Abstract
Chong-Myung-Tang (CMT) is a multi-herbal formula that has been used to improve memory. However, the potential mechanism remains unknown. The present study investigated the effects of CMT (50, 100, and 200 mg/kg) on spatial memory of aged mice. The behavioral training tests indicated that 200 mg/kg CMT treatment can significantly improve spatial memory of aged mice in the Morris water maze. Moreover, cell survival was examined by injecting bromodeoxyuridine (BrdU) on the first three days. The result showed that 200 mg/kg CMT treatment significantly increased cell survival in the dentate gyrus. Cell proliferation was determined by injecting BrdU 2 h before the mice were killed. The result suggested that CMT treatments had no influence on cell proliferation in the dentate gyrus. Thus, an increase in cell survival in the dentate gyrus stimulated by CMT may be involved in the effect of CMT on spatial memory improvement.
Graphical Abstract
The multi-herbal formula Chong-Myung-Tang increases cell survival but not cell proliferation in the dentate gyrus of aged mice.

Hippocampus, one of the few brain regions, plays a critical role in coding, consolidation, and retrieval of spatial memory.Citation1,2) This function is closely correlated with neurogenesis in the dentate gyrus of the hippocampus.Citation3–5) Neural progenitor cells in the dentate gyrus continue to proliferate and migrate from the subgranular zone to the granule cell layer.Citation6) After about 1–2 weeks, the newly generated cells mature into functional neurons which comprise hippocampal neural circuitry and participate in the formation of memory.Citation7,8) Hippocampal neurogenesis is modulated by numerous extrinsic factors such as learning, running, and enriched environment.Citation9–11) Additionally, some pathological states, such as stroke and ischemic damage, can also induce hippocampal neurogenesis.Citation12,13) Recently, many research revealed that plant extract such as green tea and grape seed could promote neurogenesis in rodent hippocampus.Citation14,15)
Many multi-herbal formulas are often used for the improvement of cognitive function in traditional oriental medicine.Citation16,17) Chong-Myung-Tang (CMT), a traditional Korean multi-herbal formula, consists of Acorus gramineus Soland, Polygala tenuifolia Willdenow, and Poria cocos Wolf as the ratio of 1:1:1 (dry weight). It has been widely used to improve memory function. Jang et al.Citation18) reported that CMT has a neuroprotective action against kainic acid-induced neurotoxicity. Afterwards, Kim et al.Citation19) reported that CMT can inhibit tumor necrosis factor-α production in the culture of astrocytes which are the most abundant glial cells in the brain. Our recent studies suggested that CMT improves cognitive performance of amnesic mice by regulation of cholinergic marker enzyme activities and the antioxidant defense system.Citation20,21) However, these studies did not report the effect of CMT on cell genesis in hippocampus. Oh et al.Citation17) reported that the multi-herbal formula Guibi-tang enhances memory and increases cell proliferation in the rat hippocampus, but cell survival in the dentate gyrus was not investigated in this research. Thus far, it remains largely unknown how oral administration of CMT influences cell survival and cell proliferation in the dentate gyrus. The objective of the present study is to investigate the effects of CMT on spatial memory and cell genesis in the dentate gyrus of aged mice.
Materials and methods
Preparation of CMT
The CMT was prepared according to our previous method.Citation21) The ingredients of CMT were A. gramineus Soland (China, 100 g), P. tenuifolia Willdenow (China, 100 g), and P. cocos Wolf (North Korea, 100 g) and purchased from Geumsan market (Korea). All these herbs were authenticated by Professor Ki Hwan Bae from the college of Pharmacy, Chungnam National University where voucher specimens are deposited. Milled powder of CMT (300 g) was extracted with boiling water (100 g/L) for 2 h. After filtration, the filtrate was concentrated with a rotary evaporator followed by freeze-drying. The yield of CMT extract was 21.2% (w/w). The obtained CMT powder was dissolved in sterile 0.9% saline to a concentration of 20 mg/mL.
Experimental animals
Male ICR mice (25–30 g), approximately 18 months old, were used in the present research. They were housed in plastic cages (eight mice per cage) and maintained at the controlled temperature (23 ± 1 °C) with 60 ± 10% humidity and a 12 h:12 h light/dark cycle as well as with free access to food and water. All behavioral experiments were carried out in an adjacent room where the mice were housed under the same conditions of temperature, humidity, and light cycle. All experimental procedures were complied with the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health, USA, and were approved and monitored by the Ethical Committee of Animal Experiments at the Chungnam National University.
Animals grouping and drug treatment
Initially, mice were randomly divided into four experimental groups: control and CMT (50, 100, and 200 mg/kg) groups. Each group was further subdivided into three subgroups (n = 8 mice per group), as described in Fig. . One group was used for the behavioral test (Fig. (A)), and the others were used for the effects of CMT on cell genesis including cell survival and cell proliferation in the dentate gyrus of aged mice. CMT was administered orally at dose of 50, 100, and 200 mg/kg/day for 14 days by gastric tube. The control mice were orally given 0.9% saline.
Fig. 1. Overview of the experimental design.
Notes: (A) The schedule of behavioral test. (B) The experiment was designed to measure cell survival in the dentate gyrus. BrdU was injected three times to mice for the first three days, and CMT (50, 100, and 200 mg/kg) was given for the 14 days. The mice were sacrificed for immunohistochemistry study on the 14th day. (C) The experiment was designed to measure cell proliferation in the dentate gyrus. CMT (50, 100, and 200 mg/kg) was given for 14 days and the mice were killed 2 h after one injection of BrdU.
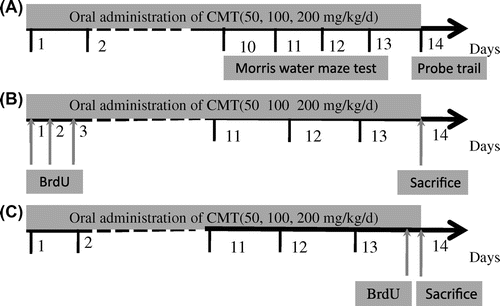
Bromodeoxyuridine (BrdU) is a thymidine analog that is incorporated into dividing cells in the S-phase of DNA synthesis.Citation22) In order to clarify the cellular mechanism of memory improvement, an appropriate protocol of BrdU was used for measuring cell proliferation and survival independently. Briefly, to determine the effect of CMT on cell survival, BrdU (50 mg/kg, i.p.) was injected three times to mice on the first three days, and CMT was given for the 14 days. The mice were sacrificed on the 14th day for immunohistochemistry study (Fig. (B)). In this case, BrdU-labeled cells on the first three days which were still surviving on the 14th day were observed. On the other hand, to examine the effect of CMT on cell proliferation, CMT was given for 14 days and the mice were killed 2 h after one injection of BrdU (Fig. (C)). In this case, newly proliferated cells in dentate gyrus of aged mice were observed.
Morris water maze test
The Morris water maze (MWM) was a red tank (diameter 90 cm, height 65 cm) filled with water (24 ± 1 °C, 30 cm deep). The water was made opaque with powdered milk. A white platform was then fixed at the southeast quadrant of the tank and submerged 1 cm below the water surface so that it was invisible at the water level. Visual cues were consisted of different shapes of posters placed equidistantly on the walls of the room. A computer-assisted video tracking device system was used to monitor and quantify the behavior performance of mice.
Firstly, the mice were habituated to the water maze for 90 s in the absence of the platform. During the learning period, mice were trained three times per day. In the experiment, the mice were allowed to swim freely until they found the hidden platform. If a mouse failed to find the platform within 120 s, it was guided to the platform and allowed to stay there for 15 s. After the swim, the mice were kept dry in a plastic holding cage filled with paper towels. The time interval between trials was 10 min. The escape latencies to reach the platform and the total distance traveled were measured in each trial for four days, and the swim speed was measured on the fourth day. The means of three trials for each day were compared.
Probe trial
On the fifth day, mice were subjected to a probe trial session in which the platform was removed from the pool, allowing the mice to swim for 120 s to search for it. A calculation was kept for the swimming time in the pool quadrant where the platform had previously been placed, and the number of crossings over the previous platform position was determined.
Immunohistochemistry
The mice were deeply anesthetized with sodium pentobarbital (50 mg/kg i.p.) and perfused transcardially with 200 mL normal saline and 200 mL 4% paraformaldehyde solution at the corresponding timepoints. Brain samples were removed and postfixed by 4% paraformaldehyde for overnight at 4 °C. The processed samples were embedded in paraffin using routine procedures, and cut transversely into 9-μm sections for immunohistochemistry study.
Immunohistochemistry was carried out using the Histostain detection system (Invitrogen, Carlsbad, CA, USA). The sections were deparaffinized and rehydrated in graded ethanol, and placed in 100 mM phosphate-buffered saline (PBS) for 10 min before starting the staining procedure. To eliminate endogenous peroxidase activity, the sections were submerged in peroxidase quenching solution (3% H2O2) for 10 min, followed by rinsing in PBS three times for 2 min. DNA was denatured by incubating the sections in 2 N HCl at 37 °C for 30 min, and then rinsed in PBS three times for 2 min. The sections were incubated with 10% non-immune goat serum for 20 min to block the non-specific binding of antibodies. The primary antibody used was BrdU mouse monoclonal IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sections were incubated with primary antibody at a 1:100 dilution in 0.01 M PBS at 37 °C for 4 h and rinsed in PBS three times for 2 min. Subsequently, the samples were incubated with a biotinylated second antibody for 20 min at 37 °C, followed by incubation with streptavidin–peroxidase conjugate for 10 min. The peroxidase activity was detected with a DAB chromogen system controlled by observing under a microscope. After counterstaining with hematoxylin, the slides were dehydrated with a graded series of alcohol, cleared in xylene, and mounted with a coverslip.
Quantification of BrdU-positive cells
The counting numbers of BrdU immunoreactive cells were performed according to the published methods with some modification.Citation9) Every sixth section throughout the hippocampus was processed for counting (six sections per animal). Using this space ensures that same cell will not be counted in two sections. All BrdU-labeled cells in hippocampal dentate gyrus of aged mice were counted in each section by an observer blind to the treatment of animals. To distinguish single cells within clusters, all counts were performed at 400× and 1000× magnification under a light microscope equipped with digital camera. Data for cell counting are represented as the mean number of BrdU-positive cells per hippocampal section.
Statistical analysis
Data from all procedures were expressed as “mean ± standard error mean.” Differences between groups were analyzed by one-way ANOVA followed by Duncan’s test for multiple comparisons. Especially, the escape latency in MWM test was analyzed by the two-way ANOVA with repeated measures. The level for a significant difference was set at p < 0.05.
Results
Behavioral test in MWM
The MWM was used to investigate the effects of CMT on spatial learning and memory of aged mice. Fig. shows the behavioral results of training in the MWM test. The mice in all groups spent less time to find the hidden platform with the increase of training. Compared with the control group, 200 mg/kg CMT treatment significantly decreased the escape latency to find the hidden platform and the total distance traveled, especially on the third and fourth day (p < 0.05), while 50 and 100 mg/kg treatments had no significant effects on the escape latency and the total distance traveled of the aged mice (Fig. (A) and (B)). Moreover, the swim speed of the aged mice was compared on the fourth test day. However, no statistically significant differences were observed between the four groups with respect to swim speed. This indicates that CMT treatments did not change the motor of the aged mice (Fig. (C)).
Fig. 2. The effects of CMT on cognitive performance in MWM test.
Notes: After 14 days of CMT treatments, spatial memory of aged mice was assessed using MWM test. 200 mg/kg CMT treatment significantly decreased the escape latency and total distance traveled (A and B, *p < 0.05 vs. the control group), but CMT treatments did not significantly change the swim speed (C). In the retention trial, 200 mg/kg CMT treatment significantly increased the percentages of time in target quadrant (D, p < 0.05) and the number of crossing (E, p < 0.05). Data are the means ± SEM. of eight mice in each group, and the means with different letters are significantly different (p < 0.05).
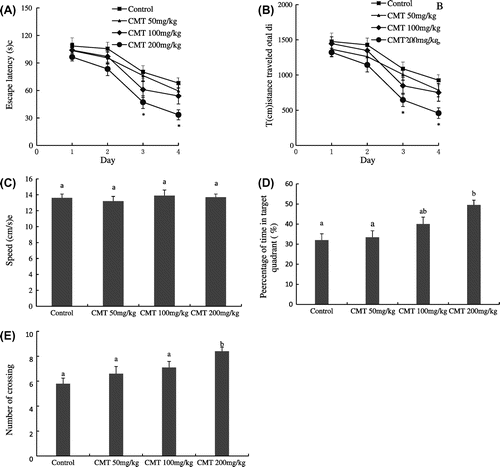
To measure the strength of spatial memory retention, the platform was removed in the probe trial session. Compared with the control group, only 200 mg/kg CMT treatment significantly increased the percentage of time in target quadrant (p < 0.05), while 50 and 100 mg/kg CMT treatments did not significantly change the time in target quadrant (Fig. (D)). Moreover, 200 mg/kg CMT treatment had more number of platform crossing than that of control group, 50 and 100 mg/kg treatments (p < 0.05) (Fig. (E)).
Effects of CMT on cell genesis in the dentate gyrus
Newborn cells in the dentate gyrus can be quantified by immunostaining of BrdU.Citation22) Fig. shows BrdU-positive cells in the dentate gyrus of aged mice. To determine the effect of CMT on cell survival in the dentate gyrus, mice were given three injections of BrdU on the first three days, and BrdU-positive cells in the dentate gyrus were analyzed after 14 days administration of CMT (Fig. (B)). Compared with control group, 200 mg/kg CMT treatment significantly increased cell survival in the dentate gyrus (p < 0.05), while 50 and 100 mg/kg had no significant effect on cell survival in the dentate gyrus of aged mice (Fig. (A)).
Fig. 3. BrdU-immunostaining.
Notes: Photomicrograph of BrdU-positive cells (arrowhead) in the dentate gyrus of aged mice (A, Control; B, 200 mg/kg CMT). Scale bar = 10 μm.
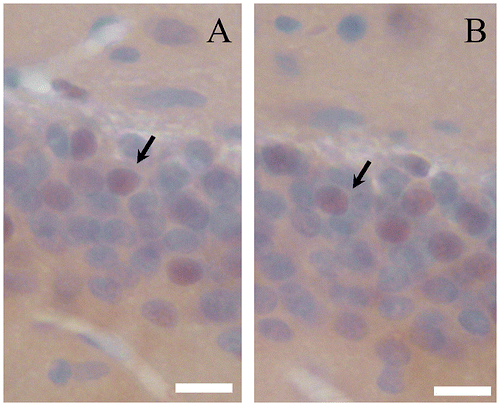
Fig. 4. Effects of CMT on cell survival (A) and cell proliferation (B) in the dentate gyrus of the aged mice.
Notes: After 14 days of CMT treatment, the mice were sacrificed for immunohistochemistry study. 200 mg/kg CMT treatment significantly increased cell survival (p < 0.05), whereas three CMT treatments (50, 100, and 200 mg/kg) had no significant effect on cell proliferation in the dentate gyrus of aged mice. Data are the means ± SEM. of eight mice in each group, and the means with different letters are significantly different (p < 0.05).
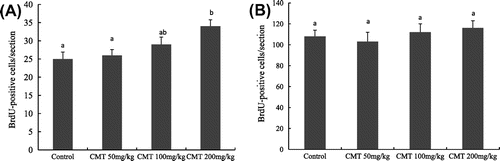
In order to investigate the effect of CMT on cell proliferation in the dentate gyrus of aged mice, CMT was given for 14 days, and then one dose of BrdU was injected to the mice 2 h before they were sacrificed for immunohistochemistry study (Fig. (C)). As a result, the number of BrdU-positive cells did not significantly change in the dentate gyrus of aged mice. This suggested that three CMT treatments (50, 100, and 200 mg/kg) had no significant influence on cell proliferation in the dentate gyrus of aged mice (Fig. (B)).
Discussion
CMT is composed of three kinds of memory enhancing herbs. P. tenuifolia has been shown to promote proliferation of neural stem cells in hippocampal CA1 region, but not in dentate gyrus.Citation23) P. cocos extract can decrease lead-induced cognitive impairments by preventing Fas antigen expression in brain.Citation24) Liu et al.Citation25) reported that volatile oil of A. gramineus stimulating olfaction can significantly enhance memory ability of rat with Alzheimer’s disease. In the present study, these herbs are combined in the form of a multi-herbal formula to exert pharmacological action. Generally, aging can induce some degree of decline in cognitive function, and aged mice were used to evaluate the effect of CMT on spatial memory. Our data showed that the aged mice treated with 200 mg/kg CMT spent less time and traveled shorter distance to find the hidden platform in the acquisition phase (p < 0.05), and had significantly more time in the target quadrant and more number of platform crossing in the probe trial (p < 0.05), while 50 and 100 mg/kg CMT treatments had no significant effect on behavioral performance of aged mice (Fig. ). Moreover, the behavioral results also showed that CMT did not change the swim speed of the aged mice. This indicated that the alteration of behavioral performance in MWM should be attributed to spatial cognitive performance of the aged mice, but not the motor. In other words, 200 mg/kg CMT treatment improved spatial memory of aged mice. The CMT concentration is usually used for patient with cognitive dysfunction, and this finding is consistent with our previous report.Citation21)
Hippocampal neurogenesis has been proved to be an important event for cognitive function.Citation26,27) Therefore, the effect of CMT on cell genesis in dentate gyrus was further investigated to elucidate the potential mechanism of memory improvement. Learning and exercise has been known to enhance neurogenesis in hippocampus, which could interfere with the memory evaluation of CMT.Citation9,10) In order to avoid the interfering factors and to reflect the true level of cell genesis stimulated by CMT, only the mice treated with CMT without training in MWM test were used for the investigation. The result showed that only 200 mg/kg CMT treatment significantly increased cell survival in the dentate gyrus (p < 0.05), whereas CMT treatments (50, 100, and 200 mg/kg) had no significant effect on cell proliferation in the dentate gyrus of aged mice (Fig. ). Qiao et al.Citation28) also observed the similar result that ginseng extract enhanced the survival of newly generated cell in rat hippocampus while not affect cell proliferation. In addition to cell proliferation, another important aspect of hippocampal neurogenesis is the neuronal differentiation and eventual integration into the hippocampal circuitry. Newly generated cells in the hippocampus are reported to have neuronal morphology and can display functional properties to those of mature neuron.Citation27) It has been reported that about 80% of BrdU-positive cells in the hippocampus expressed neuron specific marker.Citation28) It can be deduced that the newly proliferated cells stimulated by CMT in the dentate gyrus may differentiate into neurons, but the further research is required to determine the exact effects of CMT on neuronal differentiation.
On the other hand, the newly generated neurons in the dentate gyrus are involved in the acquisition of spatial memory,Citation29) but hippocampal neurogenesis decreases with the increasing age.Citation30) Wati et al. reported that a decreased survival of proliferated cells in the hippocampus is closely associated with a decline in spatial memory of aged rats.Citation31) Our research demonstrated that oral administration of CMT prolonged the survival of newly generated cells in the dentate gyrus. This may be one of the reasons why CMT could improve spatial memory of aged mice. Moreover, some evidence shows that neurogenesis induction could be a novel therapeutic approach for disease associated with cognitive dysfunction.Citation32) Therefore, CMT is a promising medicine for the treatment of aging-induced memory deficit.
In summary, the present study showed that CMT increased cell survival but not cell proliferation in the dentate gyrus of aged mice. This may be one of the mechanisms by which CMT improves the spatial cognitive function of aged mice. The further research is required to investigate the effects of individual CMT component herbs on cognitive function and cell genesis in the dentate gyrus of aged mice.
Author contributions
L. Liu and C.K. Sung conceived this study. L. Liu and M.R. Lee prepared the solution of Chong-Myung-Tang and performed behavioral test in water maze. L. Liu, Z. Wang and J.G. Hou performed immunohistochemistry experimemt. L. Liu, M.W. Zhang and R.F. Zhang analyzed the results and wrote this manuscript. All the authors made comments to this manuscript.
Funding
This work was supported in part by the National Nature Science Foundation of China [grant number 31301459].
Notes
Abbreviations: CMT, Chong-Myung-Tang; BrdU, Bromodeoxyuridine; MWM, Morris water maze; PBS, phosphate-buffered saline.
References
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Grande PD. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci. Lett. 2000;286:21–24.10.1016/S0304-3940(00)01074-0
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641.10.1016/S0896-6273(02)00830-9
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Nat. Acad. Sci. 2003;100:14385–14390.10.1073/pnas.2334169100
- Dupret D, Revest JM, Koehl M, Ichas F, DeGiorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:1–14.
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376.10.1038/35066584
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003;460:563–572.10.1002/(ISSN)1096-9861
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317.10.1038/3305
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438.10.1126/science.287.5457.1433
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999;2:260–265.10.1038/6365
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495.10.1038/386493a0
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–720.
- Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol. Dis. 2012;49:68–78.
- Shen L, Zhang J. Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of adult gerbils. Neurosci. Lett. 2003;344:1–4.10.1016/S0304-3940(03)00318-5
- Wang Y, Li M, Xu X, Song M, Tao H, Bai Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol. Nutr. Food Res. 2012;56:1292–1303.10.1002/mnfr.201200035
- Yoo DY, Kim W, Yoo KY, Lee CH, Choi JH, Yoon YS, Kim DW, Won MH, Hwang IK. Grape seed extract enhances neurogenesis in the hippocampal dentate gyrus in C57BL/6 mice. Phytother. Res. 2011;25:668–674.
- Oh MS, Park C, Huh Y, Kim HY, Kim H, Kim HM, Bae H, Ahn Dk, Park WS, Park Sk. The effects of BR003 on memory and cell proliferation in the dentate gyrus of rat hippocampus. Biol. Pharm. Bull. 2006;29:813–816.10.1248/bpb.29.813
- Oh MS, Huh Y, Bae H, Ahn DK, Park SK. The multi-herbal formula Guibi-tang enhances memory and increases cell proliferation in the rat hippocampus. Neurosci. Lett. 2005;379:205–208.10.1016/j.neulet.2004.12.077
- Jang kJ, Lee KH, Kim SL, Choi DY, Park BK, Im DH, Cho YJ, Jhoo WK, Kim HC. Chongmyungtang attenuates kainic acid-induced seizure and mortal effect in the mouse. Arch. Pharmacal Res. 1997;20:375–378.10.1007/BF02976204
- Kim HM, Lee YJ, Lyu YS. Inhibition of lipopolysaccharide plus substance P-induced tumor necrosis factor-α production from astrocytes by Chongmyung-Tang. J. Ethnopharmacol. 1999;66:295–300.10.1016/S0378-8741(99)00021-5
- Lee MR, Yun BS, Oh CJ, Kim BC, Oh HI, Sung CK. Characterization of Korean traditional medicine Chongmyungtang for cognitive function related to anti-cholinesterases and antioxidant activity. Food Sci. Biotechnol. 2011;20:1331–1336.10.1007/s10068-011-0183-6
- Lee MR, Yun BS, Park SY, Ly SY, Kim SN, Han BH, Sung CK. Anti-amnesic effect of Chong–Myung–Tang on scopolamine-induced memory impairments in mice. J. Ethnopharmacol. 2010;132:70–74.10.1016/j.jep.2010.07.041
- Gould E, Gross CG. Neurogenesis in adult mammals: some progress and problems. J. Neurosci. 2002;22:619–623.
- Park HJ, Lee K, Heo H, Lee M, Kim JW, Whang WW, Kwon YK, Kwon H. Effects of Polygala tenuifolia root extract on proliferation of neural stem cells in the hippocampal CA1 region. Phytother. Res. 2008;22:1324–1329.10.1002/ptr.v22:10
- Lu J, Yu P, Lu Y, Rao M. The effect of Poria cocos wolf extract on memory damage and related antigen expression induced by lead in mouse. J. Toxicol. 2006;20:224–225.
- Liu ZB, Niu WM, Yang XH, Wang Y, Wang WG. Study on perfume stimulating olfaction with volatile oil of Acorus gramineus for treatment of the Alzheimer’s disease rat. J. Tradit. Chin. Med. 2010;30:283–287.10.1016/S0254-6272(10)60057-X
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004;14:186–191.10.1016/j.conb.2004.03.001
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034.10.1038/4151030a
- Qiao C, Den R, Kudo K, Yamada K, Takemoto K, Wati H, Kanba S. Ginseng enhances contextual fear conditioning and neurogenesis in rats. Neurosci. Res. 2005;51:31–38.10.1016/j.neures.2004.09.004
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852.10.1016/j.neuroscience.2004.10.009
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat. Neurosci. 1999;2:894–897.10.1038/13197
- Wati H, Kudo K, Qiao C, Kuroki T, Kanba S. A decreased survival of proliferated cells in the hippocampus is associated with a decline in spatial memory in aged rats. Neurosci. Lett. 2006;399:171–174.10.1016/j.neulet.2006.01.056
- Chuang TT. Neurogenesis in mouse models of Alzheimer’s disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2010;1802:872–880.10.1016/j.bbadis.2009.12.008
