Abstract
We analyzed modes of action of ribonuclease P (RNase P) proteins, C5 in Escherichia coli and Rpr2 in Saccharomyces cerevisiae, using a pair of complementary fluorescence-labeled oligoribonucleotides. Fluorescence resonance energy transfer-based assays revealed that RNA annealing and strand displacement activities found in archaeal RNase P proteins are prevalent in eubacterial (C5) and eukaryotic (Rpr2) RNase P proteins.
Ribonuclease P (RNase P) is the ribonucleoprotein (RNP) that catalyzes the processing of 5′ leader sequences from tRNA precursors (pre-tRNA) and other noncoding RNAs in all living cells.Citation1,2) We earlier found in reconstitution experiments that RNase P RNA (PhopRNA) alone in the hyperthermophilic archaeon Pyrococcus horikoshii OT3 had no endonuclease activity, but PhopRNA and five archaeal proteins, PhoPop5, PhoRpp38, PhoRpp21, PhoRpp29, and PhoRpp30, reconstituted RNase P activity that exhibits enzymatic properties like those of the authentic enzyme.Citation3,4)
Rajkowitsch and Schroeder developed method that combines monitoring of the RNA annealing (accelerate annealing (AN)) and strand displacement (SD) activities in a single setup and detects double-stranded RNA by fluorescence resonance energy transfer (FRET),Citation5) and they proposed that RNA-binding proteins that bind non-specifically to RNA and promote SD are referred to as RNA chaperones, while proteins that AN of complementary RNAs are termed RNA annealers.Citation6) Recently, FRET-based assays using Cy3- or Cy5-labeled oligonucleotides showed that PhoPop5, PhoRpp21, PhoRpp29, and PhoRpp30 significantly enhanced both AN and SD, while PhoRpp38 had no influence on both reactions.Citation7) Thus, the four archaeal proteins, PhoPop5, PhoRpp21, PhoRpp29, and PhoRpp30, belong to the RNA chaperone and RNA annealer and may assist PhopRNA in reaching a functionally active conformation by acceleration of AN and SD. The FRET analysis was, however, carried out using non-specific oligonucleotides under binding conditions distinct from those used for pre-tRNA cleavage assay. Therefore, we could not correlate AN and SD activities with the activation of PhopRNA by the four proteins. However, a recent study has suggested that C-terminal helices of PhoPop5 are involved in the promotion of AN and SD and thereby activate PhopRNA.Citation8)
It is known that archaeal RNase P proteins are conserved in eukaryotic RNase Ps, suggesting that eukaryotic counterparts have a similar function, forming a core structure in eukaryotic RNase Ps.Citation2,9) Furthermore, despite the fact that the bacterial RNase P protein is structurally distinct from archaeal and eukaryotic RNase P proteins, the archaeal protein pair, PhoPop5 and PhoRpp30, in P. horikoshii RNase P, functions equivalently to the C5 protein in the Escherichia coli RNase P, being involved in activation of the PhopRNA C-domain.Citation10) A similar observation was reported on the corresponding protein complex from other archaea.Citation11) In this study, we first tested whether the E. coli RNase P protein C5 and yeast RNase P protein Rpr2, a homolog of PhoRpp21, could promote AN and SD using a pair of complementary fluorescence-labeled oligoribonucleotides.
Two fluorescence-labeled RNAs, Cy3-21R− (Cy3 – 5′-ACUGCUAGAGAUUUUCCACAU-3′) and Cy5-21R+ (Cy5 – 5′-AUGUGGAAAAUCUCUAGCAGU-3′) which were used by Rajkowitsch and Schroeder,Citation5) were obtained from Operon Biotechnologies (Tokyo). PhoRpp21, PhoRpp38, and C5 were prepared as described previously.Citation3,4,8) Rpr2 was overproduced in E. coli BL21 (DE3) using pET-22b, and the resulting protein was purified to homogeneity by column chromatography. An oligoribonucleotide (21R+) with a sequence (5′-AUGUGGAAAAUCUCUAGCAGU-3′) identical to Cy5-21R+ was produced by in vitro transcription with T7 RNA polymerase using a corresponding synthetic DNA oligonucleotide as a template. The resulting RNAs were purified by ion-exchange column chromatography on a HiTrap DEAE-Sepharose FF column, as described by Easton et al.Citation12) FRET assays were principally performed as described previously,Citation5) with some modifications.Citation7) The energy transfer efficiency (E) was calculated as follows: E = 1 − FDA/FD, where FD and FAD are relative fluorescence intensities of the donor (Cy3-21R−) in the absence and presence of acceptor (Cy5-21R), respectively. For normalization, Et/E1200 and (Et − E1200)/(E2400 − E1200) were plotted for phase as a function of reaction time (s). For quantitative evaluation of the activities in phases 1 and 2, half-lives (t1/2) were employed using the time course of energy transfer efficiency. They were measured as the time required for E to reach half of the maximum increase or decrease by annealing or SD. In this analysis, the P. horikoshii RNase P proteins, PhoRpp21 and PhoRpp38, were analyzed as positive and negative controls, respectively. The result showed that the presence of either C5 or Rpr2, as the case for PhoRpp21, significantly accelerated the annealing of the two RNA strands. When the t1/2 values were calculated, it was indicated that C5 and Rpr2 accelerate the annealing of the two oligonucleotides by approximately 4- and 2.2-fold, respectively (Fig. ).
Fig. 1. FRET-based assay for RNA-binding properties of E. coli C5 and yeast Rpr2.
Notes: Fluorescence spectrophotometric analysis of two complementary RNAs (Cy5-21R+ and Cy3-21R−) in the absence or presence of the proteins was carried out, as described in the text. Note: For evaluation of the activities in phases 1 and 2, half-lives (t1/2) were calculated using the time course of energy transfer efficiency and are indicated in the figure. ND indicates not detected. (1) Cy5-21R+ and Cy3-21R− with no protein; (2) Cy5-21R+ and Cy3-21R− with C5; (3) Cy5-21R+ and Cy3-21R− with Rpr2; (4) Cy5-21R+ and Cy3-21R− with PhoRpp21; (5) Cy5-21R+ and Cy3-21R− with PhoRpp38.
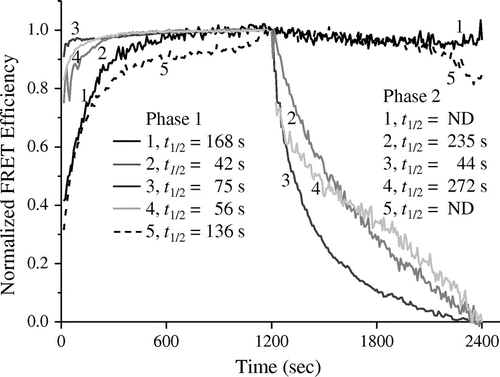
When nonfluorescence-labeled competitor RNA (21R−, 100 nM) was added (phase 2), the three proteins, C5, Rpr2, and PhoRpp21, decreased the FRET efficiency between Cy3 and Cy5 linked to the complementary RNA strands, suggesting that C5 and Rpr2 induce SD (Fig. ). It should be noted that Rpr2 promoted SD more strongly than its archaeal homolog, PhoRpp21. This result indicated that the promoting activity of AN and SD found in the four archaeal RNase P proteins is conserved in E. coli protein C5 and yeast RNase P protein Rpr2.
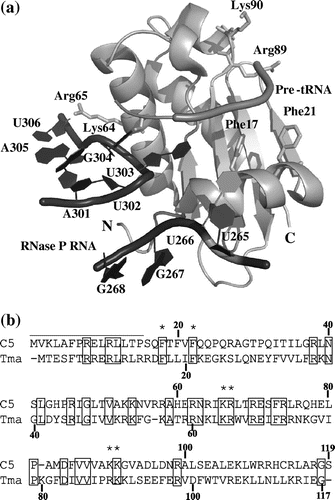
The crystal structure of the Thermotoga maritima RNase P in complex with tRNA shows that Phe17, Phe21, Arg89, and Lys90 in the RNase P protein are involved in the interaction with the leader sequence of pre-tRNA, whereas the 15 N-terminal residues and amino acids located on the helix α4 (residues 56–73) are involved in the interaction with conserved regions, CRIV and CRV, in RNase P RNA (Fig. (a)).Citation13) Since these amino acids in the T. maritima protein are highly conserved in the E. coli C5 protein (Fig. (b)), it is likely that the corresponding amino acids in C5 serve as RNA-binding sites in E. coli RNase P. We next examined whether putative RNA-binding sites in C5 could be involved in the promoting activity of AN and SD. For this purpose, three mutants, F18A/F22/A, K66A/R67A, and K90A/K91A, in which Phe18 and Phe22, Lys66 and Arg67, and Lys90 and Lys91 in C5 were replaced by Ala, were prepared. In addition, the N-terminal 15-amino acid deletion mutant (∆N15) was prepared, and the four resulting mutants were characterized with respect to pre-tRNA cleavage activity and FRET-based analysis.
Fig. 2. Structures of the T. maritima RNase P protein.
Notes: (a) The crystal structure of the T. maritima RNase P protein in complex with RNase P RNA and the leader sequence of pre-tRNA.Citation12) The 15 N-terminal residues and Lys64 and Arg65 are involved in binding to RNase P RNA, while Phe17, Phe21, Arg89, and Lys90 are involved in binding to the leader sequence of pre-tRNA. N and C termini are indicated. (b) Alignment of amino acid sequences of the T. maritima RNase P protein (Tma) and E. coli C5 (C5). Amino acids mutated in this study are indicated by asterisks, and the 15 N-terminal residues deleted are shown by the upper line. Identical residues in the two proteins are enclosed in boxes.
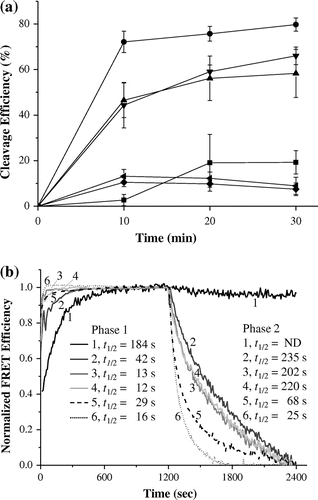
Mutations of Phe18 and Phe22, and deletion of the 15N-terminal residues strongly decreased the pre-tRNA cleavage activity in the presence of E. coli RNase P RNA (M1 RNA) (Fig. (a)). In addition, mutations of Lys66 and Arg67 or Lys90 and Lys91 moderately influenced pre-tRNA cleavage activity (Fig. (a)). This result demonstrated the involvement of possible RNA-binding sites in C5 in the catalytic activity of E. coli RNase P. We next analyzed the promoting activity of AN and SD of the mutants by the FRET-based assay. The four mutants enhanced AN more strongly than did wild-type C5 (Fig. (b)). In phase 2, K66A/R67A and K90A/K91A promoted SD comparably to wild-type C5, whereas F18A/F22A and ∆N15, which exhibited significantly reduced pre-tRNA cleavage activity, enhanced SD more significantly than did wild-type C5 (Fig. (b)). As for the activation of AN and SD by mutations, it is likely that mutated residues may be involved in a specific RNA-binding and suppress the acceleration of AN and SD. Nevertheless, these present results revealed that possible RNA binding sites in C5 are not involved in AN and SD enhancement activities, suggesting that unexamined residues are responsible for these activities. Alternatively, AN and SD enhancement activities observed in C5 might be not implicated in catalysis by the E. coli RNase P. Further studies on the C5 protein will give an answer to this issue.
Fig. 3. Characterization of C5 and its mutants.
Notes: (a) Pre-tRNA cleavage activities of the reconstituted mixtures containing M1 RNA and C5 or its mutants were assayed at 37 °C in 10 mM Tris-HCl, pH 7.5, containing 5 mM MgCl2, 400 mM CH3COONH4, and 0.01% NP-40 using P. horikoshii pre-tRNATyr as a substrate. The reaction volume was 200 μl and a small aliquot (10 μl) was withdrawn at the following time points: 10, 20, and 30 min. The reactions were stopped using a phenol extract. The cleavage products were resolved on 10% acrylamide/8 M urea/TBE gels and visualized by staining the gels in a 0.1% toluidine blue N solution. The individual bands were quantified using Multi Gauge V3.1 and the cleavage efficiency was calculated as (tRNA + 5′-leader fragment)/(pre-tRNA + tRNA + 5′-leader fragment) × 100. The experiments were carried out in triplicate, and the mean values are presented. The reconstituted particles with C5 (●), K66A/R67A (▲), ∆N15 (◆), K90A/K91A (▼), F18A/F22A (◀), or without C5 (■). (b) FRET-based assay for RNA-binding properties of C5 or its mutants was carried out as described in figure 1. Half-lives (t1/2) were calculated using the time course of energy transfer efficiency and are indicated in the figure (1) Cy5-21R+ and Cy3-21R− with no protein; (2) Cy5-21R+ and Cy3-21R− with C5; (3) Cy5-21R+ and Cy3-21R− with K66A/R67A; (4) Cy5-21R+ and Cy3-21R− with K90A/K91A; (5) Cy5-21R+ and Cy3-21R− with ∆N15; (6) Cy5--21R+ and Cy3-21R− with F18A/F22A.
Funding
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to M.K. [grant number 22380062].
Notes
Abbreviations: FRET, fluorescence resonance energy transfer; M1 RNA, Escherichia coli RNase P RNA; PhopRNA, Pyrococcus horikoshii RNase P RNA; RNP, ribonucleoprotein; pre-tRNA, tRNA precursor; RNase P, ribonuclease P.
References
- Kirsebom LA, Trobro S. RNase P RNA-mediated cleavage. Int. Union Biochem. Mol. Biol. Life. 2009;61:189–200.
- Esakova O, Krasilnikov AS. Of proteins and RNA: the RNase P/MRP family. RNA. 2010;16:1725–1747.
- Kouzuma Y, Mizoguchi M, Takagi H, Fukuhara H, Tsukamoto M, Numata T, Kimura M. Reconstitution of archaeal ribonuclease P from RNA and four protein components. Biochem. Biophys. Res. Commun. 2003;306:666–673.
- Fukuhara H, Kifusa M, Watanabe M, Terada A, Honda T, Numata T, Kakuta Y, Kimura M. A fifth protein subunit Ph1496p elevates the optimum temperature for the ribonuclease P activity from Pyrococcus horikoshii OT3. Biochem. Biophys. Res. Commun. 2006;343:956–964.
- Rajkowitsch L, Schroeder R. Coupling RNA annealing and strand displacement: a FRET-based microplate reader assay for RNA chaperone activity. Biotechniques. 2007;43:304–310.
- Rajkowitsch L, Chen D, Stampfl S, Semrad K, Waldsich C, Mayer O, Jantsch MF, Konrat R, Blasi U, Schroeder R. RNA chaperones, RNA annealers and RNA helicases. RNA Biol. 2007;4:118–130.
- Ishihara M, Nishimoto E, Yamashita S, Kakuta Y, Kimura M. A distinct binding mode of archaeal ribonuclease P proteins to RNA. Biosci. Biotechnol. Biochem. 2012;76:2335–2337.
- Hazeyama K, Ishihara M, Ueda T, Nishimoto E, Nakashima T, Kakuta Y, Kimura M. Extra-structural elements in the RNA recognition motif in archaeal Pop5 play a crucial role in the activation of RNase P RNA from Pyrococcus horikoshii OT3. Biochem. Biophys. Res. Commun. 2013;440:594–598.
- Jarrous N, Gopalan V. Archaeal/Eukaryal RNase P: subunits, functions and RNA diversification. Nucleic Acids Res. 2010;38:7885–7894.
- Honda T, Hara T, Nan J, Zhang X, Kimura M. Archaeal homologs of human RNase P protein pairs Pop5 with Rpp30 and Rpp21 with Rpp29 work on distinct functional domains of the RNA subunit. Biosci. Biotechnol. Biochem. 2010;74:266–273.
- Chen WY, Pulukkunat DK, Cho IM, Tsai HY, Gopalan V. Dissecting functional cooperation among protein subunits in archaeal RNase P, a catalytic ribonucleoprotein complex. Nucleic Acids Res. 2010;38:8316–8327.
- Easton LE, Shibata Y, Lukavsky PJ. Rapid, nondenaturing RNA purification using weak anion-exchange fast performance liquid chromatography. RNA. 2010;16:647–653.
- Reiter N, Osterman A, Torre-Larios A, Swinger KK, Pan T, Mondragon A. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature. 2010;468:784–789.
