Abstract
Colonization and oxidative metabolism of South African low-rank discard coal by the fungal strain ECCN 84 previously isolated from a coal environment and identified as Neosartorya fischeri was investigated. Results show that waste coal supported fungal growth. Colonization of waste coal particles by N. fischeri ECCN 84 was associated with the formation of compact spherical pellets or sclerotia-like structures. Dissection of the pellets from liquid cultures revealed a nucleus of “engulfed” coal which when analyzed by energy dispersive X-ray spectroscopy showed a time-dependent decline in weight percentage of elemental carbon and an increase in elemental oxygen. Proliferation of peroxisomes in hyphae attached to coal particles and increased extracellular laccase activity occurred after addition of waste coal to cultures of N. fischeri ECCN 84. These results support a role for oxidative enzyme action in the biodegradation of coal and suggest that extracellular laccase is a key component in this process.
Graphical Abstract

Neosartorya fischeri, a species of Deuteromycota (fungi imperfecti) belonging to the family Trichocomaceae, a teleomorph of the Aspergillus fischeri series,Citation1) is saprotrophic and colonizes environment rich in decaying organic material including coal.Citation2−5) Thus, this fungus is capable of colonizing and using a wide range of complex organic substrates which emphasizes its biodegradation potential for use in rehabilitation of recalcitrant materials. Furthermore, the production of heat resistant ascosporesCitation6) indicates that N. fischeri is a robust microbial catalyst and proliferates relatively easily irrespective of prevailing conditions. It is therefore, perhaps not surprising that N. fischeri has been identified as a candidate species in the development of sustainable technologies for coal mine rehabilitation.
Igbinigie et al.Citation3) first reported on the degradation of hard coal by N. fischeri and pointed toward use of this organism for sustainable rehabilitation of solid coal mine waste. More recently, Taewoo et al.Citation7) reported the breakdown of petroleum hydrocarbons by this fungus. The two studies focused on the by-products of biodegradation emphasizing the potential of N. fischeri for exploitation as a catalyst in the development of remedial bioprocess technologies. In a further study, Uribe-Alvarez et al.Citation5) investigated the mineralization of petroleum asphaltenes by N. fischeri and detected the activity of an extracellular oxidative laccase (LAC, E.C. 1.10.3.2). Ligninolytic enzymes like LAC, produced mostly by the Ascomycetes, Basidiomycetes, and Deuteromycetes, have been widely reported to play a role in modifying and degrading the complex structure of substrates such as coal and polyaromatic hydrocarbons.Citation8) In fact, since the initial discovery that fungi possess the ability to breakdown and metabolize coal and polycyclic aromatic hydrocarbons researchers have focused on elucidating the underlying mechanisms. Thus, lignite degradation by Basidiomycota, such as Trametes versicolorCitation9) and Phanerochaete chrysosporiumCitation10) appears to involve increased activity of LAC and the peroxidases: lignin peroxidase (LiP, E.C. 1.11.1.14) and Mn-dependent peroxidase (MnP, E.C. 1.11.1.13).
Industrial application of microbial catalytic mechanisms remains competent and economic due to the ability of microbial systems to perform under ambient temperatures and pressure. However, elucidation and understanding of microbial processes is crucial for their efficient exploitation and for further development of rehabilitation technologies. Fakoussa and FrostCitation9) argue that of the three ligninolytic enzymes involved in coal degradation biological LAC production are more economic and comparable with application of chemically produced agents. This is because LAC requires only molecular oxygen as a cofactor to oxidize a wide range of complex substrates while peroxidases require hydrogen peroxide as a co-substrate. Following its purification from a number of fungal strains,Citation11) application of extracellular LAC was explored in different industrial sectors including the food, textile, pulp, and paper industries as well as in medical, pharmaceutical, and bioremediation fields.Citation12,13) Furthermore, LAC production by fungi is not confined to degradation of complex molecules but is also involved in the synthesis of pigments associated with fungal development such as dihydroxynaphthalene melanins produced in response to environmental stress, formation of fruiting bodies, morphogenesis, detoxification, spore formation, and pathogenesis.Citation13) This intimate association between LAC and fungal physiology highlights the potential of N. fischeri to survive and rejuvenate under harsh environmental conditions which are the characteristic of waste coal dumps.
In the present work, the colonization and oxidative metabolism of South African low-rank coal by N. fischeri strain ECCN84 were investigated by quantifying fungal growth in response to substrate, determining the interaction between this fungal biocatalyst and coal particles, and by screening and monitoring activity of oxidative enzymes and in particular, laccase.
Materials and methods
Materials
All chemicals and reagents, unless stated otherwise, were purchased from Merck (Pty) Ltd., Modderfontein, South Africa. N. fischeri strain ECCN 84 was previously isolated from waste coal dumps,Citation3) maintained on 2.5% potato dextrose agar (PDA), and stored as mycelial plugs (5 × 5 mm) in 50% glycerol (v/v) at −20 °C. Waste coal comprising a mix of low-grade roof coal and discards from the void, following extraction of the high-grade coal seam and with the following characteristics: total organic carbon = 10.3 ± 2.0 mg kg−1, ash content = 55.5 ± 0.3 wt%, and calorific value = 8–10 MJ kg−1, was obtained from coal mines in eMalahleni (Witbank), Mpumalanga Province, South Africa. Aliquots were powdered using a HP-M 100 Pulverizer (HERZOG Maschinenfabrik GmbH Co., Osnabrück, Germany) to yield particles of approximately 0.2–0.5 mm in diameter. Powdered coal was sterilized by freeze thawing using liquid nitrogen (three cycles) to eliminate any in situ microbial activity. Confirmation of sterilization was achieved by monitoring microbial growth after plating serially diluted aliquots of the waste coal, suspended in sterile Milli-Q water, on nutrient agar which was incubated at 30 °C for 48 h.
Fungus cultivation, spore preparation, inoculation, and incubation
N. fischeri strain ECCN 84 was cultured from stock mycelial plugs on PDA at 30 °C which was determined as the optimum temperature for spore formation, established by culturing ECCN 84 on PDA at 15, 30, 35, and 45 °C, and production of spores confirmed by light microscopy (Olympus BX-50 light microscope) after staining with lactophenol blue.
Fungal spores were harvested into suspension by washing mature fungal lawns with sterile phosphate-buffered saline and using the liquid spore suspension for inoculation into basal salts media comprising K2HPO4 (1.71 g); KH2PO4 (1.32 g); NH4Cl (1.26 g); MgSO4.6H2O (0.011 g); CaCl2 (0.02 g), 4 mL trace element mixture, and 60 mL glutamate media (KH2PO4, 12.7 g; NaNO3, 3.0 g; K2HPO4, 3.1 g; MgSO4.7H2O, 0.5 g; KCl, 0.5 g; and glutamate 2.0 g, per L) diluted to 1 L and adjusted to pH 6–6.5. For biodegradation studies in liquid media, sterilized powdered waste coal was added and cultures incubated at 30 °C on a rotary shaker (130 rpm). Fungal growth was allowed to proceed for 20 d with regular monitoring of pH, biomass harvested every 4 d, and dry weight determined after tearing apart the sclerotia-like structures to remove attached coal particles and drying at 50 °C for 24 h.
For plate culture, spores were inoculated on agar prepared using a basal salts medium of KH2PO4 (1.0 g) NH4Cl (1.26 g); MgSO4∙7H2O (0.5 g); CaCl2∙2H2O (0.01 g); yeast extract (0.01 g); CuSO4∙5H2O (0.001 g); Fe2(SO4)3 (0.001 g); MnSO4∙H2O (0.001 g), glucose (0.4%, w/v) per L, and solidified with 1.6% (w/v) agar agar.Citation14) After incubation for 8 d at 30 °C, dry sterile powdered discard coal was added and the plates incubated for 7 d prior to analysis.
Light and scanning electron microscopy and energy dispersive X-ray spectroscopy
The association between the fungal biocatalyst and coal particles was investigated using both light and scanning electron microscopy (SEM) and any metabolic interaction established by energy dispersive X-ray spectroscopy (EDS).
N. fischeri strain ECCN 84 was cultured either in liquid glutamate-containing basal salts media or on agar plates prepared as described above. After addition of sterile waste coal powder, growth was allowed to proceed until sclerotia-like structures were visible. For light microscopy, sclerotia were carefully removed from the culture media, prepared as wet mounts, and examined using an Olympus BX-50 light microscope. For SEM, sclerotia from liquid-glutamate containing basal salts media were collected and fixed using 2.5% buffered glutaraldehyde at 4 °C overnight. The buffered glutaraldehyde was decanted and the samples washed twice with phosphate buffer (0.1 M, pH 7.3) and then, dehydrated using a graded alcohol series at 30, 50, 70, 80, 90%, and absolute alcohol; the specimens dried in hexamethyldisilazane, gold coated, and mounted on metal stubs for examination.Citation15) Samples were examined using a Vega 3 LMU (TESCAN, Brno, Czech Republic) analytical scanning electron microscope at 30 kV.
Elemental analysis of coal particles trapped within fungal mycelia was by EDS using an INCA Penta FET X3 assembly attached to the scanning electron microscope and was carried out at the intervals specified in results. Sample preparation was as described for SEM but without gold coating.
Oxidative enzyme screening and LAC assay
Screening for LAC activity was initially carried out by culturing the fungus ECCN 84 on 2.5% PDA plates with added 2.6-dimethoxyphenol, 2.2′[azino-bis-(3-ethylbonzthiazoline-6-sulphonic acid) diammonium salt] (ABTS) as described by Hofrichter et al.Citation16) The prepared PDA/ABTS (0.02%) and control (without ABTS) plates were inoculated with a 5 × 5 mm mycelial plug and incubated at 30 °C in darkness. The plates were monitored at regular intervals for color change (green) indicating the production of the ABTS cation radical, ABTS•+, the product of LAC activity.Citation17) For the screening of MnP, strain ECCN 84 was sub-cultured on agar plates containing 2.5% PDA and increasing concentrations of MnCl2 (250, 500, 1000, and 2000 μg/mL) and incubated in darkness at 30 °C. Formation of a black precipitate (MnO2) surrounding the fungal colony was taken to indicate oxidation of Mn2+ to Mn3+.
For analysis of extracellular LAC activity, culture filtrates of ECCN 84 growing in glutamate supplemented basal salt medium with and without adding coal or glucose were analyzed for enzyme activity by measuring the change in absorbance of ABTS at 420 nm using the molar extinction coefficient of 36.0 mM−1 cm−1 as described by Li et al.Citation18) Heat-inactivated (100 °C × 10 min) filtrates were similarly analyzed. Reaction mixtures consisted of a 1.5 mL aliquot of culture filtrate added to 1.5 mL sodium acetate buffer (1 mM, pH5) with 1.5 mL ABTS (0.5 mM) serving as substrate for enzyme catalyzed oxidation. Absorbance of the reaction mixture was monitored at intervals (1 min) over a period of 25 min. A plot of absorbance vs. time was used to derive the slope and enzyme activity expressed as nmol min−1 mL−1, calculated by multiplying the slope by the molar extinction coefficient of ABTS.
Statistical analysis
All data were processed using Sigma Plot version 11.2 (SPSS Inc., Chicago, IL). Lines of best fit were computed using the statistical function of Sigma Plot following non-linear regression using one-way analysis of variance and the Shapiro–Wilks normality test (P < 0.05). Data are presented as the mean of at least three determinations ± standard deviation (SD).
Results and discussion
Fig. shows the growth response of N. fischeri strain ECCN 84 in liquid culture either in basal salts medium or basal salts medium supplemented with glutamate. Fungal spores germinated and produced mycelial pellets or sclerotia-like structures in agitated basal salts media and in glutamate supplemented media after several days’ incubation at 30 °C. However, biomass accumulation was greater in the glutamate supplemented medium (Fig. ). Mean dry weight of fungal biomass harvested from glutamate supplemented basal salts medium with added waste coal appeared to be proportional to the amount of substrate added (Table ). Thus, cultures of N. fischeri strain ECCN 84 supplied 0.1 g of waste coal produced one and a half times the dry weight of biomass than cultures given 0.05 g indicative of a classical dose-response effect. Furthermore, formation of pellets or sclerotia-like structures after addition of waste coal substrate suggested active engulfment of the coal particle by fungal mycelia. Since entrapment of coal particles has been observed previouslyCitation19) but not analyzed in any detail, it seemed pertinent to examine this phenomenon in N. fischeri using both light and SEM.
Fig. 1. Growth curves of N. fischeri strain ECCN 84 in liquid culture.
Notes: Spores were inoculated into either basal salt medium or glutamate supplemented basal salt medium (GluM) containing sterile powdered waste coal and incubated for 20 d at 30 °C. At the specified intervals, biomass accumulation was determined after drying at 50 °C for 24 h. Data are the mean of at least three determinations ± SD.
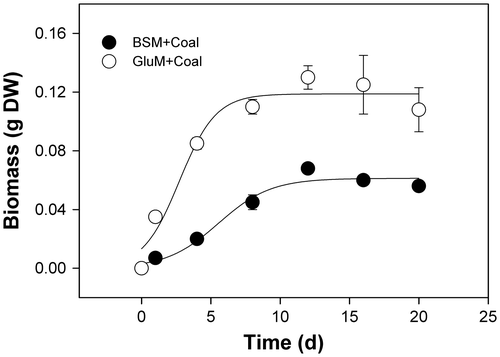
Table 1. Effect of substrate on accumulation of biomass by N. fischeri strain ECCN 84 cultured in glutamate supplemented basal salts media with either glucose (0.05 g) or coal (0.05 and 0.1 g). Data are the mean ± SD of three determinations.
Spores of N. fischeri strain ECCN 84 were inoculated into glutamate-containing basal salts media or on agar plates and cultivated for 8 and 1 d, respectively, prior to enrichment with sterile waste coal powder.
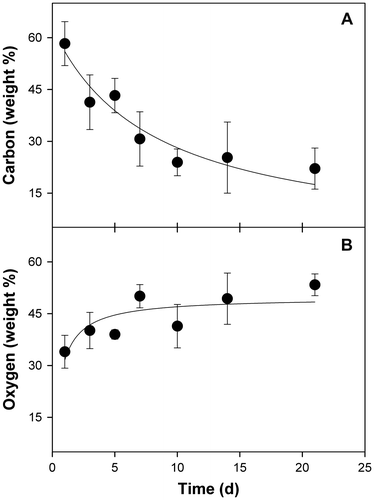
In liquid culture, spores of ECCN 84 germinated and grew to form compact spherical pellets or sclerotia-like structures (Fig. (A)). As described by SinghCitation20), fungi in liquid culture form clumps from loose hyphal aggregates, while spherical pellets form by aggregation of fungal spores, either individually or in clumps, which results in subsequent aggregation of fungal hyphae upon spore germination into non-coagulating pellets. Dissection of these individual pellets revealed some “engulfed” coal forming a nucleus at the centre (arrows, Fig. (A)) while SEM revealed coal particles entrapped by fungal mycelia (Fig. (B) and (C)). In situ elemental analysis of the coal particles trapped within fungal mycelia by EDS was carried out on 1, 3, 5, 7, 14, and 21 d after addition of coal substrate. Results showed a time-dependent decline in weight percentage of elemental carbon within the coal particles entrapped by fungal mycelia (Fig. (A)) which occurred concomitant with an increase in weight percentage of elemental oxygen (Fig. (B)). Together, these results provide strong circumstantial evidence for a direct interaction between strain ECCN 84 and waste coal in which the fungus not only colonizes and is attached to the coal particles but is able to extract elemental carbon by oxidative metabolism to sustain growth and proliferation.
Fig. 2. Entrapment of coal particles by N. fischeri strain ECCN 84 cultured in liquid media containing basal salts medium and waste coal.
Notes: (A) Light micrograph of a section through a fungal pellet (sclerotia) showing mycelial entrapment of coal particles (arrows); ((B) and (C)) scanning electron micrographs of hyphae engulfing and ramifying coal particles embedded within the fungal mycelial pellet.
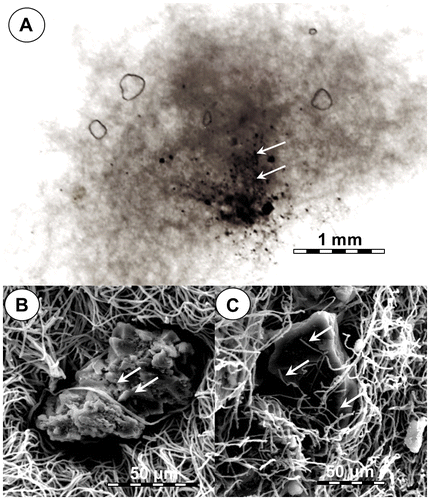
Fig. 3. Change in elemental carbon and oxygen content of coal particles engulfed by fungal mycelia within sclerotia-like structures.
Notes: Spores of N. fischeri strain ECCN 84 were inoculated in glutamate medium with added coal, biomass harvested after 21 d at 30 °C, and analyzed by EDS as described in Materials and methods. Data are the mean of at least three determinations ± SD.
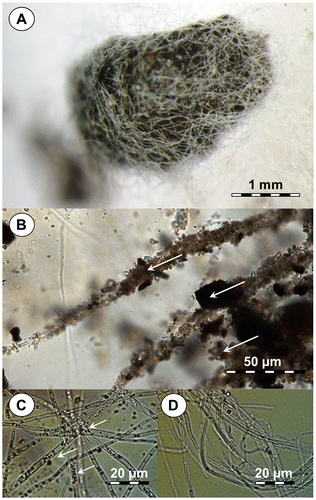
When coal particles were added to lawns of strain ECCN 84 growing on solidified basal salt media fungal hyphae covered the coal particles in a mycelial structure reminiscent of sclerotia (Fig. (A)). Light microscope examination of wet mounts of coal covered by fungal mycelium showed hyphae attached to coal particles (arrows, Fig. (B)) and, which on closer scrutiny, revealed the presences of organelles presumably peroxisomes (arrows, Fig. (C)). These organelles were absent in hyphae from N. fischeri strain ECCN 84 cultivated on medium without added coal (Fig. (D)). Typically, peroxisomes are associated with the oxidation of compounds such as fatty acids using naturally occurring peroxide, typically hydrogen peroxide (H2O2), which is reduced to water. In fungi, these organelles are known to be important for carbon source utilization, pathogenesis, development, and secondary metabolism.Citation21,22)
Fig. 4. Light micrographs of N. fischeri strain ECCN 84 cultured as a lawn on agar containing basal salts medium illustrating attachment to coal particles.
Notes: (A) Typical mycelial pellet or sclerotia-like structure formed after addition of sterile powdered waste coal; (B) attachment of hyphae to coal particles within the mycelial pellet or sclerotia-like structures; (C) high-resolution image showing the presence of peroxisome-like organelles in hyphae from cultures supplied waste coal; and (D) hyphae from cultures in the absence of waste coal substrate lacking peroxisome-like organelles.
Several studies have indicated that coal degrading fungi are able to accomplish the breakdown of this substrate by synthesizing a suite of ligninolytic enzymes and in particular, enzymes those catalyze peroxidation reactions. Chief amongst these enzymes is LiP, MnP, and the phenol oxidase, LAC.Citation23–29) Preliminary screening by the culturing of strain ECCN 84 on PDA plates in the presence and absence of substrates for MnP (MnCl2) and LAC (ABTS) indicated a positive response for LAC activity only (data not shown). Consequently, experiments were carried out to confirm the presence of extracellular LAC activity in culture filtrates of ECCN 84 and to determine whether this strain responded to the addition of waste coal by increasing activity of LAC and the results are presented in Fig. .
Fig. 5. Waste coal-induced changes in extracellular LAC activity in culture filtrates from N. fischeri strain ECCN 84.
Notes: Filtrates were assayed for extracellular LAC activity by monitoring the oxidation of ABTS spectrophotometrically at 420 nm and data represent the mean ± SD of three experiments.
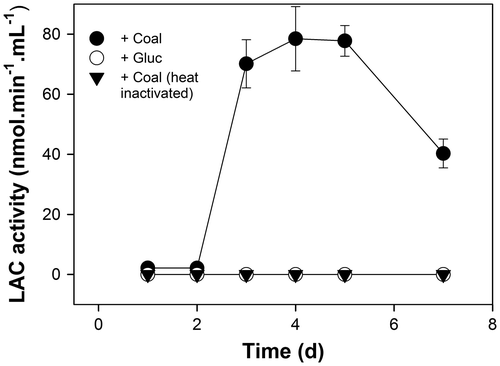
Extracellular LAC activity increased to a maximum within 5 d following addition of waste coal to liquid cultures of ECCN 84 (Fig. ). No induction of LAC activity was evident in filtrates from cultures in which an equal weight of glucose was substituted for waste coal as substrate and, LAC activity was not detectable in heat-inactivated filtrates from cultures supplied waste coal as substrate confirming that the activity detected was the result of an enzyme catalyzed reaction. These results together with the recent cloning and characterization of a β-glucosidase from N. fischeri which catalyses the hydrolysis of ρ-nitrophenyl-β-D-glucopyranoside and flavone compounds with high levels of catalytic activity,Citation30) coupled with the cloning, and sequencing of an endo-1,3(4)-β-glucanase from N. fischeri NRRL 181Citation31) illustrate the potential and versatility of this biocatalyst to degrade recalcitrant materials and in particular waste coal.
In conclusion, this work has demonstrated for the first time, the induction of an extracellular LAC in N. fischeri by coal. This coupled with EDS analysis which showed a decline in carbon and concomitant increase in elemental oxygen content, supports fungal-mediated oxidative metabolism of waste coal. In addition to searching for other oxidase enzymes that might be involved in coal biodegradation by N. fischeri, effort is also focused on the purification and characterization of the LAC activity detected in this study. Thus, further research will aim to elucidate the role of LAC and other oxidases and hydrolases as a part of the mechanism of biodegradation of coal by N. fischeri.
Funding
This research was supported by a grant from Anglo American Thermal Coal and the National Research Foundation [IFR1202220169, grant number 80879]. Ms. Lerato M. Sekhohola acknowledges financial support from Anglo American Thermal Coal in the form of a doctoral bursary.
Notes
Abbreviations: ABTS, 2.6-dimethoxyphenol, 2.2′ [azino-bis-(3-ethylbonzthiazoline-6-sulphonic acid) diammonium salt]; EDS, energy dispersive X-ray spectroscopy; LAC, laccase; LiP, lignin peroxidase; MnP, Mn-dependent peroxidase; PBS, phosphate buffered saline; PDA, potato dextrose agar; SEM, scanning electron microscopy.
References
- Varga J, Vida Z, Toth B, Debets F, Horie Y. Phylogenetic analysis of newly described Neosartorya species. Antonie van Leeuwenhoek. 2000;77:235–239.
- Girardin H, Monod M, Latgé JP. Molecular characterization of a food-borne fungus Neosartorya fischeri (Malloch and Cain). Appl. Environ. Microbiol. 1995;61:1378–1383.
- Igbinigie EE, Atkins S, van Breugel Y, van Dyke S, Davies-Coleman MT, Rose PD. Fungal biodegradation of hard coal by a newly reported isolate, Neosartorya fischeri. Biotechnol. J. 2008;3:1407–1416.
- Hong SB, Kim DH, Park I, Samson RA, Shin HD. Isolation and identification of Aspergillus section Fumigati strains from arable soil in Korea. Microbiol. 2010;38:1–6.
- Uribe-Alvarez C, Ayala M, Perezgasga L, Naranjo L, Urbina H, Vazguez-Duhalt R. First evidence of mineralization of petroleum asphaltenes by a strain of Neosartorya fischeri. Microbiol. Biotechnol. 2011;4:663–672.
- Amaeze NJ, Ugwuanyi JO, Obeta JAN. Studies of heat-resistant fungi in the soil: Talaromyces flavus isolated in Nigerian soils. N.Y. Sci. J. 2010;3:8–14.
- Taewoo Y, Eun-Hee L, Hyerim P, Kyung-Suk C. Biodegradation of petroleum hydrocarbons by Neosartorya sp. BL4. J. Environ. Sci. Health. Part A Environ. Sci. Health Part A Environ. Sci. Eng. 2011;46:1763–1768.
- Sekhohola LM, Igbinigie EE, Cowan AK. Biological degradation and solubilisation of coal. Biodegradation. 2013;24:305–318.
- Fakoussa RM, Frost PJ. In vivo decolorization of coal-derived humic acids by laccase-excreting fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1999;52:60–65.
- Ralph JP, Catcheside DEA. Transformations of low rank coal by Phanerochaete chrysosporium and other wood-rot fungi. Fuel Process. Technol. 1997;52:79–93.
- Garcia TA, Santiago MF, Ulhoa CJ. Studies on the Pycnoporus sanguineus CCT-4518 laccase purified by hydrophobic interaction chromatography. Appl. Microbiol. Biotechnol. 2007;75:311–318.
- Maciel MJM, Silva AC, Ribeiro HCT. Industrial and biotechnological applications of ligninolytic enzymes of the Basidiomycota: a review. J. Biotechnol. 2010;13:1–13.
- Dwivedi UN, Singh P, Pandey VP, Kumar A. Structure-function relationship among bacterial, fungal and plant laccases. J. Mol. Catal. B: Enzym. 2011;68:117–128.
- Pointing SB. Qualitative methods for the determination of lignocellulotic enzyme production by tropical fungi. Fungal Diversity. 1999;2:17–33.
- Weber WJ, Pirbazari M, Melson GL. Biological growth on activated carbon: an investigation by scanning electron microscopy. Am. Chem. Soc. 1978;127:817–819.
- Hofrichter M, Bublitz F, Fritsche W. Fungal attack on coal l. Modification of hard coal by fungi. Fuel Process. Technol. 1997;52:43–53.
- Steffen KT, Hofrichter M, Hatakka A. Mineralisation of 14C-labelled synthetic lignin and ligninolytic enzyme activities of litter decomposing basidiomycetous fungi. Appl. Microbiol. Biotechnol. 2000;54:819–825.
- Li A, Zhu Y, Xu L, Zhu W, Tian X. Comparative study on the determination of assay for laccase of Trametes sp. Afr. J. Biochem. Res. 2008;2:181–183.
- Haider R, Ghauri MA, SanFilipo JR, Jones EJ, Orem WH, Tatu CA, Akhtar K, Akhtar N. Fungal degradation of coal as a pretreatment for production of methane. Fuel. 2013;104:717–725.
- Singh H. Mycoremediation: fungal bioremediation. John Wiley & Sons Inc., Hoboken, New Jersey; 2006. p. 1–29.10.1002/0470050594
- Gabaldon T. Peroxisome diversity and evolution. Philos. Trans. R. Soc. B: Biol. Sci. 2010;365:765–773.
- Hynes MJ, Murray SL, Khew GS, Davis MA. Genetic analysis of the role of peroxisomes in the utilization of acetate and fatty acids in Aspergillus nidulans. Genetics. 2008;178:1355–1369.
- Bartoszewska M, Opalinski L, Veenhuis M, Klei I. The significance of peroxisomes in secondary metabolite biosynthesis in filamentous fungi. Biotech. Lett. 2011;33:1921–1931.
- Hatakka A. Ligin modifying enzymes from selected white rot fungi: production and role from lignin degradation. FEMS Microbiol. Rev. 1994;13:125–135.
- Thurston CF. The structure and function of fungal laccases. Microbiol. 1994;140:19–26.
- Silva-Stenico ME, Vengadajellum CJ, Janjua HA, Harrison STL, Burton SG, Cowan DA. Degradation of low rank coal by Trichoderma atroviride ES11. J. Ind. Microbiol. Biotechnol. 2007;34:19–26.
- Mot AC, Silaghi-Dumitrescu R. Laccases: complex architectures for one-electron oxidation. Biochem. (Moscow). 2012;77:1395–1407.
- Yadav M, Singh SK, Yadava S. Purification, characterisation and coal depolymerisation activity of lignin peroxidase from Lenzitus betulina MTCC-1183. Appl. Biochem. Microbiol. 2012;48:583–589.
- Shevchenko EA, Bessolitsyna EA, Darmov IV. Identification of genes encoding ligninolytic enzymes in naturally occurring basidiomycete isolates. Appl. Biochem. Microbiol. 2013;49:280–286.
- Ramachandrana P, Tiwari MK, Singh RK, Haw J-R, Jeya M, Lee J-K. Cloning and characterization of a putative β-glucosidase (NfBGL595) from Neosartorya fischeri. Process Biochem. 2012;47:99–105.
- Hua C, Yan Q, Jiang Z, Li Y, Katrolia P. High-level expression of a specific β-1,3-1,4-glucanase from the thermophilic fugus Paecilomyces thermophila in Pichia pastoris. Appl. Microbiol. Biotechnol. 2010;88:509–518.
