Abstract
To obtain basic information on how microbial cells absorb cadmium from aqueous solution, we examined cadmium absorption in various micro-organisms. Of 51 micro-organism strains tested, we found that some Gram-positive bacteria, such as, Arthrobacter nicotianae and Bacillus subtilis, and some actinomycetes, such as, Streptomyces flavoviridis and S. levoris were highly capable of absorbing cadmium from an aqueous solution. A. nicotianae absorbed the largest amount of cadmium, over 800 μmol cadmium per gram of dry wt. cells. However, cadmium absorption by A. nicotianae was affected by the solution pH, cadmium concentration, and cell density. The absorption of cadmium was very rapid. Some factors that affected cadmium absorption by A. nicotianae cells were also discussed.
Graphical Abstract
We examined cadmium absorption of 51 microorganisms and found Arthrobacter nicotianae absorbed the largest amount of cadmium. Some factors that affected cadmium absorption by A. nicotianae cells were also discussed.

The pollution of the natural environment by heavy metals is a potential menace to the health and welfare of mankind. Therefore, attention has been focused on how heavy metal pollution affects the food chain.
Cadmium is known to be one of the most toxic elements to human health. The effect of cadmium on water microbial communities grown in continuous culture has been previously investigated.Citation1,2) Those studies showed that 0.5 ppm of cadmium in the culture medium did not hinder microbial development or biodegradation processes. Other studies conducted in a treatment plant on a pilot scale produced results that led to a similar conclusion.Citation3,4)
Microbes can concentrate heavy metals to a great extent.Citation5–9) Several authors consider micro-organisms as natural bioadsorbents.Citation10–12)
Several studies have described the accumulation of cadmium by organisms in aqueous solutions, such as marine algae,Citation13–15) sawdust,Citation16,17) and baker’s yeast.Citation18) However, few reports have determined which types of organisms absorb the largest amounts of cadmium.
Earlier investigations determined micro-organism accumulation of heavy metal elements, such as uraniumCitation19) and thorium,Citation20) from the points of view of environmental control and resource recovery.
This study aimed to screen micro-organisms for their capacity to absorb cadmium from an aqueous cadmium solution; we then focused the study on cadmium absorption by Arthrobacter nicotianae which showed the maximum cadmium absorption of all tested strains.
Materials and methods
Materials
The strains used in this study were generously donated by the University of Tokyo (IAM) Culture Collection, Center for Cellular and Molecular Research, Institute of Molecular and Cellular Biosciences, at the IAM; the Faculty of Engineering, Hiroshima University (HUT); and the Faculty of Agriculture, Hokkaido University (AHU). All chemicals (guaranteed reagents) were obtained from Nacalai Tesque, Inc., Kyoto.
Micro-organism cultures
The medium for growing bacteria contained 3 g/L meat extract, 5 g/L peptone, and 5 g/L NaCl in deionized water. The medium for growing actinomycetes, fungi, and yeasts contained 4 g/L yeast extract, 10 g/L malt extract, and 4 g/L glucose in deionized water, pH 7.1 (for actinomycetes) or pH 5.7 (for fungi and yeasts). The micro-organisms were maintained on agar slants and grown in 300 mL of the appropriate medium in a 500-mL flask with continuous shaking (120 rpm) for 72 h at 30 °C. Cells were collected by centrifugation (for bacteria and yeasts) or by filtration through filter paper (for actinomycetes and fungi), washed thoroughly with deionized water, and then used in the following absorption experiments.
Cadmium absorption experiments (Batch system)
Cadmium was supplied in the form of Cd(NO3)2. Nitrate was used in this study, because anions, such as nitrate, chloride, sulfate, and thiosulfate, were affected little by the absorption of uranium; in contrast, carbonate and fluoride were strongly affected.Citation19) Therefore, we considered nitrate suitable for cadmium removal. The pH of the solution was adjusted to the desired value with 0.1 M HNO3 or 0.1 M NaOH. Unless otherwise stated, the absorption experiments were conducted as follows. Resting micro-organisms (15 mg dry wt. basis) were suspended in a 100 mL solution (pH 5.0) that contained 10.0 ppm cadmium. These amounts of cells (15 mg dry wt. basis) and cadmium (10 ppm 100 mL) were suitable for comparison (not too little and not excessive). The suspension was shaken for 1 h at room temperature. The temperature was not adjusted exactly, because the effect of temperature within 10–25 °C was insubstantial on uranium absorption.Citation21) In this study, the effect of temperature was also examined. The micro-organisms were then collected by filtration through a membrane filter (pore size 0.2 μm). The amount of cadmium ions that accumulated in the cells was determined by measuring the cadmium content of the filtrate with an atomic absorption spectrometer (AA-6300; Shimadzu, Kyoto).
Zeta potential measurements
Zeta potentials of bacterial cells were measured with a ZEECOM ZE-2000 apparatus equipped with a video system. After conditioning at pH values ranging from 1.0 to 5.0, the zeta potential of the bacterial cells (5.0 × 108 cells/mL) was measured. All measurements were conducted at the same ionic strength (10−3 M KNO3).
Immobilization of microbial cells
Precultured A. nicotianae cells (5.0 g fresh wt.) were suspended in 4.5 mL of isotonic sodium chloride solution. Next, we added 680 mg of acrylamide monomer, 34 mg of N,N′-methylene-bis(acrylamide), 0.30 mL of 3-dimethylaminopropionitrile solution (5.0%), and 0.34 mL of potassium persulfate solution (2.5%). After solidification, the gel was crushed into small pieces (50–100 mesh), washed thoroughly with isotonic sodium chloride solution, washed again with deionized water, and then used in the following absorption experiments.
Cadmium absorption experiments (column system)
Unless otherwise stated, 50 mL of cadmium solution (5 ppm cadmium, Cd(NO3)2, pH 5.0) was passed through a column (diameter 10 mm and bed volume 30 mL) of immobilized A. nicotianae cells at a flow rate of 10 mL/min. The cells in the immobilized cells at a concentration was 0.66 g dry wt. cells/g dry wt. immobilized cells. The absorbed cadmium was desorbed with 50 mL of 1 M sodium carbonate solution at space velocity of 10 mL/min.
Results and discussion
Screening micro-organisms for cadmium absorption from the cadmium nitrate solution
To determine which strain of micro-organism had the highest capacity for absorbing cadmium from an aqueous solution, we screened 51 strains of 43 species (17 bacteria, 5 actinomycetes, 15 fungi, and 14 yeasts).
We examined the absorptions of rare earths in both growing and restingCitation22) cells. The amount of rare earths removed by growing cells was far smaller than that removed by resting cells. Similarly, the amount of rare earth absorbed by growing cellsCitation23) was smaller than that using resting cells.Citation22) These results could be explained by the high amounts of chelate present in the growing cultures. Therefore, it was reasonable that the amount of metal ion absorbed by growing cells was smaller than that absorbed by resting cells. A previous study showed that growing Citrobacter sp. removed 0.6 μg Cd/dry wt. cells and resting same cell removed 140 μg Cd/dry wt. cells.Citation24) According to those results, we screened micro-organisms for cadmium removal with resting cells.
We found that the amount of absorbed cadmium increased with increasing pH in the range of pH 1–5 with Bacillus subtilis IAM1026.Citation25) A precipitate of cadmium hydroxide was produced at pH 6. Therefore, the pH of the solution was adjusted to 5.0 throughout the screening.
The results obtained are summarized in Tables . The different micro-organisms varied significantly in their effectiveness at absorbing cadmium from the aqueous solution.
Table 1. Absorption of cadmium from the cadmium nitrate solution using bacteria.
Table 2. Absorption of cadmium from the cadmium nitrate solution using actinomycetes.
Table 3. Absorption of cadmium from the cadmium nitrate solution using fungi.
Table 4. Absorption of cadmium from the cadmium nitrate solution using yeasts.
Among the micro-organisms tested, the highest cadmium absorption efficiencies were exhibited by the Gram-positive bacterial strains, A. nicotianae IAM12342 and B. subtilis IAM11060 and by the Actinomycetes strain, Streptomyces flavoviridis HUT6147. The best absorption was exhibited by A. nicotianae cells; they absorbed 492 μmol cadmium ions per gram dry wt. micro-organism cells in 1 h.
Effect of the initial pH on cadmium absorption from the cadmium nitrate solution by A. nicotianae cells
Fig. shows that, the amount of cadmium absorbed by A. nicotianae IAM12342 cells increased, while the Zeta potential of the cell surface decreased, with increasing pH. The highest amount of cadmium was absorbed at pH 5. Thus, the absorption of cadmium by these cells was significantly affected by the pH of the solution. In a low pH solution, the anionic group is easily protonated, and the negative charge is lost. Therefore, the amount of absorbed cadmium decreased with increases in the acidity of the solution.
Fig. 1. The effect of initial pH on cadmium absorption (%) and the Zeta potential (mV) of A. nicotianae IAM12342 cell surface in a cadmium nitrate solution.
Cells were assayed with the batch system described in the methods, in the presence of 10 mg/L of cadmium. Closed symbols indicate the percent adsorption and open symbols indicate the Zeta potential of the cell surface.
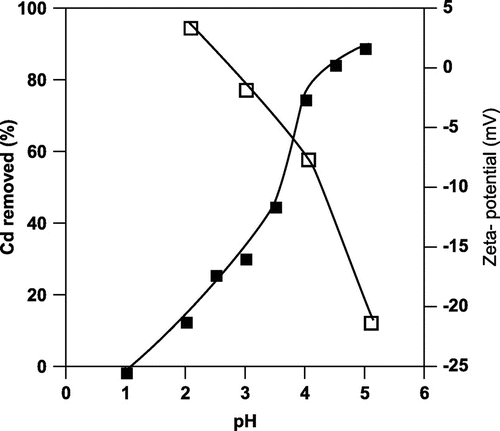
We reasoned that the amount of cadmium absorbed might decrease with increasing acidity to levels below pH 4, because high acidity would reduce the negative charges on the phosphate groups in the teichoic acid polymers on the surface of Gram-positive bacteria.Citation26) Accordingly, we postulated that the high capacity of A. nicotianae cells to absorb cadmium at pH 4–5 was mainly due to the negative charges on the teichoic acid polymers.
Cadmium absorption capacity of A. nicotianae cells from the cadmium nitrate solution
As shown in Fig. (A), the amount of cadmium absorbed by A. nicotianae cells (μmol Cd/g dry wt. cells) increased with increasing cadmium concentration. However, the ratio of absorbed cadmium to cadmium concentration decreased. When the cadmium concentration was 200 ppm, extremely high cadmium absorption (about 800 μmol Cd/g dry wt. cells) was observed.
Fig. 2. Effect of cadmium concentration on absorption from a cadmium nitrate solution by A. nicotianae IAM12342 cells.
Cells were assayed with the batch system described in the methods, in the presence of the indicated Cd concentrations (Ce). (A) Cadmium absorption and percent absorption by A. nicotianae cells. Symbols: Open squares indicate specific cadmium absorption (μmol Cd/g dry wt. cells); closed squares indicate total cadmium absorbed (%); dotted line: calculated cadmium absorbed (μmol Cd/g dry wt. cells) based on the Langmuir isotherm. (B) Langmuir’s and (C) Freundlich’s isotherm of the effect of cadmium concentration on the absorption of cadmium by A. nicotianae IAM12342 cells.
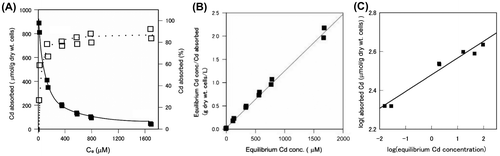
Fig. (B) shows the relationship between the residual cadmium concentration in solution and the amount of cadmium absorbed. Clearly, the absorption of cadmium by A. nicotianae cells obeyed the Langmuir isotherm, which states that Ce/Q = mCe + n. In this equation, Q is the amount of cadmium absorbed (μmol Cd/g dry wt. cells), Ce is the residual cadmium in the solution (μM), and m and n are constants (m = 0.00122; n = 0.0309). The maximum amount of cadmium absorbed was 819 μmol Cd/g dry wt. cells, estimated from the slope of the line.
Fig. (C) shows the relationship between log Q and log Ce. The absorption of cadmium by A. nicotianae cells also obeyed the Freundlich isotherm, which states that log Q = log (0.0863Ce + 2.48).
These results suggested that the absorption of cadmium by A. nicotianae cells was independent of biological activity. On the basis of these findings, it seems reasonable to postulate that the absorption of cadmium by micro-organisms is mostly dependent on physicochemical binding to cell components. Additionally, the highest amount of cadmium removed (819 μmol Cd/g dry wt. cells) was comparable with the relatively high amounts reported for other organisms, such as marine algae,Citation13) sawdust,Citation16) and baker’s yeastCitation18) (712, 655, and 816 μmol Cd/g dry wt. cells, respectively).
Effect of cell density on cadmium absorption from the cadmium nitrate solution by A. nicotianae
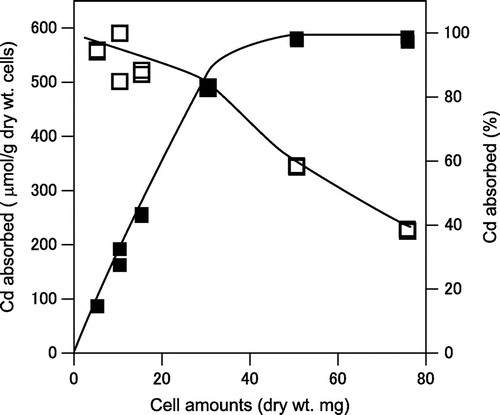
We examined cadmium absorption with different densities of A. nicotianae cells. As shown in Fig. , the amount of cadmium absorbed by A. nicotianae cells (μmol Cd/g dry wt. cells) decreased with increasing cell density, whereas the ratio of the total absorbed cadmium to cell density increased.
Fig. 3. Effect of cell density on the absorption of cadmium from the cadmium nitrate solution by A. nicotianae IAM12342 cells.
The indicated amounts of resting cells were assayed with the batch system described in the methods, in the presence of 20 mg/L of cadmium. In order to confirm the effect of cell amounts, twice amounts of cadmium was used in this experiment. Symbols are described in Fig. (A).
Time course of cadmium absorption by A. nicotianae cells
Next, we examined time course of cadmium absorption by A. nicotianae cells. As shown in Fig. , cadmium absorption was very rapid, and it reached equilibrium within 3 h. These results were fitted with an adsorption model based on the time-dependent Langmuir equation:
Fig. 4. Time course of cadmium absorption from the cadmium nitrate solution by A. nicotianae IAM12342 cells.
Cells were assayed with the batch system described in the methods. Dotted line indicates the time dependence of cadmium absorption (μmol Cd/g dry wt. cells), calculated with the Langmuir’s isotherm.
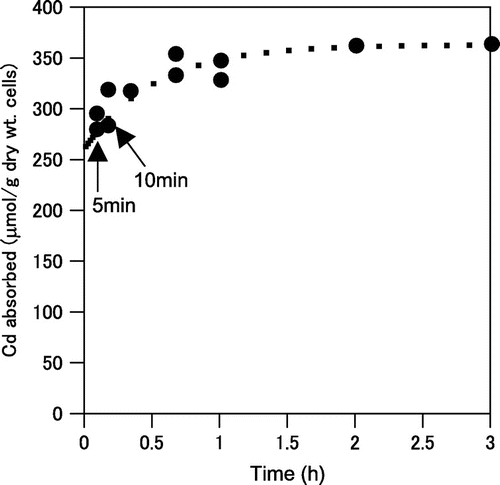
where Q is the amount of absorbed cadmium, Qe is the absorbed amount at equilibrium, and j is the sum of the absorption and desorption rate constant, i.e. j = ka + kd, where ka is the absorption rate constant and kd is the desorption rate constant.Citation27) Qe and j were estimated to be 250 μmol Cd/g dry wt. of adsorbents and 1.24 min−1, respectively.
Effect of temperature on cadmium absorption from the cadmium nitrate solution by A. nicotianae
We examined the effect of temperature on cadmium absorption by A. nicotianae cells. As shown in Fig. (A), the amount of cadmium absorbed by A. nicotianae cells (%) increased gradually as the temperature increased between 10 and 50 °C. Cadmium absorption became constant at temperatures over 50 °C.
Fig. 5. Effect of temperature on cadmium absorption from the cadmium nitrate solution by A. nicotianae IAM12342 cells.
Cells were assayed with the batch system described in the methods, in the presence of 20 mg/L of cadmium. In order to confirm the effect of temperature, twice amounts of cadmium was used in this experiment. (A) Percent cadmium absorbed at different temperatures. (B) The logarithm of the Kd (absorbed cadmium concentration/residual cadmium concentration in solution) plotted against the reciprocal of the temperature to estimate the enthalpy of Cd absorption.
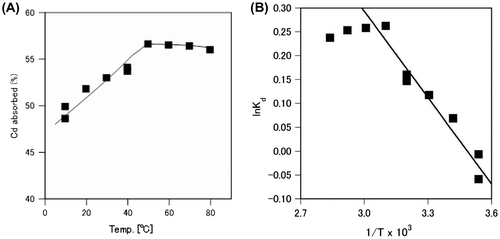
Fig. (B) shows the logarithm of the distribution coefficient, Kd (absorbed cadmium/residual cadmium in solution) was plotted against the reciprocal of the temperature. This displayed a linear relationship which indicated that the cadmium absorption by A. nicotianae cells caused a change in enthalpy of 5.01 kJ/mol. This result suggested that the absorption of cadmium by A. nicotianae IAM12342 cells is an endothermic reaction.
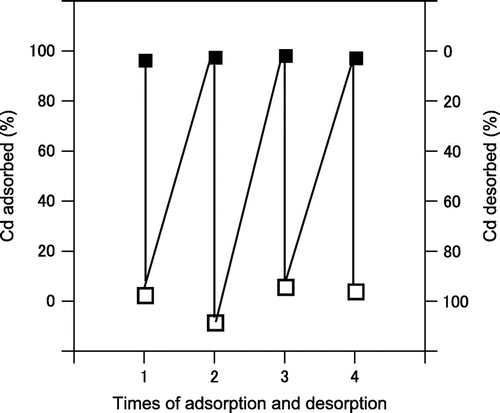
Cadmium adsorption and desorption by immobilized A. nicotianae
We obtained basic information on the recovery of cadmium by immobilized A. nicotianae cells from aqueous systems. A. nicotianae cells were immobilized in a column system, and we studied the adsorption–desorption cycle. Fig. shows that cadmium was adsorbed quantitatively onto the immobilized cells. Moreover, all the cadmium adsorbed onto the immobilized cells could be desorbed with a Na2CO3 solution. Thus, cadmium adsorbed by the immobilized cells was easily released by washing the cells with a 1 M Na2CO3 solution.
Fig. 6. Test of repeated cadmium adsorption–desorption by immobilized A. nicotianae IAM12342 cells.
Closed symbols indicate the percent adsorption and open symbols indicate the percent desorption.
In practical applications, some metal ions, such as copper,Citation16) uranium,Citation16) and rare earths, can affect cadmium absorption. Therefore, these metals must be pre-removed with micro-organisms before cadmium absorption can be achieved.
Acknowledgment
We wish to thank the graduated student Mr Yuki Aiba of Kyushu University for his measurements of the Zeta potential.
References
- Remacle J. Cadmium uptake by freshwater bacterial communities. Water Res. 1980;115:67–71.
- Remacle J, Houba C, Ninane J. Cadmium fate in bacterial microcosms. Water Air Soil Pollut. 1982;18:455–465.
- Bagby MM, Sherrard JH. Combined effects of Cd and Ni on the activated sludge process. J. Water Pollut. Control Fed. 1981;53:1609–1619.
- Weber AS, Sherrard JH. Effects of cadmium on the completely mixed activated sludge process. J. Water Pollut. Control Fed. 1980;52:2378–2388.
- Babich H, Stotzky G. Effects of cadmium on the biota: influence of environmental factors. Adv. Appl. Microbiol. 1978;23:55–117.10.1016/S0065-2164(08)70065-0
- Beveridge TJ, Koval SF. Uptake of metal ions by Rhizopus arrhizus biomass. Appl. Environ. Microbiol. 1981;42:325–335.
- Chopra I. Decreased uptake of cadmium by a resistant strain of Staphylococcus aureus. J. Gen. Microbiol. 1971;63:265–267.
- Matthews TH, Doyle RJ, Streips UN. Contribution of peptidoglycan to the binding of metal ions by the cell wall of Bacillus subtilis. Curr. Microbiol. 1979;3:51–53.10.1007/BF02603134
- Remacle J, Houba C. The influence of cadmium upon freshwater saprophytic bacteria. Environ. Technol. Lett. 1980;1:193–200.10.1080/09593338009383967
- Kurek E, Czaban J, Bollag JM. Sorption of cadmium by microorganisms in competition with other soil constituents. Appl. Environ. Microbiol. 1982;43:1011–1015.
- Nakajima A, Horikoshi T, Sakaguchi T. Studies on the accumulation of heavy metal elements in biological systems. Eur. J. Appl. Microbiol. Biotechnol. 1981;12:76–83.10.1007/BF01970038
- Tsezos M, Volesky B. Biosorption of uranium and thorium. Biotechnol. Bioeng. 1981;23:583–604.10.1002/(ISSN)1097-0290
- Herrero R, Cordero B, Lodeiro P, Rey-Castro C. Interactions of cadmium(II) and protons with dead biomass of marine algae Fucus sp. Mar. Chem. 2006;99:106–116.10.1016/j.marchem.2005.05.009
- Sherif YE, Ashmawy A, Badr S. Biosorption of cadmium and nickel by Nilewater algae. J. Appl. Sci. Res. 2008;4:391–396.
- Sakaguchi T, Tsuji T, Nakajima A, Horikoshi T. Accumulation of cadmium by green microalgae. Eur. J. Appl. Microbiol. Biotechnol. 1979;8:207–215.10.1007/BF00506184
- Memon SQ, Memon N, Shah SW, Khuhawar MY, Bhanger MI. Sawdust—a green and economical sorbent for the removal of cadmium(II) ions. J. Hazard. Mater. 2007;139:116–121.10.1016/j.jhazmat.2006.06.013
- Taty-Costodes VC, Fauduet H, Porte C, Delacroix A. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J. Hazard. Mater. 2003;105:121–142.10.1016/j.jhazmat.2003.07.009
- Vasudevan P, Padmavathy V, Dhingra SC. Kinetics of biosorption of cadmium on Baker’s yeast. Bioresour. Technol. 2003;89:281–287.10.1016/S0960-8524(03)00067-1
- Tsuruta T. Removal and recovery of uranyl ion using various microorganisms. J. Biosci. Bioeng. 2002;94:23–28.10.1016/S1389-1723(02)80111-6
- Tsuruta T. Cell-associated adsorption of thorium or uranium from aqueous system using various microorganisms. Water Air Soil Pollut. 2004;159:35–47.10.1023/B:WATE.0000049190.05993.3b
- Nakajima A, Horikoshi T, Sakaguchi T. Recovery of uranium by immobilized microorganisms. Eur. J. Appl. Microbiol. Biotechnol. 1982;16:88–91.10.1007/BF00500732
- Tsuruta T. Selective accumulation of light or heavy rare earth elements using gram-positive bacteria. Colloids Surf., B. 2006;52:117–122.10.1016/j.colsurfb.2006.04.014
- Kamijo M, Suzuki T, Kawai K, Murase H. Accumulation of yttrium by Variovorax paradoxus. J. Ferment. Bioeng. 1998;86:564–568.10.1016/S0922-338X(99)80007-5
- Macaskie LE, Dean ACR. Cadmium accumulation by a Citvobacter sp. the chemical nature of the accumulated metal precipitate and its location on the bacterial cells. J. Gen. Microbiol. 1984;130:53–62.
- Tsuruta T, Umenai D, Hatano T. Accumulation of cadmium ion from aqueous cadmium solution using Bacillus subtilis. Proceedings of the 15th International Conference on Environment and Mineral Processing. 2011;II:303–308.
- Conn EE, Stumpf PK, Bruening G, Doi RH. Outlines of biochemistry. 5th ed. New York, NY, Wiley; 1987. p. 292–293.
- Kondoh S, Ishikawa T, Adachi I. Science of adsorption. Tokyo: Maruzen; 1988. Chapter 4; p. 90.
