Abstract
In this study, we revealed that a Mekabu (Udaria pinnantifida) extract enhanced immunoglobulin (Ig) production of mouse spleen lymphocytes. Furthermore, it was suggested that water-soluble and high molecular weight ingredients in the Mekabu extract have significant enhancing effect on Ig production. Therefore, fucoidan was estimated as the active component.
Immune system consists of innate and acquired immunity and has the crucial roles in maintaining our health. Acquired immune system is further classified into cellular and humoral immunity. In cellular immunity, cytotoxic T lymphocytes play important roles in eliminating abnormal substances such as virus-infected cells and cancer cells. On the other hand, in humoral immunity, foreign substances are mainly excluded by antibodies, which are produced by B lymphocytes. Antibody has 5 classes of immunoglobulin (Ig) named IgA, IgD, IgE, IgG, and IgM, and each class of Ig has different functions. IgA has significant role primarily in the mucosal immune system.Citation1) They are secreted into mucosa of digestive and respiratory tract, saliva, and tears, and inhibit the absorption and invasion of foreign materials such as virus, bacteria, and allergens.Citation1,2) IgE is famous for cause of type I allergy, but usually contributes to the resolution of parasite infection.Citation3) IgG is contained mostly in the plasma and has the protective effect against bacteria, virus, and toxin. IgG also exerts anti-type I allergic effect by inhibiting the action of IgE. IgM also involves in the suppression of allergic reaction as with IgG, and it acts as primary antibody of humoral immune response to rapidly eliminate harmful foreign substances. IgM forms an antigen–antibody complex to agglutinate antigen. The immune complex activates complement system and enhances elimination of antigen by phagocytosis. Monomer of IgM is expressed on the cell surface of B lymphocytes and function as antigen receptors.
However, immune function attenuates for the various reasons such as stress,Citation4) unbalanced diet, smoking, and aging. Anti-cancer agents also weaken the immune function. The immune-compromised peoples are sensitive to infection of virus and bacteria, and fall prey easily to diseases including cancer. For these reasons, the study on immunoenhancing effect of food component is worthwhile for proposing a means of maintaining our health status. In this context, we have found in previous studies that vegetable extracts and water-soluble dietary fibers exert stimulating effect on Ig production of rat lymphocytes both in vitro and in vivo.Citation5–7)
Blown algae like Wakame (Undaria pinnatifida) and Kombu (Laminaria sp.) are popular foods in East Asia. These edible algae contain abundant minerals, vitamins, and dietary fibers. Mekabu is sporophyll of U. pinnatifida, which has been eaten as a safe and savory food in Japan from old days, and attracted recently because of their multiple functions. For example, potential ability for chemoprevention of human breast cancer has been revealed as water extract of Mekabu powder exhibited strong suppressive effect on rat mammary carcinogenesis.Citation8)
A promising candidate for bioactive component containing in Mekabu is fucoidan. Fucoidan is fucose-rich sulfated polysaccharides and constitutes extracellular component of brown algae. Fucoidan is a sort of water-soluble dietary fiber, and has a broad molecular weight distribution (approx. 10–1000 kD). It is well known that fucoidan has various biological effectsCitation9–11) such as anti-tumor,Citation12–18) anti-virusCitation19,20), and anti-inflammatory effects.Citation21) Furthermore, Mekabu-derived fucoidan showed anti-tumor effect in vivo and its mechanism was mediated by IFN-γ activated NK cells.Citation22) However, few data have been reported in relation to the effect of Mekabu components on Ig production. Hence, the present study was performed to determine Ig production regulating activity of a Mekabu extract.
Spleen lymphocytes were obtained from 7 to 12-week-old BALB/c female mice (Kyudo, Fukuoka. Japan). Isolated spleens were mashed to make single cell suspension and treated with ammonium chloride-based reagent for lysis of red blood cells. After twice washing, lymphocytes were separated using Lympholyte-M (Cedarlane, Ontario, Canada) and re-suspended at a density of 1.5 × 106 cells/mL in RPMI1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% fetal calf serum (Intergen Co., Purchase, NY). The spleen lymphocyte suspensions were seeded into wells of 24-well plates, and cultured for 72-h at 37 °C with 5% CO2 after sample adding. This experiment was carried out according to the guidelines for animal experiments at the Faculty of Agriculture, Kyushu University. After the 72-h culture, Ig content of the culture supernatant which secreted by spleen lymphocytes was measured by enzyme-linked immunosorbent assay (ELISA) using mouse IgA, IgE, IgG, and IgM BD ELISA kit (Bethyl Laboratories, Inc., Montgomery, TX) following the manufacture’s instruction. Ig concentrations are calculated using the standard curve obtained for each ELISA plates. Data are analyzed by Student’s t-test to evaluate significant differences.
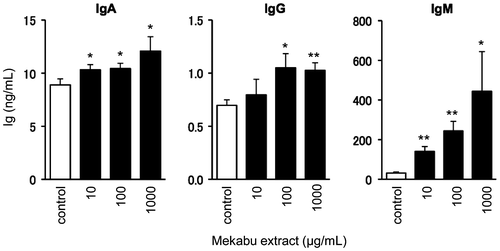
First we examined the effect of a Mekabu water extract (Mekabu extract) on Ig production of mouse spleen lymphocytes. Dried powder of the Mekabu extract was provided from NPO Research Institute of Fucoidan. It is known that fucoidan derived from Mekabu is relatively highly sulfated galactofucan composed of almost equal ratio of fucose and galactose residues.Citation19) Composition of the Mekabu extract used in this study was 22.1% fucose, 22.4% galactose, and 30.8% ester sulfate as percentage of the dry weight. Therefore, fucoidan content of the Mekabu extract was estimated to be above 75%. On the other hand, uronic acids are also minor components of fucoidan, and alginate which is another major polysaccharide contained in Mekabu entirely consists of uronic acids. The content of uronic acids in the Mekabu extract is only 2.5%. The extract powder was dissolved in water at room temperature for 15 min with magnetic stirrer, and centrifuged at 3200 × g for 20 min to remove insoluble materials. The obtained supernatant was lyophilized and re-dissolved in ultra pure water. This sample was added in culture medium by 10% at final concentration 10, 100, 1000 μg/mL, and ultra pure water was added as control. After 72-h culture, we collected these culture media, and Ig production regulating effect of the Mekabu extract was evaluated by measuring concentration of Ig secreted in the culture supernatant. As shown in Fig. , Mekabu extract significantly increased contents of IgA, IgG, and especially IgM at 10 and 100 μg/mL. The concentration of IgE secreted in the media was below the detection limit of the ELISA kit. On the other hand, numbers of viable cells at after the 72-h culture were evaluated by measuring intracellular adenosine triphosphate (ATP) contents with a luminescence ATP detection assay system (ATPlite 1step, PerkinElmer, Inc., Waltham, MA). As a result, the value of luminescence intensity (mean ± SD) corresponding to the viable cell number in each culture condition was as follows; control: 24,078 ± 1696, 10 μg/mL: 64,221 ± 3630, 100 μg/mL: 94,466 ± 4451, 1000 μg/mL: 70,491 ± 4330. Therefore, it was suggested that the Mekabu extract acted in protection of spleen lymphocyte during the in vitro culture, but exerted a little cytotoxicity at high concentration over 1000 μg/mL. From the results, one mechanism for the enhancement of Ig production by the Mekabu extract was inferred to maintain and/or stimulate growth of spleen lymphocytes, whereas effects of the Mekabu extract on Ig production and cell viability were not exactly correlated with each other. It is well known that IgM antibody acting in early stage of immune response has important roles in immune surveillance. IgA is primary humoral factor in mucosal immunity, and we revealed that some kinds of water-soluble dietary fibers stimulated IgA production of intestinal immune cells.Citation5–7) Katayama et al. also reported that oral administration of highly viscous polysaccharides extracted from Gagome alga augmented IgA production of spleen and Payer’s patch cells.Citation23) Therefore, the above-mentioned results suggest that Mekabu extract has potential of enhancing effective initial immune responses.
Fig. 1. Effect of Mekabu extract on Ig production by mouse spleen lymphocytes.
Notes: Mekabu extract is water extract from U. pinnatifida. Mouse spleen lymphocytes were cultured for 72-h in the presence of the extracts (10, 100, 1000 μg/mL). After the cultivation, Ig contents in the culture media were measured by ELISA. Data are expressed as mean ± SE (n = 3). *p < 0.05, **p < 0.01 compared to the control by Student’s t-test. Experiments were repeated at least three times with similar results.
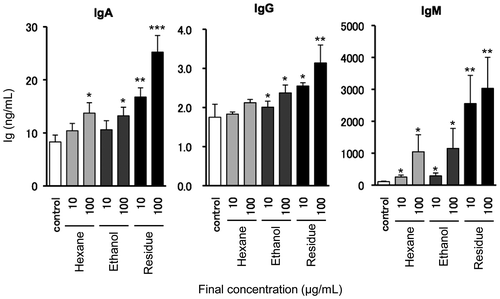
Mekabu extract used in above experiment is crude material and contains multiple components. Therefore, we undertook fractionation of the ingredients that contributes to increase Ig production by sequential (solid-liquid) extraction with hexane and ethanol as solvent. Mekabu extract powder was suspended in 20-times volume of hexane and centrifuged (3200 × g, 3 min) after strenuous agitation. These processes were repeated another two times and all supernatants were got together as hexane fraction. After completely removing hexane contained in the residue, we re-suspended the residue in 10-times volume of ethanol by shaking for 24 h with light blocking and collected the supernatant. These processes were repeated three times and all supernatants were got together as ethanol fraction. The residue resulting from the ethanol extraction was used as residue fraction. Each fraction was air-dried to completely remove organic solvent, and the resultant dry matters were dissolved in ultra pure water and lyophilized. The rate of each lyophilizate of hexane, ethanol, and residue fraction was 0.22, 0.84, and 98.94%, respectively. Each sample was dissolved in 1% dimetylsulfoxide at 10 mg/mL and added in culture media at 100 μg/mL final concentration. As shown in Fig. , residue fraction had the strongest effect of the 3 fractions. On the other hand, hexane and ethanol fractions slightly increased Ig production of mouse spleen lymphocytes compared to residue fraction. This result suggests that main active component in the Mekabu extract has relatively low lipid solubility, since the water-soluble ingredients cannot generally dissolved in hexane. Moreover, as it is assumed that fucoidan is contained in residue fraction because of its high water soluble nature, the convincing candidate for the main active ingredient is fucoidan but not other Mekabu components that dwelled in making processes. However, recovery rate of hexane and ethanol fraction was very low. Therefore, unidentified components that tightly associated with fucoidan might modify the effect of fucoidan on the lymphocyte function.
Fig. 2. Effect of fractionated Mekabu extract components on Ig production by mouse spleen lymphocytes.
Notes: Each fraction differ in their solubility was prepared from Mekabu extract powder by sequential (solid–liquid) extraction with hexane and ethanol as solvent. Mouse spleen lymphocytes were cultured for 72-h after adding each fraction (10, 100 μg/mL). After the cultivation, Ig contents in the culture media were measured by ELISA. Data are expressed as mean ± SE (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control by Student’s t-test. Experiments were repeated three times with similar results.
Finally, we further fractionated the each extracts according to their molecular weight by ultrafiltration. For ultrafiltration, we used VIVACON 2 (Sartorius Stedim biotech, Goettingen, Germany) that could roughly divide materials into two fractions on the bases of 2000 molecular weight (MW). Low molecular weight fractions (LMWF) were used just as they were after ultrafiltration. As the high molecular weight ingredients are concentrated, we filled them with solvent up to the volume before ultrafiltration. As shown in Fig. , high molecular weight fractions (HMWF) exhibited the enhancing effect on Ig production as equivalent intensity to the whole fraction before ultrafiltration. However, the LMWF did not increase Ig production. These results suggest that active components in the fractionated Mekabu extract have relatively high molecular weight at least above 2000 MW. As these characteristics accord with those of fucoidan, it became more likely that Mekabu fucoidan increase Ig production of mouse spleen lymphocytes. As IgA, IgG, and IgM are major effector proteins in humoral immune system, it is expected that Mekabu fucoidan is useful for maintaining host defense through increasing Ig production.
Fig. 3. Effect of high and low molecular weight components in Mekabu extract on Ig production by mouse spleen lymphocyte.
Notes: HMWF and LMWF were obtained from each sample used in Fig. by ultrafiltration. H = Hexane fraction, E = Ethanol fraction, and R = Residue fraction. Mouse spleen lymphocytes were cultured for 72-h after adding fractions (100 μg/mL). After the cultivation, Ig contents in the culture media were measured by ELISA. Data are expressed as mean ± SE (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control by Student’s t-test. Experiments were repeated three times with similar results.
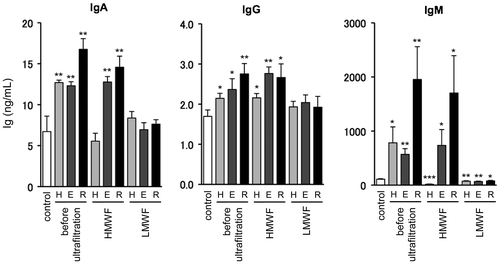
Although we did not reveal detail mechanisms on the immune regulatory effect of the Mekabu-derived fucoidan, it has been reported that fucoidan prepared from Mekabu induced blast formation of splenic B cells from herpes simplex virus (HSV)-1-infected mice.Citation24) As mentioned above, we also found that Mekabu extract supported in vitro growth of spleen lymphocytes. However, it was still unclear if fucoidan directly stimulated B lymphocytes to proliferate and secrete Ig because spleen lymphocytes consist of multiple types of immune cells such as monocytes, granulocytes, T lymphocytes and B lymphocytes, and so on. On the other hand, the most IgG production was observed in the culture with ethanol-extracted HMWF, whereas IgA and IgM production were potently stimulated by residual HMWF (Fig. ). In addition, the enhancing effect of the Mekabu extract and the fraction was more potently observed in production of IgM than IgA and IgG (Figs. ). These results might be indicating that the Ig production was class-specifically regulated by the Mekabu extract. Identification of the regulatory pathway, target cells, and active components other than fucoidan remains unsolved subjects.
In conclusion, we elucidated that the Mekabu extract has significant enhancing effect on production of each class antibody by mouse spleen lymophocytes. Furthermore, it was estimated that water-soluble and high molecular weight molecules including fucoidan were the main active components. It is noteworthy that fucoidan is expected to be useful in prevention of influenza epidemics because it was reported that anti-viral antibody titer after influenza vaccination in elderly Japanese peoples was boosted up by intake of fucoidan.Citation20) For all that, future investigations that define the in vivo feeding effect and the mechanisms on enhancement of basal Ig production are required for evaluating genuine efficacy of the Mekabu fucoidan.
Notes
Abbreviations: Ig, immunoglobulin; ELISA, enzyme-linked immunosorbent assay; MW, molecular weight; LMWF, low molecular weight fractions; HMWF, high molecular weight fractions.
References
- Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013;4 ( Article 185):1–11.
- Blutt SE, Miller AD, Salmon SL, Metzger DW, Conner ME. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol. 2012;5:712–719.10.1038/mi.2012.51
- Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245.10.1038/349243a0
- Miyazaki Y, Yamasaki M, Mishima H, Mansho K, Tachibana H, Yamada K. Oxidative stress by visible light irradiation suppresses immunoglobulin production in mouse spleen lymphocytes. Biosci. Biotechnol. Biochem. 2001;65:593–598.10.1271/bbb.65.593
- Lim BO, Yamada K, Nonaka M, Kuramoto Y, Hung P, Sugano M. Dietary fibers modulate indices of intestinal immune function in rats. J. Nutr. 1997;127:663–667.
- Yamada K, Tokunaga Y, Ikeda A, Ohkura K, Mamiya S, Kaku S, Sugano M, Tachibana H. Dietary effect of guar gum and its partially hydrolyzed product on the lipid metabolism and immune function of Sprague-Dawley rats. Biosci. Biotechnol. Biochem. 1999;63:2163–2167.10.1271/bbb.63.2163
- Yamada K, Tokunaga Y, Ikeda A, Ohkura K, Kaku-Ohkura S, Mamiya S, Lim BO, Tachibana H. Effect of dietary fiber on the lipid metabolism and immune function of aged Sprague-Dawley rats. Biosci. Biotechnol. Biochem. 2003;67:429–433.10.1271/bbb.67.429
- Funahashi H, Imai T, Mase T, Sekiya M, Yokoi K, Hayashi H, Shibata A, Hayashi T, Nishikawa M, Suda N, Hibi Y, Mizuno Y, Tsukamura K, Hayakawa A, Tanuma S. Seaweed prevents breast cancer? Cancer Sci. 2001;92:483–487.10.1111/cas.2001.92.issue-5
- Rocha de Souza MC, Marques CT, Guerra Dore CM, Ferreira da Silva FR, Oliveira Rocha HA, Leite EL. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007;19:153–160.
- Hong SW, Jung KH, Lee HS, Zheng HM, Choi MJ, Lee C, Hong SS. Suppression by fucoidan of liver fibrogenesis via the TGF-β/Smad pathway in protecting against oxidative stress. Biosci. Biotechnol. Biochem. 2011;75:833–840.10.1271/bbb.100599
- Ustyuzhanina NE, Ushakova NA, Zyuzina KA, Bilan MI, Elizarova AL, Somonova OV, Madzhuga AV, Krylov VB, Preobrazhenskaya ME, Usov AI, Kiselevskiy MV, Nifantiev NE. Influence of fucoidans on hemostatic system. Mar. Drugs. 2013;11:2444–2458.10.3390/md11072444
- Zhu C, Cao R, Zhang SX, Man YN. Fucoidan inhibits the growth of hepatocellular carcinoma independent of angiogenesis. Evid. Based Complement. Altern. Med. 2013;2013 ( Article 692549):1–7.
- Azuma K, Ishihara T, Nakamoto H, Amaha T, Osaki T, Tsuka T, Imagawa T, Minami S, Takashima O, Ifuku S, Morimoto M, Saimoto H, Kawamoto H, Okamoto Y. Effects of oral administration of fucoidan extracted from Cladosiphon okamuranus on tumor growth and survival time in a tumor-bearing mouse model. Mar. Drugs. 2012;10:2337–2348.10.3390/md10102337
- Park HS, Hwang HJ, Kim GY, Cha HJ, Kim WJ, Kim ND, Yoo YH, Choi YH. Induction of apoptosis by fucoidan in human leukemia U937 cells through activation of p38 MAPK and modulation of Bcl-2 family. Mar. Drugs. 2013;11:2347–2364.10.3390/md11072347
- Hyun JH, Kim SC, Kang JI, Kim MK, Boo HJ, Kwon JM, Koh YS, Hyun JW, Park DB, Yoo ES, Kang HK. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009;32:1760–1764.10.1248/bpb.32.1760
- Yamasaki Y, Yamasaki M, Tachibana H, Yamada K. Important role of beta1-integrin in fucoidan-induced apoptosis via caspase-8 activation. Biosci. Biotechnol. Biochem. 2012;76:1163–1168.
- Banafa AM, Roshan S, Liu YY, Chen HJ, Chen MJ, Yang GX, He GY. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013;33:717–724.
- Boo HJ, Hong JY, Kim SC, Kang JI, Kim MK, Kim EJ, Hyun JW, Koh YS, Yoo ES, Kwon JM, Kang HK. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar. Drugs. 2013;11:2982–2999.10.3390/md11082982
- Lee JB, Hayashi K, Hashimoto M, Nakano T, Hayashi T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004;52:1091–1094.10.1248/cpb.52.1091
- Negishi H, Mori M, Mori H, Yamori Y. Supplementation of elderly japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013;143:1794–1798.10.3945/jn.113.179036
- Kyung J, Kim D, Park D, Yang YH, Choi EK, Lee SP, Kim TS, Lee YB, Kim YB. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab. Anim. Res. 2012;28:91–97.10.5625/lar.2012.28.2.91
- Maruyama H, Tamauchi H, Hashimoto M, Nakano T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo. 2003;17:245–249.
- Katayama S, Nishio T, Kishimura H, Saeki H. Immunomodulatory properties of highly viscous polysaccharide extract from the gagome alga (Kjellmaniella crassifolia). Plant Foods Hum. Nutr. 2012;67:76–81.
- Hayashi K, Nakano T, Hashimoto M, Kanekiyo K, Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008;8:109–116.10.1016/j.intimp.2007.10.017
