Abstract
The relationship between l-tryptophan to nicotinamide metabolism and the menstrual cycle of Japanese women was investigated. Nine metabolism intermediates from urine samples collected during the preovulatory and postovulatory phases were measured. Only urine 3-hydroxykynurenine was higher in the postovulatory phase than in the preovulatory phase. This increase in 3-hydroxykynurenine suggests a decreased reaction of 3-hydroxykynurenine → 3-hydroxyanthranilic acid catalyzed by kynureninase, a vitamin B6 enzyme.
Pellagra is a niacin deficiency that frequently occurs in underprivileged people who eat a lot of corn. In the United States, a pellagra pandemic was reported during 1925–1945; 7,000 people died in 1930, and eventually 59,391 women and 28,022 men died of pellagra during 1920–1960.Citation1) The death rate from pellagra is twofold higher in women than in men.
The vitamin nicotinamide (Nam) is known to biosynthesize from the essential amino acid, l-tryptophan (Trp). We have previously studied an outbreak of pellagra based on the hypothesis that a primary factor would be the decrease in the conversion of l-Trp to Nam but not a simple deficiency of niacin and l-Trp. The conversion was affected by the quality of food and not by the quantity of food. For example, it was decreased with increasing dietary protein and with increasing dietary unsaturated fatty acids, and affected by the dietary sources of carbohydrates.Citation2) We recently reported that the conversion of l-Trp to Nam was significantly reduced by moderate calorie restriction.Citation3) We also confirmed that the conversion of l-Trp to Nam is affected by quality of food as well as by quantity of food in rats. Furthermore, the conversion is affected by many kinds of hormones in rats.Citation4–6) In these reports, we clarified that single administration of progesterone did not reduce the conversion, but that of estrone reduced the conversion to 50% and co-administration of progesterone and estrone reduced it to 25%.Citation4–6)
In humans, a l-Trp metabolic disorder similar to vitamin B6-deficiency has been reported in pregnant women since the 1950s when l-Trp was administered.Citation7–9) In 1966, RoseCitation10,11) reported that the administration of 2 g or 5 g l-Trp to women taking birth control pills that contain estrogen and progesterone induced abnormally increased urinary excretion of xanthurenic acid (XA). Price et al. in 1967,Citation12) Toseland and Price in 1969,Citation13) and Luhby et al.Citation14) in 1971 reported a similar finding. On the contrary, Wertz et al.Citation15) and LojkinCitation16) reported that the urinary excretion of N1-methylnicotinamide (MNA) was higher in pregnant women and rats than in non-pregnant women and rats. We also clarified that the conversion of l-Trp to Nam was higher in the pregnant state than in non-pregnant state in women and female rats.Citation17) El-Zoghby et al.Citation18) reported an interesting finding that l-Trp–kynurenine metabolism is different among prepubertal, sexually mature, and menopausal females. These reports encouraged us to examine the relationship between the menstrual cycle and l-Trp catabolism. Here, we describe a comparison of urinary excretion of l-Trp and its catabolites during the preovulatory and postovulatory phases.
Our study was reviewed and approved by the Ethical Committee of the University of Shiga Prefecture and was conducted according to guidelines of the Declaration of Helsinki. Female Japanese university students were recruited. The purpose and protocol of this study were explained to all participants, and written informed consent was obtained. Participants diagnosed with cold or influenza, and those who had taken multi-vitamin supplements at least once during the previous month, were excluded. All subjects passed a regular medical examination. Thirteen subjects, aged 21 to 25 years, (means ± SD = 22.2 ± 1.1 years, height 158 ± 5 cm, body weight 54.8 ± 6.5 kg, BMI 21.9 ± 1.5 kg/m2) completed the study. The 24-h urine samples were collected during the preovulatory phase, determined as the 9th day of the menstrual cycle, and during the postovulatory phase, determined as the 21st day of the menstrual cycle. The collected urine samples were mixed with 1 mol/L HCl and stored at −25 °C until analysis.
Daily urinary excretion amounts (nmol/24 h) of anthranilic acid (AnA),Citation19) kynurenic acid (KA),Citation20) 3-hydroxykynurenine (3-HK),Citation21) XA,Citation22) 3-hydroxyanthranilic acid (3-HA),Citation22) Nam,Citation23) N1-methylnicotinmide (MNA),Citation24) N1-methyl-2-pyridone-5-carboxamide (2-Py),Citation23) and N1-methyl-4-pyridone-5-carboxamide (4-Py)Citation23) were measured using HPLC.
Significant differences in the mean values between the preovulatory and postovulatory phase were tested using Paired Student’s two-tailed t-test. Differences of p < 0.05 were considered to be statistically significant. GraphPad Prism (version 5.00; obtained from GraphPad software, Inc., San Diego, CA, USA) was used for statistical analysis.
The association between l-Trp to Nam metabolism and the menstrual cycle in Japanese females were investigated. Nine metabolic intermediates (AnA, KA, 3-HK, XA, 3-HA, Nam, MNA, 2-Py, 4-Py) in each 24-h urine sample at preovulatory and postovulatory phases were measured. As shown in Table , the urinary excretion of 3-HK was higher in the postovulatory phase than in the preovulatory phase. A possible explanation for this difference may be that the formation of 3-HK from kynurenine (catalyzed by kynurenine 3-monooxygenase, which is found in the outer membrane of the mitochondria), where NADPH and FAD are involved,Citation25) varies with the menstrual cycle. Fig. shows the metabolic pathway of l-Trp to Nam. An increase in 3-HK suggests a decrease of reaction 3-HK → 3-HA catalyzed by kynureninase, a vitamin B6 enzyme. Although named kynureninase, human kynureninase has very high substrate specificity for 3-HK rather than kynurenine;Citation26) the hydrolytic cleavage of 3-HK to produce 3-HA and alanine. The enzymes concerned with the pathway l-Trp → 3-HA exists in many kinds of tissues,Citation26) however, the enzymes concerned with 3-HA → α-amino-β-carboxymuconate-ε-semialdehyde (ACMS) → QA → nicotinic acid mononucleotide (NaMN) → nicotinic acid adenine dinucleotide (NaAD) →NAD → Nam → MNA → 2-Py + 4-Py localize mainly in liver.Citation27) 3-HA is catalyzed by 3-HA 3,4-dioxygenase, which has an over 100-fold higher activity compared with other enzymes involved in the l-Trp to ACMS metabolismCitation27) (see Fig. ). Organic acids that have not been metabolized are generally excreted into urine.Citation28)
Table 1. Comparison of urinary excretion of l-Trp and Its catabolites between preovulatory and postovulatory phases.
Fig. 1. Metabolic Pathway of l-Trp.
Notes: 2-AAA, 2-aminoadipic acid; ACMS, α-amino-β-carboxymuconate-ε-semialdehyde; AMS, α-aminomuconate-ε-semialdehyde; AnA, anthranilic acid; N-FK, N-formylkynurenine; 3-HA, 3-hydroxyanthranilic acid; 3-HK, 3-hydroxykynurenine; KA, kynurenic acid; Kyn, kynurenine; MNA, N1-methylnicotinamide; 2-Py, N1-methyl-2-pyridone-5-carboxamide; 4-Py, N1-methyl-4-pyridone-3-carboxamide; Nam, nicotinamide; NaAD, nicotinic acid adenine dinucleotide; NaMN, nicotinic acid mononucleotide; NMN, nicotinamide mononucleotide; 2-OAA, 2-oxoadipic acid; QA, quinolinic acid; Trp, l-tryptophan. (1), kynurenine 3-monooxygenase; (2), kynureninase; (3), kynurenine aminotransferase; (4) 3-hydroxyanthranilic acid 3,4-dioxygenase; (5), α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase; (6) quinolinic acid phosphoribosyltransferase.
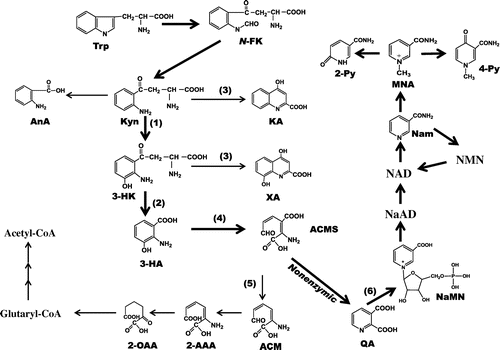
In the liver, the metabolic ability of Trp is much stronger compared with non-hepatic tissues; most of the intermediates can be almost completely metabolized into acetyl-CoA via ACMS although a little part of ACMS is non-enzymatically converted into QA. Thus, the accumulation of intermediates such as AnA, KA, 3-HK, XA, and 3-HA in the liver does not occur. On the contrary, non-hepatic tissues can metabolize l-Trp into 3-HA, but the metabolic ability is very weak. Thus, intermediates such as AnA, KA, 3-HK, XA, and 3-HA accumulate, and therefore the urinary excretion amounts of such intermediates reflect the metabolic ability of non-hepatic tissues. Urine MNA, 2-Py, and 4-Py (Table ) not affected by the menstrual cycle suggests this consideration. Lower urine 3-HK during the preovulatory phase compared with the postovulatory phase suggests that the activity of kynurenine 3-monooxygenase in non-hepatic tissues is lower during the preovulatory phase than in the postovulatory phase or activity of kynureninase in non-hepatic tissues is higher in the preovulatory phase than in the postovulatory phase.
El-Zoghby et al.Citation18) reported that urine 3-HK in prepubertal, sexually mature, and menopausal females was 56.5 ± 17.4 μmol/d, 4.9 ± 2.4 μmol/d, and 14.5 ± 4.3 μmol/d, respectively. This suggests that female hormones suppress the formation of 3-HK. A cause of the increase in 3-HK could be due to an insufficiency of vitamin B6 that would result in a decrease of the pyridoxal phosphate coenzyme of kynureninase.
The conversion of l-Trp to Nam was not affected by the menstrual cycle and reflects the ability of l-Trp metabolism in the liver. The metabolism of kynurenine (l-Trp → 3-HA), which reflects the ability of l-Trp metabolism in non-hepatic tissues changed during the postovulatory phase when vitamin B6 nutritional status in non-hepatic tissues might be insufficient. Thus, it can be recommended that sexually mature women take vitamin B6 supplements during the postovulatory phase of the menstrual cycle with a recommended average allowance 0.023 mg/g vitamin B6.Citation29)
Funding
This study was part of the “Studies on the nutritional evaluation of amino acids and B-group vitamins” (Principal Investigator, Katsumi Shibata). Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science supported this study.
Notes
Abbreviations: ACMS, α-amino-β-carboxymuconate-ε-semialdehyde; ACMSD, α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase; AMS, α-aminomuconate-ε-semialdehyde; AnA, anthranilic acid; 3-HA, 3-hydroxyanthranilic acid; 3-HK, 3-hydroxykynurenine; HPLC, high-performance liquid chromatography; KA, kynurenic acid; MNA, N1-methylnicotinamide; 2-Py, N1-methyl-2-pyridone-5-carboxamide; 4-Py, N1-methyl-4-pyridone-3-carboxamide; Nam, nicotinamide; Trp, tryptophan; XA, xanthurenic acid.
References
- Miller DF. Pellagra deaths in the United States. Am. J. Clin. Nutr. 1978;31:558–559.
- Shibata K. Nutritional factors that regulate on the conversion of l-tryptophan to niacin. Adv. Exp. Med. Biol. 1999;467:711–716.10.1007/978-1-4615-4709-9
- Shibata K, Nakata C, Fukuwatari T. Moderate food restriction suppresses the conversion of l-tryptophan to nicotinamide in weaning rats. Biosci. Biotechnol. Biochem. Published online: 2014;78:478–481.
- Shibata K, Kondo T, Onodera M. Comparison of tryptophan-nicotinamide metabolism between male and female rats. Biosci. Biotechnol. Biochem. 1993;57:858–859.10.1271/bbb.57.858
- Shibata K, Kondo T. Effect of progesterone and estrone on the conversion of tryptophan to nicotinamide in rats. Biosci. Biotechnol. Biochem. 1993;57:1890–1893.10.1271/bbb.57.1890
- Shibata K, Toda S. Effects of sex hormones on the metabolism of tryptophan to niacin and to serotonin in male rats. Biosci. Biotechnol. Biochem. 1997;61:1200–1202.10.1271/bbb.61.1200
- Sprince H, Lowry RS, Folsome CE, Behrman JS. Studies on the urinary excretion of “xanthurenic acid” during normal and abnormal pregnancy: a survey of the excretion of “xanthurenic acid” in normal nonpregnant, normal pregnant, pre-eclamptic, and eclamptic women. Am. Obstet. Gynecol. 1951;62:84–92.
- Vandelli I, Cinoulhiac E, Tenconi LT. Urinary elimination of xanthurenic acid after tryptophan overload in relation to various hormonal conditions. Ann. Obstet. Ginecol. 1955;77:324–328.
- Wachstein M, Lobel S. Abnormal tryptophan metabolites in human pregnancy and their relation to deranged vitamin B6 metabolism. Exp. Biol. Med. 1954;86:624–627.10.3181/00379727-86-21183
- Rose DP. Excretion of xanthurenic acid in the urine of women taking progestogen-oestrogen preparations. Nature. 1966;210:196–197.10.1038/210196a0
- Rose DP, Adams PW. Oral contraceptives and tryptophan metabolism: effects of oestrogen in low dose combined with a progestagen and of a low-dose progestagen (megestrol acetate) given alone. J. Clin. Pathol. 1972;25:252–258.10.1136/jcp.25.3.252
- Price JM, Thornton MJ, Mueller LM. Tryptophan metabolism in women using steroid hormones for ovulation control. Am. J. Clin. Nutr. 1967;20:452–456.
- Toseland PA, Price S. Tryptophan and oral contraceptives. Br. Med. J. 1969;1(5646):777.10.1136/bmj.1.5646.777-a
- Luhby AL, Brin M, Gordon M, Davis P, Murphy M, Spiegel H. Vitamin B6 metabolism in users of oral contraceptive agents. I. Abnormal urinary xanthurenic acid excretion and its correction by pyridoxine. Am. J. Clin. Nutr. 1971;24:684–693.
- Wertz AW, Lojkin ME, Bouchard BS, Derby MB. Tryptophan-niacin relationships in pregnancy. J. Nutr. 1958;64:339–353.
- Lojkin ME. Effect of levels of nitrogen intake on tryptophan metabolism and requirement for pregnancy of the rat. J. Nutr. 1967;91:89–98.
- Fukuwatari T, Murakami M, Ohta M, Kimura N, Jin-no Y, Sasaki R, Shibata K. Changes in the urinary excretion of the metabolites of the tryptophan-niacin pathway during pregnancy in Japanese women and rats. J. Nutr. Sci. Vitaminol. 2004;50393–50398.
- El-Zoghby SM, El-Kholy ZA, El-Swedy SM, Abdel-Tawab GA. The spontaneous urinary excretion of tryptophan metabolites “via kynurenine” in women with regards to the prepuberty, sexual maturity and menopause. Acta Vitaminol. Enzymol. (Milan). 1978;32:155–158.
- Shibata K, Onodera M. Measurement of 3-hydroxyanthranilic acid and anthranilic acid in urine by high-performance liquid chromatography. Agric. Biol. Chem. 1991;55:143–148.10.1271/bbb1961.55.143
- Shibata K. Fluorimetric micro-determination of kynurenic acid, an endogenous blocker of neurotoxicity, by high-performance liquid chromatography. J. Chromatogr. B. 1988;430:376–380.10.1016/S0378-4347(00)83173-4
- Shibata K, Onodera M. High-performance liquid chromatographic determination of 3-hydroxykynurenine with fluorimetric detection; comparison of preovulatory phase and postovulatory phase urinary excretion. J. Chromatogr. B. 1991;570:13–18.10.1016/0378-4347(91)80196-J
- Shibata K, Onodera M. Simultaneous high-performance liquid chromatographic measurement of xanthurenic acid and 3-hydroxyanthranilic acid in urine. Biosci. Biotechnol. Biochem. 1992;56:974.10.1271/bbb.56.974
- Shibata K, Kawada T, Iwai K. Simultaneous micro-determination of nicotinamide and its major metabolites, N1-methyl-2-pyridone-5-carboxamide and N1-methyl-4-pyridone-3-carboxamide, by high-performance liquid chromatography. J. Chromatogr. B. 1988;424:23–28.10.1016/S0378-4347(00)81072-5
- Shibata K. Ultramicro-determination of N1-methylnicotinamide in urine by high-performance liquid chromatography. Vitamins (Japan). 1987;61:599–604.
- Uemura T, Hirai K. L-kynurenine 3-monooxygenase from mitochondrial outer membrane of pig liver: purification, some properties, and monoclonal antibodies directed to the enzyme. J. Biochem. 1998;123:253–262.10.1093/oxfordjournals.jbchem.a021930
- Lima S, Khristoforov R, Momany C, Phillips RS. Crystal structure of Homo sapiens kynureninase. Biochemistry. 2007;46:2735–2744.10.1021/bi0616697
- Terakata M, Fukuwatari T, Kadota E, Sano M, Kanai M, Nakamura T, Funakoshi H, Shibata K. The niacin required for optimum growth can be synthesized from l-tryptophan in growing mice lacking tryptophan-2,3-dioxygenase. J. Nutr. 2013;143:1046–1051.10.3945/jn.113.176875
- Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol. Res. 2012;65:254–260.10.1016/j.phrs.2011.11.003
- Shibata K, Fukuwatari T, Imai E, Hayakawa T, Watanabe F, Takimoto H, Watanabe T, Umegaki K. Dietary reference intakes for Japanese 2010: water-soluble vitamins. J. Nutr. Sci. Vitaminol. 2013;59:S67–S82.
