats control group.
Statistical analysis
All data are expressed as means ± SDs. Statistical significance was evaluated using the Tukey–Kramer HSD difference (JMP; SAS Institute, Cary, NC, USA) test. Results were considered to be statistically significant at the p < 0.05 level.
Results
Effect of CFA on CYP2B transcriptional activity
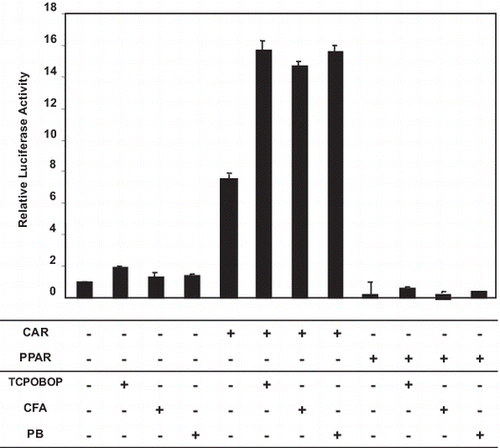
Treatment of HepG2 cells with TCPOBOP, CFA, or PB, without CAR transfection induced CYP2B transcriptional activity compared to the control. Transfection of HepG2 cells with mCAR expression vector induced CYP2B transcriptional activity even without any treatment. Treatment of CAR-transfected HepG2 cells with TCPOBOP or PB produced a further increase in CYP2B transcriptional activity compared to CAR-transfected untreated HepG2 cells. Importantly, CFA increased CYP2B transcriptional activity to the same level as that induced by TCPOBOP or PB. The PPARα-transfected HepG2 cells did not show any induction with or without ligand treatment (Fig. ).
Fig. 1. Effect of CFA on CYP2B tanscriptional activity.
Notes: HepG2 cells were transfected with pCR3-mCAR expression vector or pDEST-h PPARα full-length hPPARα expression vector, using 100 ng of vector per well, or with the empty pDEST vector using an equal amount of DNA. The cells were also co-transfected with NR1 reporter plasmid (50 ng/well) and PRL40 vector (5 ng/well). Sixteen hours later, the media were replaced with fresh medium containing DMSO (control), 750 μM PB, 2 mM CFA or 250 nM TCPOBOP for 24 h. Cells were lysed, and firefly and Renilla luciferase activities were measured from six independent transfections using the Dual-Luciferase Reporter Assay System. The data presented are the means ± SD of three experiments. *Significantly higher than control, p < 0.05. **Significantly higher than its level in mCAR-transfected HepG2 cells without treatment, p < 0.05.
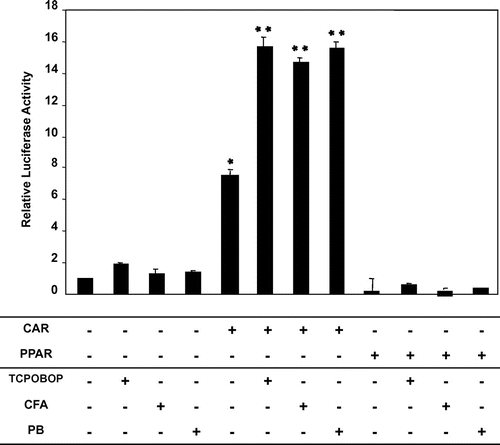
CYP2B protein and mRNA expression in female Wistar Kyoto rats compared to female Wistar rats
We demonstrated in our previous study that CFA is able to induce CYP2B expression in Wistar rat livers.Citation17) To investigate the mechanism of CYP2B induction by CFA, we used female Wistar Kyoto rats,which are known to have low-expression levels of CAR protein. Treatment with PB showed very low expression of CYP2B protein in WKY female rats compared to Wistar female rats (Fig. (A)). CYP2 mRNA expression measurement showed that it also follows the same pattern of protein levels, being very low in WKY female rats compared to Wistar female rats (Fig. (B)).
Fig. 2. Protein levels and CYP2B mRNA expression in female Wistar Kyoto rats compared to Wistar rats.
Notes: Female Wistar or Wistar Kyoto rats were treated with 80 mg/kg PB i.p. 24 h later rats were sacrificed with CO2, and microsomal and nuclear protein extracts were prepared from liver samples. (A) Microsomal protein (12 μg/lane) samples were applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with CYP2B antibodies, as described in the Materials and Methods.[W] denotes Wistar rats while [WKY] denotes Wistar Kyoto rats, [C] denotes cytosolic protein, and [N] denotes nuclear protein. (B) CYP2B mRNA expression. Semi-quantitative PCR was performed to estimate the expression levels of CYP2B mRNA in both rat strains. Total RNA was isolated and RT-PCR was performed as described in the Materials and Methods. cDNA samples were amplified in PCR tubes using 25 cycles for G3PDH mRNA (lower panel), and 30 cycles for CYP2B1 mRNA amplification (upper panel). CYP2B1 mRNA upper panel was normalized to its corresponding G3PDH mRNA bands and then calculated relative to the mean values in Wistar rats. Data are presented in columns representing Mean + SD (n = 5). *Significantly lower than Wistar rats p < 0.05. (C) CAR mRNA expression. Real-time PCR was performed to estimate the expression levels of CAR mRNA in both rat strains as described in the Materials and Methods. Data are presented in columns representing Mean + SD (n = 5). *Significantly lower than Wistar rats p < 0.05. (D) Nuclear protein extracts were prepared from liver samples. Cytosol or nuclear protein samples (12 μg/lane) were applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with CAR antibodies, as described in the Materials and Methods. [W] denotes Wistar rats [WKY] denotes Wistar Kyoto rats, [C] denotes cytosolic protein, and [N] denotes nuclear protein.
![Fig. 2. Protein levels and CYP2B mRNA expression in female Wistar Kyoto rats compared to Wistar rats.Notes: Female Wistar or Wistar Kyoto rats were treated with 80 mg/kg PB i.p. 24 h later rats were sacrificed with CO2, and microsomal and nuclear protein extracts were prepared from liver samples. (A) Microsomal protein (12 μg/lane) samples were applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with CYP2B antibodies, as described in the Materials and Methods.[W] denotes Wistar rats while [WKY] denotes Wistar Kyoto rats, [C] denotes cytosolic protein, and [N] denotes nuclear protein. (B) CYP2B mRNA expression. Semi-quantitative PCR was performed to estimate the expression levels of CYP2B mRNA in both rat strains. Total RNA was isolated and RT-PCR was performed as described in the Materials and Methods. cDNA samples were amplified in PCR tubes using 25 cycles for G3PDH mRNA (lower panel), and 30 cycles for CYP2B1 mRNA amplification (upper panel). CYP2B1 mRNA upper panel was normalized to its corresponding G3PDH mRNA bands and then calculated relative to the mean values in Wistar rats. Data are presented in columns representing Mean + SD (n = 5). *Significantly lower than Wistar rats p < 0.05. (C) CAR mRNA expression. Real-time PCR was performed to estimate the expression levels of CAR mRNA in both rat strains as described in the Materials and Methods. Data are presented in columns representing Mean + SD (n = 5). *Significantly lower than Wistar rats p < 0.05. (D) Nuclear protein extracts were prepared from liver samples. Cytosol or nuclear protein samples (12 μg/lane) were applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with CAR antibodies, as described in the Materials and Methods. [W] denotes Wistar rats [WKY] denotes Wistar Kyoto rats, [C] denotes cytosolic protein, and [N] denotes nuclear protein.](/cms/asset/8149d7a8-ca6e-482d-a71e-8121275ad92c/tbbb_a_923302_f0002_oc.gif)
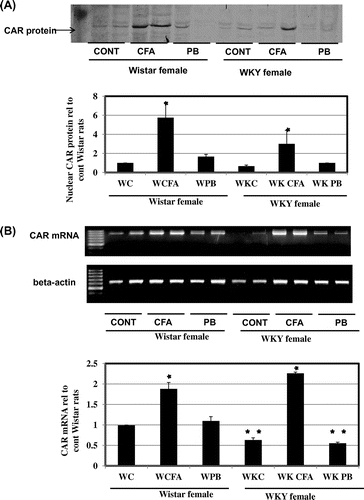
CAR mRNA and protein expression in female Wistar Kyoto and Wistar rats.
Measurement of CAR mRNA by real-time PCR showed that its mRNA expression is very low compared to its levels in female Wistar rats (Fig. (C)). Moreover, Investigation of the nuclear translocation of CAR protein showed low levels of CAR protein in the nuclear extract of female Wistar Kyoto rats compared to those of female Wistar rats (Fig. (D)), confirming the low level of CAR protein expression in female Wistar Kyoto rats and their validity for use in this study.
Effect of CFA on CYP2B expression in female Wistar and female Wistar Kyoto rats
Treatment with PB highly induced CYP2B protein expression compared to controls in female Wistar rats, while in female Wistar Kyoto rats, PB treatment only slightly induced CYP2B expression compared to controls. CYP2B expression was highly induced in female Wistar rats treated with CFA compared to controls. However, this induction did not reach the same level as that induced by PB. Both CFA and PB treatment slightly induced CYP2B expression in female WKY rats (Fig. (A)). Measurement of CYP2B mRNA levels showed that CFA induces CYP2B mRNA expression higher than control in female Wistar rats however, the increase in CYP2BmRNA due to PB was higher than control and CF treated groups. CYP2B mRNA pattern was following the same pattern of protein induction due to each treatment in both rats strains (Fig. (B)).
Fig. 3. Effect of CFA and on CYP2B protein levels and mRNA expression in female Wistar and Wistar Kyoto rats.
Notes: Female Wistar or Wistar Kyoto rats were treated with 300 mg/kg CFA for 3 days or 80 mg/kg PB i.p. 24 h prior to sacrifice with CO2. (A) CYP2B protein expression. Microsomal protein (12 μg/lane) was applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with CYP2B antibodies as described in the Materials and Methods. Data are presented in columns representing Mean + SD (n = 5). *Significantly higer than Wistar rats p < 0.05 (B) CYP2B mRNA expression. Semi-quantitative PCR was performed to estimate the expression level of CYP2B mRNA in both rat strains. Total RNA was isolated and RT-PCR was performed as described in the Materials and Methods. cDNA samples were amplified in PCR tubes using 25 cycles for β-actin mRNA (lower panel), and 30 cycles for CYP2B1 mRNA amplification (upper panel). CYP2B1 mRNA upper panel was normalized to its corresponding G3PDH mRNA bands and then calculated relative to the mean values in Wistar rats. Data are presented in columns representing Mean + SD (n = 5). *Significantly higher than Wistar rats p < 0.05.
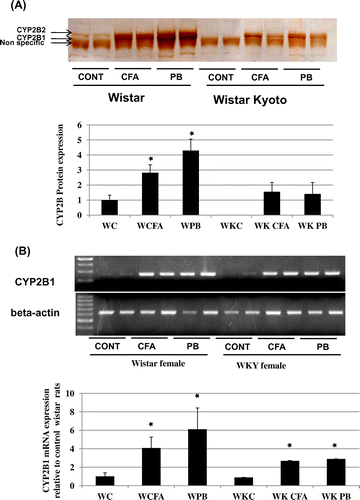
Effect of CFA treatment on CAR protein nuclear translocation and CAR mRNA expression
To investigate the mechanism of CYP2B induction by CFA further, we analyzed the nuclear translocation of CAR protein. CFA treatment induced nuclear translocation of CAR to a greater extent than in the control Group of female Wistar and PB-treated groups in both rat strains (Fig. (A)). RT-PCR measurement of CAR mRNA showed that CFA highly induced CAR mRNA expression in both rat strains higher than control and even higher than that of PB-treated groups in both rat strains (Fig. (B)).
Fig. 4. Effect of CFA treatment on CAR protein nuclear translocation and CAR mRNA expression.
Notes: Female Wistar or Wistar Kyoto rats were treated with 300 mg/kg CFA for 3 days or 80 mg/kg PB i.p. 24 h prior to sacrifice with CO2. (A) Nuclear translocation of CAR protein in response to CFA or PB treatment in female Wistar and Wistar Kyoto rats. Nuclear protein extracts were prepared from liver samples as described in the Materials and Methods. Nuclear protein (12 μg/lane) was applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with CAR antibodies as described in the Materials and Methods. Treatment groups are denoted as follows: control (C), clofibric acid (CFA), and PB. Data are presented in columns representing Mean + SD (n = 5). *Significantly higher than control group in Wistar rats p < 0.05. (B) mRNA expression of CAR Semi-quantitative PCR was performed to estimate the expression level of CAR mRNA in both rat strains. Total RNA was isolated and RT-PCR was performed as described in the Materials and Methods. cDNA samples were amplified in PCR tubes using 25 cycles for β-actin mRNA (lower panel), and 30 cycles for CAR mRNA amplification (upper panel). CAR mRNA upper panel was normalized to its corresponding G3PDH mRNA bands lower panel and then calculated relative to the mean values in control group in Wistar rats. Data are presented in columns representing Mean + SD (n = 5). *Significantly higher than Wistar rats p < 0.05. **Significantly lower than control group in Wistar rats p < 0.05.
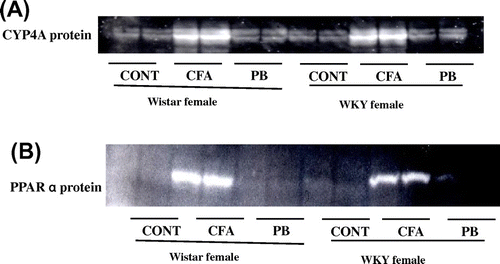
Effect of CFA and PB on microsomal CYP4A protein levels and PPARα nuclear protein levels
To confirm this effect of CFA, we measured CYP4A protein levels. CYP4A protein was induced by CFA to the same levels in both rat strains meanwhile, no CYP4 induction has resulted due to PB treatment in either rat strains (Fig. (A)). Induction of CYP4A has been shown to be mediated by PPARαCitation24). Analysis of the nuclear translocation of PPARα protein showed that its nuclear translocation was induced only by CFA and that occurred at the same levels in both rat strains (Fig. (B)).
Fig. 5. Effects of CFA or PB on CYP4A protein and PPARα nuclear protein expression.
Notes: Female Wistar or Wistar Kyoto rats were treated with 300 mg/kg CFA for 3 days or 80 mg/kg PB i.p. for 24 h prior to sacrifice with CO2. (A) Effects of CFA or PB treatment on CYP4A. Microsomal protein (12 μg/lane) was applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with CYP4A1 antibodies as described in the Materials and Methods. (B) PPARα nuclear protein expression. Nuclear protein extracts were prepared from liver samples as described in the Materials and Methods. Nuclear protein (12 μg/lane) was applied to 10% SDS-PAGE, transblotted onto nitrocellulose membranes, and reacted with PPARα antibodies as described in the Materials and Methods. Treatment groups are denoted as follows: control (C), CFA, and PB.
Discussion
We have previously demonstrated the ability of clofibrate to induce CYP2B in the rat liver,Citation17) however, the mechanism behind this induction was not investigated. In this report, we have demonstrated that clofibric acid causes activation of CAR, resulting in induction of CYP2B transcription. The CYP2B transcriptional factor, NR1, was induced with transfection of mCAR expression vector in HepG2 cells without application of any ligand (Fig. ). The ability of non-activated CAR to induce the transcription of CYP2B agrees with the findings of Bae et al.Citation25) In HepG2 cells transfected with mCAR and NR1, reporter activities were increased significantly with PB or TCPOBOP treatment compared to levels seen in non-treated mCAR-transfected HepG2 cells. These data also agree with a previous findings.Citation25) Notably, treatment with CFA increased the reporter activity to levels similar to those induced by PB or TCPOBOP. HepG2 cells transfected with PPARα failed to exhibit any CYP2B reporter activity before or after CFA treatment. The induction of CYP2B transcription with CFA in mCAR-transfected HepG2 cells and its absence in PPARα-transfected cells (Fig. ) indicates the induction of CYP2B by CFA occurs most likely through CAR not PPARα. Indeed, di (n-butyl) phthalate (a PPARα agonist) was reported to induce CYP2B reporter activity in HepG2 cells transfected with CAR.Citation26) The expression levels of CAR protein were reported to be extremely low in the cytosol and nucleus in female WKY rat livers.Citation21) Therefore, female WKY rats and female Wistar rats, which have low and normal levels of CAR protein, respectively, are good models for comparison in our study. Treatment of rats of both strains with PB revealed low-CYP2B protein levels and mRNA expression in female WKY rats (Fig. (A) and (B)). This was associated with low-CAR mRNA expression (Fig. (A)) and low-nuclear CAR protein in female WKY rat livers compared to female Wistar rat livers (Fig. (B)).
CAR has been reported to form a complex with Hsp90 and cochaperone in the cytoplasm.Citation27) In response to PB exposure, CAR translocates to the nucleus, forms a heterodimer with the RXRCitation28) and activates the 51-bp PB-responsive enhancer element that is conserved in the rat,Citation29) mouseCitation30), and human CYP2B genes.Citation31) Fibrates, which are PPARα agonists, are widely used in humans for the treatment of dyslipidemia in patients with or without type 2 diabetes.Citation1) In this study, treatment of both rat strains showed that CYP2B protein level was induced by CFA to a nearly similar level as that induced by PB when compared to control values. However, CYP2B protein induction was very low in female WKY rats compared to Wistar rats (Fig. (A)). When both rat strains were treated with CFA or PB, the lower levels of CYP2B protein induction in female WKY rats compared to female Wistar rats were associated with lower CYP2B mRNA levels (Fig. (B)). Nuclear CAR protein measurements showed that CFA or PB treatment induced higher levels of nuclear CAR protein translocation compared with control levels. However, it was also noted that low-nuclear CAR protein (Fig. (A)) was associated with impaired induction of CYP2B mRNA in female WKY rats compared with female Wistar rats (Fig. (A)). This indicates the low level of CYP2B induction was due to lower CAR protein expression in female WKY rats compared with female Wistar rats. The induction of CYP2B was reported to be mediated by CAR.Citation28,32) To further confirm the effect of CFA, we measured the induction of CYP4A1 protein expression, which was only seen in the CFA-treated groups in both rat strains. Nuclear protein expression of PPARα was induced only by CFA treatment in both rat strains. These results, combined with the difference, seen in CYP2B induction in both rat strains after CFA treatment, confirm that CYP2B induction by CFA was due to stimulation of CAR, and eliminate the possibility that CYP2B induction by CFA occurred through PPARα stimulation. It is worthy to note that the nuclear CAR protein expression caused by CFA treatment was higher compared to that induced by PB treatment in both rat strains. Nevertheless, its expression was still lower in female WKY rats compared with Wistar rats after treatment with CFA (Fig. (A)). It has been reported that PB is not a ligand of CAR.Citation33) However, PB-induced expression of CYP2B6 has been reported to be a CAR/NR1I3-mediated event in which the inducer triggers nuclear translocation of CAR/NR1I3, possibly via receptor dephosphorylation.Citation34) In the current study, CFA elicited induction of CAR protein expression in both rat strains. This indicates that CFA is most likely a ligand of CAR and is able to induce its protein expression, in addition to its ability to induce its nuclear translocation, activation, and subsequent effects on its target genes (Fig. ). The results of this study are in agreement with recent data reporting that the PPARα ligand, di-(2-ethylhexyl) phthalate are able to activate CAR.Citation35) Our assumption of CAR activation by CFA is in contrast to the explanation of CYP2B induction by PPARα ligands assumed by Saito et al.Citation36) who proposed that PPARα activation was the cause of CAR protein induction however, that proposal still need stronger evidences. Moreover, in our study HepG2 cells transfected with PPARα failed to exhibit any CYP2B reporter activity before or after CFA treatment (Fig. ), which eliminates the possibility of CAR protein induction by PPARα activation at least in our model. In line with the ability of CFA to activate CAR independent of PPARα, it was demonstrated that in PPARα-null mice that a number of Peroxisome proliferator chemicals including di-(2-ethylhexyl) phthalate (DEHP), exhibit transcriptional responses independent of PPARα but dependent on CAR including Cyp2b10, Cyp3a11, and Cyp3a41a. The authors added that CAR mRNA expression was increased in PPARα-null mice while was being not increased in the wild type mice.Citation35) It has been reported that not only can clinically used drugs serve as CYP2B6 substrates, but so can endogenous components, pesticides, and environmental chemicals.Citation37) CYP2B6 can metabolize 8–10% of clinically used drugs.Citation38,39) This is because CYP2B6 induction is an important source of drug–drug interactions, which may result in drug toxicity or therapeutic failure.Citation34) Consequently, our results, which indicate that CFA induces activation of CAR and its target genes, including CYP2B, may be of clinical concern since fibrates are used as hypolipidemic drugs. Interestingly, PB treatment was reported to decrease plasma glucose and improve insulin sensitivity in a rodent model of diabetes,Citation40) and also in human T2D patients.Citation41) Moreover, treatment with the CAR activators, PB and TCPOBOP, decreased the expression of the hepatic gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in mouse liversCitation42) and rat hepatocytes.Citation43) Recently, the anti-diabetic effects of the CAR agonist, TCPOBOP, were reported to be lost in CAR_/_ double mutant ob/ob mice.Citation44) The same authors also stated that loss of CAR function in the ob/ob background had no effect on such parameters as body weight, food intake, fat-to-body weight ratio, and serum levels of triglycerides, cholesterol, and free fatty acids. This may be explained by our finding that CFA is a ligand of CAR, as well as being a PPARα agonist.Citation2) The insulin sensitization effect of the hypolipidemic fibrates may occur through CAR, while their hypolipidemic effect may operate through PPARα. Recently, there has been a strong evidence indicate that CAR is among a number of nuclear receptors that regulate key metabolic pathways in the liver and therefore represents a potential target for metabolic disease.Citation45) Consequently, the discovery of the ability of fibrates to activate CAR is to be considered in light of the emerging roles of CAR in the metabolism.
Conclusion
CFA and/or its derivatives have the ability to bind to CAR and activate the signal cascade leading to the induction of CYP2B and other CAR-target genes. This indicates that the insulin sensitization effect of the hypolipidemic fibrates may occur through CAR, while their hypolipidemic effect may operate through PPARα. This finding is to be considered during the use of fibrates as hypolipidemic drugs in light of the newly discovered major roles of CAR in metabolism as recently reported.Citation44)
Acknowledgments
The authors would like to thank Professor Masahiko Negishi, Laboratory of Reproductive and Developmental Toxicology, National Institutes of Health (Research Triangle Park, North Carolina 27709, USA) for his generous gifts of mCAR expression plasmid and CYP2B reporter NR1. The authors would also like to thank Professor K. Kimura Laboratory of Biochemistry, Graduate School of Veterinary Medicine, Hokkaido University (N18, W9, Kita-ku, 060-0818 Sapporo, Japan) for his generous gift of full-length human PPARα expression plasmid.
Funding
This study was partly supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) awarded to Zein Shaban Ibrahim [grant number P05214].
Notes
Abbreviations: CAR, constitutive androstane receptor; CFA, clofibric acid; NR, nuclear receptor binding site; P450, Cytochrome P450; PB, phenobarbital; PBREM, phenobarbital-response enhancer module; PGC-1alpha, peroxisome proliferator-activated receptor-gamma coactivator-1alpha; PPARα, peroxisome proliferator receptor alpha; RXR, retinoid X receptor; TCPOBOP, 3,3′,5,5′-tetrachloro-1,4-bis(pyridyloxy)benzene or 1,4-bis[2-(3,5-dichloropyridyloxy) benzene;WKY, Wistar Kyoto.
This article was originally published with errors. This version has been updated. Please see Erratum. (http://dx.doi.org/10.1080/09168451.2014.945725).
References
- Chang F, Jaber LA, Berlie HD, O'Connell MB. Evolution of peroxisome proliferator-activated receptor agonists. Ann. Pharmacother. 2007;41:973–83.
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–2093.
- Watts GF, Dimmitt SB. Fibrates, dyslipoproteinaemia and cardiovascular disease. Curr. Opin. Lipidology. 1999;10:561–574.
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell Biol. 1995;6:3012–3022.
- Peters JM, Hennuyer N, Staels B, Fruchart JC, Fievet C, Gonzalez FJ, Auwerx J. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor alpha-deficient mice. J. Biol. Chem. 1997;272:27307–27312.
- Willhite CC. Weight-of-evidence versus strength-of-evidence in toxicologic hazard identification: Di(2-ethylhexyl)phthalate (DEHP). Toxicology. 2001;160:219–226.
- Hoekstra M, Kruijt JK, Van Eck M, Van Berkel TJ. Specific gene expression of ATP-binding cassette transporters and nuclear hormone receptors in rat liver parenchymal, endothelial, and Kupffer cells. J. Biol. Chem. 2003;278:25448–25453.
- Ashby J, Brady A, Elcombe CR, Elliott BM, Ishmael J, Odum J, Tugwood JD, Kettle S, Purchase IF. Mechanistically-based human hazard assessment of peroxisome proliferator-induced hepatocarcinogenesis. Hum. Exp. Toxicol. 1994;2:117.
- Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, Tugwood J, Wahli W. Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans? Regul. Toxicol. Pharmacol. 1998;1:47–60.
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmaco. 2202;6:1–6.
- Kodama S, Negishi M. Phenobarbital confers its diverse effects by activating the orphan nuclear receptor car. Drug Metab. Rev. 2006;38:75–87.
- Lamba JK, Lamba V, Yasuda K, Lin YS, Assem M, Thompson E, Strom S, Schuetz E. Expression of constitutive androstane receptor splice variants in human tissues and their functional consequences. J. Pharmacol. Exp. Ther. 2004;311:811–821.
- Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem. J. 1992;281:577–592.
- Honkakoski P, Moore R, Gynther J, Negishi M. Characterization of phenobarbital-inducible mouse Cyp2b10 gene transcription in primary hepatocytes. J. Biol. Chem. 1996;271:9746–9753.
- Diwan BA, Lubet RA, Ward JM, Hrabie JA, Rice JM. Tumor-promoting and epatocarcinogenic effects of 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) in DBA/2NCr and C57BL/6NCr mice and an apparent promoting effect on nasal cavity tumors but not on hepatocellular tumors in F344/NCr rats initiated with N-nitrosodiethylamine. Carcinogenesis. 1992;10:1893–901.
- Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–71200.
- Shaban Z, Soliman M, El-Shazly S, El-Bohi K, Abdelazeez A, Kehelo K, Kim HS, Muzandu K, Ishizuka M, Kazusaka A, Fujita S. AhR and PPARalpha: antagonistic effects on CYP2B and CYP3A, and additive inhibitory effects on CYP2C11. Xenobiotica. 2005;35:51–68.
- Omura T, Sato R. the carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. chem. 1964;239:2370–2378.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275.
- Carey DJ, Rafferty CM, Schramm MM. Association of heparan sulfate proteoglycan and laminin with the cytoskeleton in rat liver. J. Biol. Chem. 1987;262:3376–3381.
- Xiong H, Yoshinari K, Kim I, Brouwer R, Negishi M. Role of constitutive androstane receptor in the in vivo induction of Mrp3 and cyp2b1/2 by Phenobarbital. Drug Metabolism and Disposition. 2002;30:918–923.
- Ibrahim ZS, Ishizuka M, Soliman M, ElBohi K, WSobhy, Muzandu K, Elkattawy AM, Sakamoto KQ, Fujita S. Protection by Nigella sativa against carbon tetrachloride-induced downregulation of hepatic cytochrome P450 isozymes in rats. Jpn. J. Vet. Res. 2008;56:119–128.
- Yoshinari K, Sueyoshi T, Moore R, Negishi M. Nuclear receptor CAR as a regulatory factor for the sexually dimorphic induction of CYB2B1 gene by phenobarbital in rat livers. Mol. Pharmacol. 2001;59:278–284.
- Johnson EF, Hsu MH, Savas U, Griffin KJ. Regulation of P450 4A expression by peroxisome proliferator activated receptors. Toxicology. 2002;181–182:203–206.
- Bae Y, Kemper JK, Kemper B. Repression of CAR-mediated transactivation of CYP2B genes by the orphan nuclear receptor, short heterodimer partner (SHP). DNA Cell Biol. 2004;2:81–91.
- Wyde ME, Kirwan SE, Zhang F, Laughter A, Hoffman HB, Bartolucci-Page E, Gaido KW, Yan B, You L. Di-n-butyl phthalate activates constitutive androstane receptor and pregnane X receptor and enhances the expression of steroid-metabolizing enzymes in the liver of rat fetuses. Toxicol. Sci. 2005;86:281–290.
- Kobayashi K, Sueyoshi T, Inoue K, Moore R, and Negishi M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol. Pharmacol. 2003;64:1069–1075.
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, et al. Mol. Cell. Biol. 1999;19:6318–6322.
- Trottier E, Belzil A, Stoltz C, Anderson A. Gene. 1995;158:263–268.10.1016/0378-1119(94)00916-G
- Honkakoski P, Moore R, Gynther J, Negishi M. J. Biol. Chem. 1996;271:9746–9753.
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. J. Biol. Chem. 1999;274:6043–6046.10.1074/jbc.274.10.6043
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. Mol. Cell. Biol. 1998;10:5652–5658.
- Goodwin B, Moore LB, Stoltz CM, McKee DD, Kliewer SA. Mol. Pharmacol. 2001;60:427–431.
- Mo SL, Liu YH, Duan W, Wei MQ, Kanwar JR, et al. Curr. Drug Metab. 2009;10:730–753.10.2174/138920009789895534
- Ren H, Aleksunes LM, Wood C, Vallanat B, George MH, et al. Toxicol. Sci. 2010;113:45–59.10.1093/toxsci/kfp251
- Saito K, Kobayashi K, Mizuno Y, Fukuchi Y, Furihata T, et al. Drug Metab Pharmacokinetics. 2010;25:108–111.10.2133/dmpk.25.108
- Hodgson E, Rose RL. J. Biochem. Mol. Toxicol. 2007;21:182–186.10.1002/(ISSN)1099-0461
- Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Pharmacol Ther. 2007;116:496–526.10.1016/j.pharmthera.2007.09.004
- Lewis DF. Curr. Med. Chem. 2003;10:1955–1972.10.2174/0929867033456855
- Karvonen I, Stengard JH, Huupponen R, Stenback FG, Sotaniemi EA. Diabetes Res. 1989;10:85–92.
- Lahtela JT, Arranto AJ, Sotaniemi EA. Diabetes. 1985;34:911–916.10.2337/diab.34.9.911
- Manenti G, Dragani TA, Porta G. Chem. Biol. Interact. 1987;64:83–92.10.1016/0009-2797(87)90062-7
- Argaud D, Halimi S, Catelloni F, Leverve XM. Biochem J. 1991;280:663–669.
- Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, et al. Proc. Nat. Acad. Sci. U.S.A. 2009;106:18831–18836.10.1073/pnas.0909731106
- Maglich JM, Lobe DC, Moore JT. J. Lipid Res. 2009;50:439–445.
