Abstract
Pollen Typhae total flavone (PTF), the extract from Pollen Typhae, is reported to enhance glucose uptake in C2C12 myotubes in vitro, but the convincing evidence is lacking in vivo. In this study, PTF ameliorated insulin resistance and dyslipidemia, but failed to significantly increase body weight in type 2 diabetic rats induced by high-fat diet and low-dose streptozotocin.
Type 2 diabetes mellitus (T2DM), one of the most serious public health threats throughout the world, is increasing dramatically in prevalence,Citation1) and has received considerable attention. It is well known that insulin resistance, a hallmark of the disease, is characterized by a defect of the peripheral tissues in response to insulin, including liver, adipose tissues, and skeletal muscles,Citation2) thus leading to metabolic disturbance of glucose and fat, showing hyperglycemia, hyperinsulinemia, and dyslipidemia.Citation3) Therefore, improving insulin resistance is a key strategy for the treatment of type 2 diabetes.
Pollen Typhae, a Chinese herb, has been adopted to treat type 2 diabetes in Chinese medical clinical practice. It is believed that Pollen Typhae improves insulin resistance, but convincing evidence is lacking. Researches show that the components of Pollen Typhae are flavones, unsaturated fatty acids, and so on.Citation4–6) Our recent study has revealed that Pollen Typhae total flavone (PTF), the extract from Pollen Typhae, enhances glucose uptake in C2C12 myotubes in vitro, suggesting the potential action of PTF in ameliorating insulin resistance.Citation6) The present study aimed to determine the effects of PTF on insulin sensitivity in high-fat diet (HFD) and low-dose streptozotocin (STZ)-induced type 2 diabetic rats, which would provide the convincing evidence for the use of Pollen Typhae in clinical practice.
Male Sprague-Dawley rats were purchased from Hunan Slaccas Jingda Laboratory Animal Company Limited (Changsha, China). After 10 days of acclimatization, the rats were randomly divided into normal pellet diet (NPD) group given NPD with 4.6% fat (about 10% of calories as fat), and HFD group given HFD (about 40% of calories as fat). Two kinds of feed were provided by Shanghai Slaccas Laboratory Animal Company Limited (Shanghai, China). The diet was kept until the end of the experiment. After 6 weeks of dietary manipulation, HFD-fed rats were injected intraperitoneally with STZ (35 mg/kg) dissolved in citrate buffer (0.1 M, pH 4.4), while the rats in NPD group were only received the intraperitoneal injection of citrate buffer, which was served as the control group. Seventy-two hours after STZ injection, rats with blood glucose level of 16.7 mmol/L or greater were considered to be diabetes.Citation7,8) The study was approved by the Ethics Committee of Guangxi University of Chinese Medicine. All procedures were conducted in accordance with the internationally accepted principles for laboratory animal use and care, as found in the European Community guidelines (EEC Directive of 1986; 86/609/EEC) and the US guidelines (NIH publication #85-23, revised in 1985).
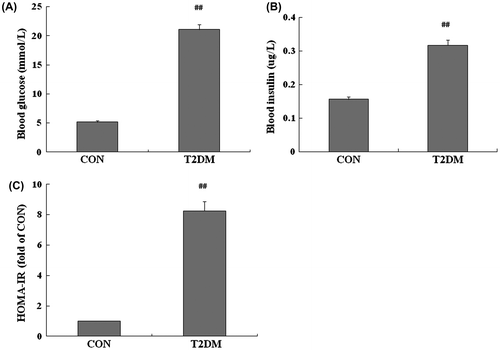
Fig. shows the characteristic of the rats at day 7 after STZ or vehicle injection. The rats induced by HFD and low-dose STZ, whose blood glucose was higher than 16.6 mmo/L (Fig. (A)), had increased fasting insulin level (Fig. (B)) and elevated homeostasis model assessment of basal insulin resistance (HOMA-IR) (Fig. (C)) when compared to the control group, suggesting that type 2 diabetic rats were successfully induced.Citation7,8)
Fig. 1. The characteristic of diabetic rats induced by HFD and low-dose STZ.
Notes: Rats with T2DM (n = 42) were induced by HFD and low-dose STZ. After 1 week of STZ or vehicle injection, fasting blood glucose (A) and insulin (B) were checked, and HOMA-IR (fasting glucose × fasting insulin/22.5) was evaluated (C). All data are presented as means ± SE. Student’s t test was used to analyze the differences between two groups. A value of p < 0.05 was regarded as statistically significant. ##p < 0.01 vs. CON (n = 9).
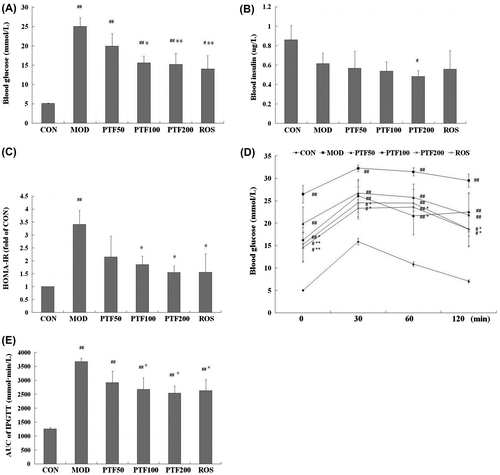
After 2 weeks of STZ injection, type 2 diabetic rats were randomly divided into 5 subgroups based on different treatment: the PTF50 group (n = 8) receiving 50 mg/kg·d PTF by intragastric administration, the PTF100 group (n = 9) receiving 100 mg/kg·d PTF, the PTF200 group (n = 9) receiving 200 mg/kg·d PTF, the rosiglitazone group (ROS, n = 8) receiving 4 mg/kg·d rosiglitazone, and the model group (MOD, n = 8). PTF and rosiglitazone were dissolved in purified water, respectively. The rats in the model group and the control group (CON, n = 9) were given an equal volume of purified water per day, respectively. Administration of PTF for 4 weeks markedly decreased blood glucose (Fig. (A)) and to some extent reduced insulin (Fig. (B)) in a dose-dependent manner. Moreover, HOMA-IR (Fig. (C)) was also reduced by PTF in the same way, which was similar to rosiglitazone, an insulin sensitizer, suggesting that PTF effectively treats type 2 diabetes. As is well known, insulin resistance is a hallmark of type 2 diabetes. In addition to elevated HOMA-IR, glucose intolerance also suggests insulin resistance in type 2 diabetes.Citation9,10) In order to further confirm the antidiabetic action of PTF, intraperitoneal glucose tolerance test (IPGTT) was carried out (Fig. (D)). As is expected, blood glucose level in the model group was much higher than that in the control group at 0, 30, 60, and 120 min points, respectively. And the areas under the glucose curves (AUC) in the model group were also significantly greater than that in the control group (Fig. (E)), which was compatible with the report.Citation11) After 4 weeks of treatment, PTF effectively improved IPGTT in a dose-dependent manner, showing lower glucose level at different time points (Fig. (D)) and AUC (Fig. (E)) than those in the model group, which was also similar to rosiglitazone and consistent with our precious study in vitro.Citation6) The results indicate that PTF has antidiabetic action through improving insulin resistance in type 2 diabetic rats.
Fig. 2. Effects of PTF on insulin sensitivity of type 2 diabetic rats induced by HFD and low-dose STZ.
Notes: After treatment for 4 weeks, biochemical parameters including blood glucose (A) and insulin (B) were checked, insulin sensitivity was evaluated by HOMA-IR (C) and IPGTT (D). IPGTT: after an overnight fast, the rats were administered by intraperitoneal injection with glucose (2 g/kg). Blood glucose was measured at 0, 30, 60, and 120 min. AUC of IPGTT (E) were calculated for each parameter by the trapezoidal rule. All data are presented as means ± SE. The significance among multiple groups was analyzed by one-way analysis of variance. #p < 0.05, ##p < 0.01 vs. CON; *p < 0.05, **p < 0.01 vs. MOD.
Type 2 diabetes often coexists with dyslipidemia including elevated triglyceride (TG), total cholesterol, low-density lipoprotein cholesterol (LDL-c), and decreased high-density lipoprotein cholesterol, which are partly responsible for the contribution to insulin resistance and the development of type 2 diabetes.Citation12,13) Furthermore, dyslipidemia such as increased LDL-c is an independent risk factor for cardiovascular disease (CVD).Citation14) In this study, the rats in the model group had higher levels of TG and LDL-c than the control group (Fig. (A)). Administration of PTF significantly decreased TG and LDL-c in a dose-dependent manner. We have reported that free fatty acid (palmitate) contributes to insulin resistanceCitation15) and that PTF prevents palmitate-induced impairment of glucose uptake in C2C12 myotubes.Citation6) Interestingly, rosiglitazone also obviously reduced TG and LDL-c levels, which was in accordance with PTF and the past reports.Citation7,16)
Fig. 3. Effects of PTF on blood lipid and body weight in type 2 diabetic rats.
Notes: (A) TG and LDL-c levels after treatment for 4 weeks. (B) Body weight before (after 2 weeks of STZ injection) and after treatment. (C) Body weight gain between before and after treatment. #p < 0.05, ##p < 0.01 vs. CON; *p < 0.05, **p < 0.01 vs. MOD; &p < 0.05 vs. ROS.
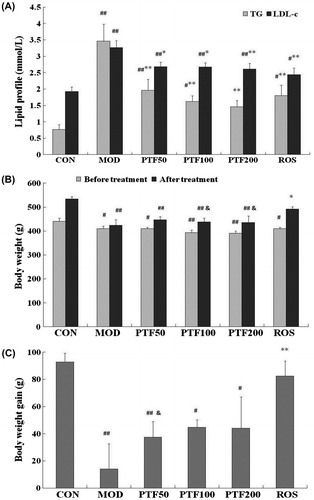
At present, although thiazolidinediones, the agonists of peroxisome proliferator-activated receptors-γ such as rosiglitazone and pioglitazone, have been widely used to treat type 2 diabetes, some deleterious side effects including weight gain, and increasing risk of CVD in type 2 diabetes patients also coexist.Citation17,18) In this study, all rats had free access to food. After STZ injection (before treatment), there was an increase in food intake (data not shown) and a decrease in body weight in 5 groups of type 2 diabetic rats (Fig. (B)). After 4 weeks of treatment, body weight in 3 PTF groups was all similar to the model group, but much lower than that in the control group, and the body weight in the PTF100 and PTF200 groups was also by far lower than that in the rosiglitazone group (Fig. (B)). Furthermore, weight gain between before and after treatment in 3 PTF groups was also similar to the model group, and the weight gain in the PTF50 group was even lower than that in the rosiglitazone group (Fig. (C)). While body weight and weight gain in the rosiglitazone group were much higher than that in the model group, and similar to the control group. Meanwhile, the amount of food intake in 3 PTF groups and the rosiglitazone group was decreased when compared to the model group. The results show that different from rosiglitazone, PTF does not significantly increase body weight when it performs the insulin sensitization characteristic, and that maybe there is little relationship between food intake and the changes of body weight during PTF treating type 2 diabetes. It was also reported that Pollen Typhae prevents and controls coronary heart diseases and myocardial infarction.Citation19)
Because of the improvement of insulin sensitivity and few side effects, it is likely that PTF is a potential choice for the treatment of type 2 diabetes. We have reported that PTF improves insulin resistance involving the β-arrestin-2-mediated signaling in C2C12 myotubes,Citation6) which is different from rosiglitazone that mainly activates peroxisome proliferator-activated receptors-γ referring to the phosphatidylinositol-3 kinase (PI3K) insulin signal pathway.Citation20)
In conclusion, PTF exhibits the insulin sensitization characteristic like rosiglitazone, but fails to promote significant increases in body weight.
Funding
This work was supported by the Guangxi Natural Science Foundation [No. 2012GXNSFBA053103]; the Guangxi Department of Science and Technology [No. 11-31-11]; the National Natural Science Foundation of China [No. 81202677]; and the Guangxi Key Laboratory of Chinese Medicine Foundation Research, Guangxi University of Chinese Medicine. No potential conflict of interest relevant to this article was reported.
References
- Romdhane HB, Ali SB, Aissi W, Traissac P, Skhiri Aounnallah H, Bougatef S, Maire B, Delpeuch F, Achour N. Prevalence of diabetes in Northern African countries: the case of Tunisia. BMC Public Health. 2014;14:1–13.
- Bloomgarden ZT. Developments in diabetes and insulin resistance. Diabetes Care. 2006;29:161–167.10.2337/diacare.29.01.06.dc06-zb01
- Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J, China National Diabetes and Metabolic Disorders Study Group. Prevalence of diabetes among men and women in China. New Engl. J. Med. 2010;362:1090–1101.10.1056/NEJMoa0908292
- Yang Y, Wang S, Zhang S. Determination of flavonoids in Pollen Typhae (Puhuang) by HPCE and HPLC. Zhongguo Zhong Yao Za Zhi. 1999;24:682–684, 703. (in Chinese).
- Ma HF, Liu B, Zhang GY, Shi RB, Ma CH, Yu MM. GC-MS analysis of the fatty components of Pollen Typhae before and after being carbonized. Zhongguo Zhong Yao Za Zhi. 2006;31:200–202. (in Chinese).
- Feng XT, Wang TZ, Chen Y, Liu JB, Liu Y, Wang WJ. Pollen Typhae total flavone improves insulin-induced glucose uptake through the β-arrestin-2-mediated signaling in C2C12 myotubes. Int. J. Mol. Med. 2012;30:914–922.
- Zhang Z, Xue HL, Liu Y, Wang WJ. Yi-Qi-Zeng-Min-Tang, a Chinese medicine, ameliorates insulin resistance in type 2 diabetes. World J. Gastroenterol. 2011;17:987–995.
- Salman ZK, Refaat R, Selima E, El Sarha A, Ismail MA. The combined effect of metformin and l-cysteine on inflammation, oxidative stress and insulin resistance in streptozotocin-induced type 2 diabetes in rats. Eur. J. Pharmacol. 2013;714:448–455.10.1016/j.ejphar.2013.07.002
- Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rats model. Exp. Diabetes Res. 2008;2008:1–9.
- Karaman A, Bayram F, Gundogan K, Ozsan M, Karaman H, Kelestimur F. Prevalence of diabetes mellitus and glucose metabolism disorders in the first degree relatives of type 2 diabetic patients. Bratisl. Lek. Listy. 2012;113:361–367.
- Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J. Nutr. Metab. 2012;2012:1–8.
- Ståhlman M, Fagerberg B, Adiels M, Ekroos K, Chapman JM, Kontush A, Borén J. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the HDL lipidome in type 2 diabetic subjects in the DIWA cohort: impact on small HDL particles. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2013;1831:1609–1617.10.1016/j.bbalip.2013.07.009
- Dickson-Humphries T, Bottenberg B, Kuntz S. Lipoprotein abnormalities in patients with type 2 diabetes and metabolic syndrome. J. Am. Acad. Phys. Assist. 2013;26:13–18.
- Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the atherosclerosis risk in communities (ARIC) study. Circulation. 2001;104:1108–1113.10.1161/hc3501.095214
- Feng XT, Wang TZ, Leng J, Chen Y, Liu JB, Liu Y, Wang WJ. Palmitate contributes to insulin resistance through downregulation of the Src-mediated phosphorylation of Akt in C2C12 myotubes. Biosci. Biotechnol. Biochem. 2012;76:1356–1361.10.1271/bbb.120107
- Ahmed D, Sharma M, Pillai KK. The effects of triple vs. dual and monotherapy with rosiglitazone, glimepiride, and atorvastatin on lipid profile and glycemic control in type 2 diabetes mellitus rats. Fundam. Clin. Pharmacol. 2012;26:621–631.10.1111/fcp.2012.26.issue-5
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. New Engl. J. Med. 2007;356:2457–2471.10.1056/NEJMoa072761
- Kikuchi M, Kaku K, Odawara M, Momomura S, Ishii R. Efficacy and tolerability of rosiglitazone and pioglitazone in drug-naïve Japanese patients with type 2 diabetes mellitus: a double-blind, 28 weeks’ treatment, comparative study. Curr. Med. Res. Opin. 2012;28:1007–1016.10.1185/03007995.2012.694361
- Zhao J, Zhang CY, Xu DM, Huang GQ, Xu YL, Wang ZY, Fang SD, Chen Y, Gu YL. The antiatherogenic effects of components isolated from Pollen Typhae. Thromb. Res. 1990;57:957–966.10.1016/0049-3848(90)90162-6
- Standaert ML, Kanoh Y, Sajan MP, Bandyopadhyay G, Farese RV. Cbl, IRS-1, and IRS-2 mediate effects of rosiglitazone on PI3K, PKC-lambda, and glucose transport in 3T3/L1 adipocytes. Endocrinology. 2002;143:1705–1716.
