Abstract
We describe the syntheses of the proposed structure of diphenyl ether oxyneolignan, apteniol A and its derivatives. The diphenyl ether moiety of proposed apteniol A was formed via Ullmann ether synthesis, but the spectral data of the synthesized apteniol A did not agree with that in previous studies. The dimethyl ester derivative of the proposed apteniol A was found to enhance neurite outgrowth in PC12 cells and inhibit antigen-induced degranulation in RBL-2H3 cells.
Graphical Abstract
Proposed apteniol A (1) and its derivatives were synthesized and the dimethyl ester (6) was found to enhance neurite outgrowth and inhibit antigen-induced degranulation.
Apteniols A–GCitation1,2) are phytotoxic metabolites that have been isolated by DellaGreca et al. from Aptenia cordifolia (Aizoaceae), a perennial herb native to South Africa and that has now largely spread throughout Europe, and a well-known groundcover or creeping plant. Devi et al. have also reported an isolation of apteniol A from the marine bacterium Bacillus licheniformis SAB1 as an antimicrobial metabolite.Citation3) However, the 1H- and 13C-NMR data provided by these reports did not agree with each other. Therefore, to solve this contradiction, we synthesized the proposed apteniol A.
Results and discussion
This paper describes the synthesis of the proposed structure of apteniol A (1). As shown in Scheme , the diphenyl ether formation, which is the key step in this synthesis, was performed via Ullmann ether synthesis.Citation4) This reaction is a conventional method for diphenyl ether formation. However, it requires harsh reaction conditions, such as high temperatures and stoichiometric quantities of copper or copper salts, and produces low to moderate yields, greatly limiting its use.Citation5) In this reaction, the use of appropriate ligands can reduce the required copper and copper salt quantities to catalytic amounts. In 2003, Ma and Cai found that under the action of N,N-dimethylglycine, the CuI-catalyzed coupling reaction of aryl halides and phenols the corresponding diaryl ethers in good to excellent yields at 90 °C.Citation4) These conditions reportedly applicable to a wide variety of substrates with different functional groups suggesting that this method was efficient for synthesis of apteniols and analogs. A first attempt at the Ullmann etherification of ethyl p-hydroxycinnamate and ethyl p-bromocinnamate produced the desired diphenyl ether with low yield. In contrast, the coupling of p-hydroxybenzaldehyde and p-bromobenzaldehyde gave the desired ether in moderate yield. Therefore, apteniol A (1) was synthesized using these aldehydes as starting materials.
As shown in Scheme , Ullmann etherification of p-hydroxybenzaldehyde and p-bromobenzadehyde gave diphenyl ether 4. Both formyl groups in 4 were converted into α,β-unsaturated esters through Horner–Wadsworth–Emmons reaction. Catalytic hydrogenation and subsequent hydrolysis of these ester groups of afforded the desired dicarboxylic acid corresponding to the proposed apteniol A (1) as a white powder. The synthesized apteniol A was further converted into dimethyl ester 6 by an esterification. The melting point of 6 (50–51 °C) agreed with known data (48.5–51 °C) reported by Schimelpfenig and Ford,Citation5) who followed a different synthetic approach. Therefore, this synthesized 6 presents the structure of the dimethyl ester of the proposed apteniol A (1). However, the 1H- and 13C-NMR data of 1 (Table ) differed from the data reported. Particularly, in spite of the same measurement conditions (solvent and measurement frequency), the C-1 carbon signal shows a difference more than 7 ppm in 13C-NMR. In addition, the seeds germination inhibitory activity and the roots elongation inhibitory activity against Lactuca sativa at 10−4 M were reported by DellaGreca et al. However, the bioassay under the same concentration (10−4 M) did not show any seeds germination inhibitory activity and showed elongation of seminal roots (Table ). These data suggest the possibility that the structure proposed by DellaGreca is an error.
Table 1. NMR Data of 1 and reported apteniol A (in CD3OD).
Table 2. Effect of compound 1 on germination of L. sativa.
Diphenyl ether derivatives are attractive target compounds in organic synthesis because of their numerous biological applications. They exhibit a wide range of biological activities that include anti-cancer,Citation6,7) anti-inflammatory,Citation8,9) anti-fungal,Citation10,11) and anti-bacterial effects.Citation12) Although apteniols could be considered as potential biologically active compounds, there are no reports of such activity. (Fig. .)
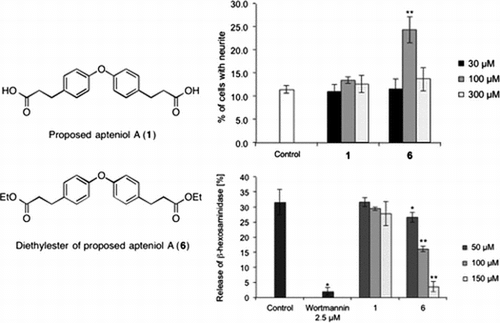
Fig. 1. Biological assay.
Notes: (A) Effects of proposed apteniol A and its derivatives on Bt2cAMP-induced neurite outgrowth in PC12 cells. PC12 cells were plated at 4.0 × 103 cells/well and cultured with indicated test compound concentrations in the presence of 0.5 mM Bt2cAMP. The extent of neurite outgrowth was measured after 24 h and expressed as the mean percentage of 300–400 cells from triplicate cultures. The bars represent SD. **p < 0.01 (Dunnett’s test) compared with the control (Bt2cAMP 0.5 mM only). (B) Inhibitory effects of proposed apteniol A and its derivatives on antigen-induced degranulation in RBL-2H3 cells. DNP-IgE sensitized RBL-2H3 cells were incubated with indicated test compound concentrations for 20 min and stimulated with DNP-HAS for 1 h. Released β-hexosaminidase was measured. Data correspond to means of triplicate cultures and the bars represent SD. *p < 0.05, **p < 0.01 (Dunnett’s test) compared with control.
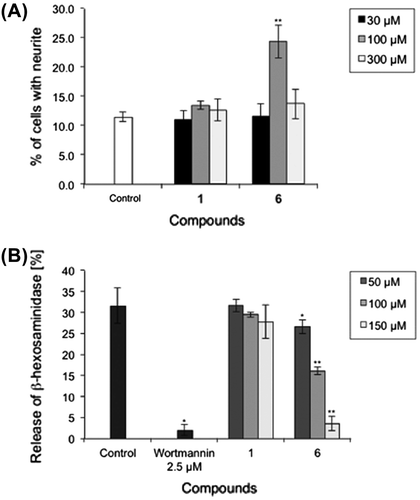
Scheme 1. Synthesis of proposed apteniol A and its derivatives.
Notes: Reagents and conditions: (a) CuI, N,N-dimethylglycine HCl salt, Cs2CO3, DMF, 100 °C (82%); (b) triethyl phosphonoacetate, NaH, benzene, rt (96%); (c) Pd/C, H2, MeOH, 50 °C (98%); (d) NaOH, THF/H2O, rt (62%); (e) H2SO4, MeOH, reflux (90%); (f) methylamine (40% in methanol), NaH, 100 °C (40%).
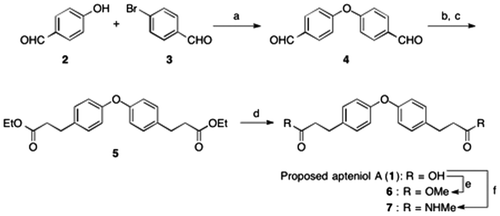
With the proposed structure of apteniol A (1) and methyl ester 6 in hand, we evaluated neurite outgrowth-promoting and degranulation-inhibiting activities. Compound 6 showed neurite outgrowth-promoting activity at a concentration of 100 μM and degranulation-inhibiting activity at concentrations ranging from 50 to 150 μM. On the other hand, the proposed apteniol A (1) exhibited degranulation-inhibiting activity at higher concentrations.
The neurite outgrowth-promoting and degranulation-inhibiting activities of the methyl amide of the proposed apteniol A (7), 3-(4-hydroxyphenyl) propionic acid, methyl 3-(p-hydroxyphenyl)propionate (8), and 3-(4-hydroxyphenyl)-N-methylpropionamide (9) were also evaluated for comparison. None of these compounds displayed any neurite outgrowth-promoting activity. However, compound 7 showed a weak degranulation-inhibiting activity at 150 μM (data not shown).
In summary, the synthesis of proposed structure of apteniol A (1) was achieved in 4 steps with an overall yield of 48%. The biological activity and the 1H/13C-NMR data of the synthesized compound differed from reported data, suggesting that there is possibility that the structure proposed apteniol A is an error. In addition, the diester derivative 6 showed neurite outgrowth-promoting and significant degranulation-inhibiting activities.
Experimental
General experimental procedures
Melting points were determined using an ATM-01 apparatus (AS ONE, Osaka, Japan). IR spectra were measured using a Spectrum GX FT-IR System 2000 spectrometer (Parkin-Elmer, Waltham, MA, USA). NMR spectra were recorded using ECA-500 (JEOL, Tokyo, Japan) and Agilent 400-MR DD2 instruments (Agilent, Santa Clara CA, USA) using tetramethyl silane as an internal standard. Mass spectra were recorded using JMS-700 instrument (JEOL). Column chromatography was performed on silica gel 60 N (100–210 mesh, Kanto Chemical Co., Tokyo, Japan).
4,4’-Oxybisbenzaldehyde (4)
A mixture of p-bromobenzaldehyde (746 mg, 4.03 mmol), p-hydroxybenzaldehyde (734 mg, 6.01 mmol), Cs2CO3 (2.61 g, 8.01 mmol), copper iodide (76 mg, 0.40 mmol), N, N-dimethylglycine HCl salt (168 mg, 1.20 mmol), and DMF (6 mL) was heated to 100 °C in a sealed tube under nitrogen atmosphere for 40 h. The mixture was cooled and partitioned between CH2Cl2 and water in a separatory funnel. The organic layer was separated and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine and dried over Na2SO4. Evaporation of the extract and purification of the residue by column chromatography (n-hexane/ethyl acetate = 4:1) on silica gel gave dialdehyde 4 as a white powder (0.74 g, 3.3 mmol, 82%), mp 61–63 °C (lit.Citation13) 59.5–64.0 °C). IR νmax (cast film) cm−1: 3070, 3035, 2829, 2781, 1694, 1590, 1497, 1247, 1155, 833. NMR δH (400 MHz, CDCl3): 9.98 (2H, s), 7.93 (4H, d, J = 8.8 Hz), 7.18 (4H, d, J = 8.8 Hz).
4,4’-Oxybis-(ethyl 3-phenylpropenoate)
Sodium hydride (60% dispersion in mineral oil, 286 mg, 7.15 mmol) was washed with dry n-hexane and suspended in dry benzene (10 mL). This mixture was added dropwise to a solution of triethyl phosphonoacetate (1.6 g, 7.0 mmol) at ice-cooled temperature. After the addition, the solution was stirred at room temperature until gas evolution ceased. This yellow solution was added dropwise to a solution of aldehyde 4 (654 mg, 2.89 mmol) in dry benzene at ice-cooled temperature. During this addition, a gummy precipitate appeared. The solution was stirred at room temperature for 1 h and partitioned between diethyl ether and water. The organic layer was separated and the aqueous layer was extracted with diethyl ether. The combined organic layers were washed with brine and dried over CaCl2. Evaporation of the extract and purification of the residue by column chromatography CH2Cl2 (100%) on silica gel gave diester as a colorless crystal (1.0 g, 2.8 mmol, 96%), mp 73–77 °C (lit.Citation14) 77–78 °C). IR νmax (cast film) cm−1: 2983, 1713, 1635, 1593, 1243, 1169, 1032, 834, 680. NMR δH (400 MHz, CDCl3): 1.34 (6H, t, J = 7.1 Hz), 4.26 (4H, q, J = 7.1 Hz), 6.37 (2H, d, J = 16.0 Hz), 7.03 (4H, d, J = 8.8 Hz), 7.53 (4H, d, J = 8.8 Hz), 7.66 (2H, d, J = 16.0 Hz).
4,4’-Oxybis-(ethyl phenylpropanoate) (5)
A suspension of 4,4’-oxybis-(ethyl 3-phenylpropenoate) (550 mg 1.50 mmol) and Pd/C (55.7 mg, cat.) in dry methanol was stirred 50 °C under hydrogen for 1 d. The reaction mixture was filtered and concentrated in vacuo to afford diester 5 as colorless oil (1.47 mmol, 542 mg, 98.0%). IR νmax (film) cm−1: 2981, 1732, 1603, 1505, 1240, 1102, 1016, 875. NMR δH (400 MHz, CDCl3): 1.24 (6H, t, J = 7.1 Hz), 2.61 (4H, t, J = 8.0 Hz), 2.92 (4H, t, J = 8.0 Hz), 4.13 (4H, q, J = 7.1 Hz), 6.90 (4H, d, J = 8.5 Hz), 7.15 (4H, d, J = 8.5 Hz). HREIMS m/z (M+): Calcd. for C22H26O5: 370.1780, Found: 370.1784.
4,4’-Oxybis(3-phenylpropanoic acid) (proposed apteniol A) (1)
A mixture of the ester 5 (775 mg, 2.09 mmol) and 95 mg of NaOH in a 15 mL of THF/H2O (1:2) was stirred at room temperature for 5 h. The solution was partitioned between ethyl acetate and 1.3 M HCl. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine and dried over Na2SO4. Evaporation of the extract and purification of the residue by column chromatography (CHCl3/methanol = 10:1) on silica gel gave 1 as a white crystal (409 mg, 1.30 mmol, 62.2%), mp 191–192 °C. HREIMS m/z (M+): Calcd. for C18H18O5: 314.1154, Found: 314.1157. 1H- and 13C-NMR spectra are reported in Table .
4,4’-Oxybis-(methyl 3-phenylpropanoate) (6)
A solution of proposed apteniol A (1) (157 mg, 0.499 mmol) was refluxed for 3 h in the presence of 180 mg of H2SO4 in dry methanol. The cooled solution was evaporated in vacuo. The dry residue was partitioned between ethyl acetate and water. The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic layers were washed with brine, dried over Na2SO4, and concentration of the extract and purification by preparative TLC (n-hexane/ethyl acetate = 6:1) gave diester 6 as a colorless crystal (153 mg, 0.447 mmol, 89.6%), mp 50–51 °C (lit.Citation5) 48.5–51 °C). IR νmax (cast film) cm−1: 3030, 2954, 1899, 1739, 1603, 1504, 1436, 1370, 1194, 875. NMR δH (400 MHz, CDCl3): 2.62 (4H, t, J = 7.8 Hz), 2.93 (4H, t, J = 7.8 Hz), 3.67 (6H, s), 6.91 (4H, d, J = 8.6 Hz), 7.14 (4H, d, J = 8.6 Hz).
4,4’-Oxybis-(N-methyl 3-phenylpropionamide) (7)
A suspension of 6 (38 mg, 0.11 mmol), NaH (60% dispersion in mineral oil, 10 mg, 0.26 mmol), and methylamine (40% in methanol, ca. 9.8 mol/L, 40 µL) in a sealed tube was heated at 100 °C for 2 h. The cooled solution was partitioned between CH2Cl2 and 1.3 M HCl. The organic layer was separated and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine and dried over Na2SO4. Evaporation of the extract and purification of the residue by column chromatography (CHCl3/methanol = 10:1) on silica gel gave amide 7 as a white powder (15 mg, 0.040 mmol, 40%), mp 185–186 °C. NMR δH (400 MHz, CDCl3): 2.45 (4H, t, J = 7.8 Hz), 2.78 (6H, d, J = 4.8 Hz), 2.94 (4H, t, J = 7.8 Hz), 5.36 (2H, s), 6.90 (4H, d, J = 8.6 Hz), 7.14 (4H, d, J = 8.6 Hz). HREIMS m/z (M+): Calcd. for C20H24O3N2: 340.1787, Found 340.1787.
3-(4-Hydroxyphenyl)-N-methylpropionamide (9)
A solution of methyl 3-(p-hydroxyphenyl)propionate (8) (197 mg, 1.09 mmol), NaH (60% dispersion in mineral oil, 94 mg, 2.3 mmol), and methylamine (40% in methanol, ca. 9.8 mol/L, 500 µL) in a sealed tube was heated at 100 °C for 1 h. Evaporation of the reaction mixture and purification of the residue by column chromatography (CHCl3/methanol = 10:1) on silica gel gave 9 as a pale yellow crystal (70 mg, 0.39 mmol, 36%), mp 84.5–85 °C. IR νmax (cast film) cm−1: 3357, 2924, 1713, 1644, 1516, 1446. NMR δH (400 MHz, CD3OD): 2.39 (2H, t, J = 7.8 Hz, 7.3 Hz), 2.66 (3H, s), 2.79 (2H, t, J = 7.8 Hz), 6.67 (2H, d, J = 8.6 Hz), 7.00 (2H, d, J = 8.6 Hz). HREIMS m/z (M+): Calcd. for C10H13O2 N: 179.0946, Found 179.0945.
Biological assay
Evaluation of germination activitiy
Activity of the compound 1 was assessed in a germination assay using L. sativa. Experiments were carried out as described by Macias et al.Citation15) using 15 seeds per Petri dish of 80 mm moistened with 2.5 mL of test solution. MES (2-[N-morpholino]ethanesulfonic acid) buffer (pH 6) served as a control, and synthesized compound was tested individually (10−4 M). Experiments were carried out at least twice (per synthesized compound) and the mean germination value (n = 6) was determined after 5 days at 25 °C in the dark. Germination data were arcsine transformed and analyzed using Student’s t-test, at a significance level of p < 0.05.
Evaluation of neurite outgrowth-promoting activityCitation16)
Culture conditions were set as described by Greene and Tischler. Briefly, PC12 cells (RIKEN Cell Bank, Tsukuba, Japan) were grown in RPMI 1640 medium supplemented with 5% heat-inactivated fetal bovine serum, 10% heat-inactivated horse serum, 100 units/mL penicillin G sodium salt, and 100 μg/mL streptomycin sulfate, and incubated in a humidified atmosphere containing 95% air and 5% CO2 at 37 °C. The suspended PC12 cells in the RPMI 1640 medium as described above were seeded in 96-well plates coated with porcine tendon collagen at a density of 4.0 × 103 cells/90 µL/well. After 24 h, 10 µL of a medium containing Bt2cAMP (0.5 mM final concentration) and the test compound was added to the cultured cells. Neurite formation was examined 24 h after treatments. The incubation was stopped by the addition of 1% glutaraldehyde and staining using Giemsa solution. The number of cells bearing neurites longer than one cell body diameter after treatments was divided by the total number of cells, which amounted to 300–400 cells per well in triplicate cultures.
Evaluation of degranulation-inhibiting activity
The inhibitory activity against the release of β-hexosaminidase from RBL-2H3 cells was evaluated by a modifying of the method of Watanabe et al.Citation17) RBL-2H3 cells were purchased from the JCRB Cell Bank (Osaka, Japan). Dulbecco’s-modified Eagle’s medium containing 10% heat-inactivated fetal bovine serum was used as a growth medium. The cells were cultured in a 96-well plate (5.0 × 104 cells/well) for 24 h at 37 °C under a humidified 5% CO2 atmosphere and incubated in a growth medium containing 50 ng/mL of mouse monoclonal anti-dinitrophenyl (DNP) IgE for 2 h. The cells were washed with the modified Tyrode buffer (MT) before the test compounds or wortmannin (2.5 μM) was added. Test compounds and wortmannin were dissolved in DMSO and diluted with MT buffer to obtain a final DMSO concentration of 0.25%. After 20 min of incubation, DNP-labeled human serum albumin (50 ng/mL final concentration) was added to the cells and the culture was incubated for 1 h. The supernatant was collected and the cells were lysed with MT buffer containing 0.1% Triton X-100. The β-hexosaminidase activities of the supernatant and cell lysate were measured by the method of Demo et al.Citation18) Supernatant or the cell lysate (20 µL) was mixed with 3.3 mM p-nitrophenyl-2-acetamide-2-deoxy-β-D-glucopyranoside (40 µL) in 100 mM citrate buffer (pH 4.5), and the mixture was incubated in a 96-well plate at 37 °C for 90 min. The reaction was terminated by adding a 2 M glycine buffer (pH 10.4, 40 µL), and the absorbance at 405 nm was measured with a microplate reader.
Data analysis of neurite outgrowth-promoting and degranulation-inhibiting assays
Results are expressed as means and SD of triplicate cultures. Multiple data comparisons were performed by analysis of variance followed by Dunnett’s t-test. P values inferior to 5% were regarded as significant.
Acknowledgments
This research was financially supported by the Sasakawa Scientific Research Grant from The Japan Science Society and BRAIN: Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry. The authors would like to thank Enago (www.enago.jp) for the English language review.
Notes
Abbreviations: DNP, dinitrophenyl; MT, modified Tyrode.
References
- DellaGreca M, Di Marino C, Previtera L, Purcaro R, Zerrelli A. Apteniols A–F, oxyneolignans from the leaves of Aptenia cordifolia. Tetrahedron. 2005;61:11924–11929.10.1016/j.tet.2005.09.054
- DellaGreca M, Fiorentino A, Izzo A, Napoli F, Purcaro R, Zerrelli A. Phytotoxicity of secondary metabolites from Aptenia cordifolia. Chem. Biodivers. 2007;4:118–128.
- Devi P, Wahidullah S, Rodrigues C, Souza LD. The sponge-associated bacterium Bacillus licheniformis SAB1: a source of antimicrobial compounds. Mar. Drugs. 2010;8:1203–1212.10.3390/md8041203
- Ma D, Cai Q. N,N-Dimethyl glycine-promoted Ullmann coupling reaction of phenols and aryl halides. Org. Lett. 2003;5:3799–3802.10.1021/ol0350947
- Schimelpfenig CW, Ford RR. Synthesis and properties of two dioxadioxoparacyclophanes. J. Org. Chem. 1981;46:1210–1212.10.1021/jo00319a035
- Boger DL, Patane MA, Zhou J. Total synthesis of bouvardin, O-methylbouvardin, and O-methyl-N9-desmethylbouvardin. J. Am. Chem. Soc. 1994;116:8544–8556.10.1021/ja00098a015
- Chomcheon P, Wiyakrutta S, Sriubolmas N, Ngamrojanavanich N, Kengtong S, Mahidol C, Ruchirawat S, Kittakoop P. Aromatase inhibitory, radical scavenging, and antioxidant activities of depsidones and diaryl ethers from the endophytic fungus Corynespora cassiicola L36. Phytochemistry. 2009;70:407–413.10.1016/j.phytochem.2009.01.007
- Akama T, Baker SJ, Zhang Y-K, Hernandez V, Zhou H, Sanders V, Freund Y, Kimura R, Maples KR, Plattner JJ. Discovery and structure–activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg. Med. Chem. Lett. 2009;19:2129–2132.10.1016/j.bmcl.2009.03.007
- Morikawa T, Tao J, Toguchida I, Matsuda H, Yoshikawa M. Structures of new cyclic diarylheptanoids and inhibitors of nitric oxide production from Japanese folk medicine Acer nikoense. J. Nat. Prod. 2003;66:86–91.10.1021/np020351m
- Tamai S, Kaneda M, Nakamura S. Piperazinomycin, a new antifungal antibiotic. I. Fermentation, isolation, characterization and biological properties. J. Antibiot. 1982;35:1130–1136.10.7164/antibiotics.35.1130
- Kaneda M, Tamai S, Nakamura S, Hirata T, Kushi Y, Suga T. Piperazinomycin, a new antifungal antibiotic. II. Structure determination by X-ray crystallography. J. Antibiot. 1982;35:1137–1140.10.7164/antibiotics.35.1137
- Lakshmi Niranjan Reddy V, Ravinder K, Srinivasulu M, Venkateshwar Goud T, Malla Reddy S, Srujankumar D, Prabhakar Rao T, Suryanarayana Murty U, Venkateswarlu Y. Two new macrocyclic diaryl ether heptanoids from Boswellia ovalifoliolata. Chem. Pharm. Bull. 2003;51:1081–1084.10.1248/cpb.51.1081
- Gawroński J, Brzostowska M, Kwit M, Plutecka A, Rychlewska U. Rhombimines – Cyclic tetraimines of trans-1,2-diaminocyclohexane shaped by the diaryl ether structural motif. J. Org. Chem. 2005;70:10147–10150.10.1021/jo051687e
- Sengupta S, Sadhukhan SK, Bhattacharyya S, Guha J. Palladium-catalyzed reactions of bisarenediazonium salts: two-fold Heck reaction, carbonylation and cross-coupling regimen. J. Chem. Soc. Perkin Trans. 1998;1:407–410.10.1039/a707378j
- Macías FA, Castellano D, Molinillo JMG. Search for a standard phytotoxic bioassay for allelochemicals. selection of target species. J. Agric. Food. Chem. 2000;48:2512–2521.10.1021/jf9903051
- Zhou X, Tai A, Yamamoto I. Enhancement of neurite outgrowth in PC12 cells stimulated with cyclic AMP and NGF by 6-acylated ascorbic acid 2-O-α-glucosides (6-Acyl-AA-2G), novel lipophilic ascorbate derivatives. Biol. Pharm. Bull. 2003;26:341–346.10.1248/bpb.26.341
- Watanabe J, Shinmoto H, Tsushida T. Coumarin and flavone derivatives from estragon ant thyme as inhibitors of chemical mediator release from RBL-2H3 cells. Biosci. Biotechnol. Biochem. 2005;69:1–6.10.1271/bbb.69.1
- Demo SD, Masuda E, Rossi AB, Throndset BT, Gerard AL, Chan EH, Armstrong RJ, Fox BP, Lorens JB, Payan DG, Scheller RH, Fisher JM. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry. 1999;36:340–348.10.1002/(ISSN)1097-0320
