Abstract
The antioxidative properties of ascorbigen, one of the major indole-derived compounds of Brassica vegetables, were systematically evaluated using multiple assay systems with comparison to the well-known antioxidants ascorbic acid and Trolox. We first performed assays using model radicals, DPPH radical, galvinoxyl radical, and ABTS radical cation (ABTS•+). Ascorbigen showed stronger activity than that of ascorbic acid in the ABTS•+-scavenging assay but showed no activity in the DPPH radical- and galvinoxyl radical-scavenging assays. In the ABTS•+-scavenging assay, the indole moiety of ascorbigen contributed to scavenging of the radicals to produce indole-3-aldehyde as one of the final reaction products. The activity of ascorbigen was then evaluated by an oxygen radical absorbance capacity assay and an oxidative hemolysis inhibition assay using physiologically relevant peroxyl radicals, AAPH-derived radicals. Ascorbigen showed much stronger antioxidant activity than did ascorbic acid and Trolox. Therefore, antioxidant activity of ascorbigen might be more beneficial than has been thought for daily health care.
Graphical Abstract
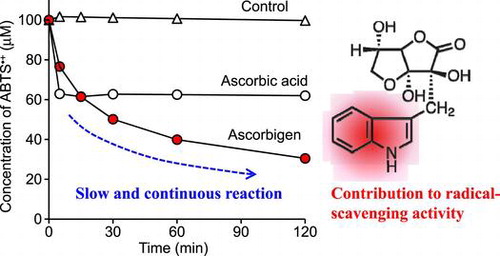
Ascorbigen slowly and continuously reacted with ABTS radical cations over the experimental period and showed stronger activity than that of ascorbic acid.
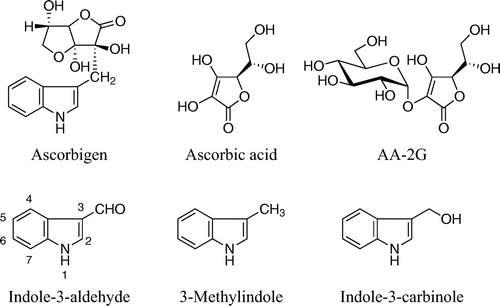
Ascorbigen is formed from 3-indolylmethylisothiocyanate (or indole-3-carbinole) and ascorbic acid in Brassica vegetables including cabbage, broccoli, cauliflower, and Chinese cabbage.Citation1−4) Ascorbigen is not present in intact plant tissues. Its formation takes two consecutive steps: the first step is enzymatic hydrolysis of glucobrassicin by myrosinase and the second step is a spontaneous reaction of emerging intermediates, 3-indolylmethylisothiocyanate and indole-3-carbinole, with ascorbic acid. The biological activity of ascorbigen has been described in numerous publications. Ascorbigen has been shown to exhibit antiscorbutic activity by releasing ascorbic acid under physiological conditions.Citation5,6) Thus, ascorbigen plays a role as a source of ascorbic acid in vivo. However, only a part of ascorbic acid bound in ascorbigen can be utilized as free ascorbic acid. Ascorbigen has also been shown to exhibit anticarcinogenic, immunomodulating, skin-protective, and antioxidative effects.Citation3,7−9) However, results of studies on the antioxidative effect of ascorbigen are not consistent. Ge et al. reported that ascorbigen inhibited copper-mediated oxidation of LDL in a concentration-dependent manner, suggesting that ascorbigen can act as an antioxidant in vitro.Citation8) Wagner et al. reported that ascorbigen did not scavenge superoxide anion-free radicals and showed little scavenging activity toward DPPH radical and ABTS radical cation (ABTS•+) and that ascorbigen prevented tert-buthylhydroperoxide-induced cytotoxicity by inhibition of lipid peroxidation in cultured human keratinocytes.Citation9) These findings indicate that ascorbigen effectively prevented tert-buthylhydroperoxide cytotoxicity in cultured cells, though it seems that ascorbigen does not play a potent role as a free radical scavenger in vitro. There seems to be a discrepancy between results showing that ascorbigen had no or little radical-scavenging activity against several radical species and results showing that ascorbigen protected biomolecules from damage caused by free radicals. We speculate that scavenging free radicals contribute to the prevention of oxidative injury to biomolecules. Therefore, it is necessary to further investigate what kind of antioxidative property is exhibited by ascorbigen.
It has been reported that there are two types of antioxidants that scavenge radicals quickly and quench many radicals, and it has been proposed that reactivity should be assessed on the basis of both reaction rate and stoichiometry and that multiple methods should be used since the activities of some antioxidants vary depending on the assay method.Citation10−13) We have assessed antioxidant activities with attention to the above-mentioned proposals and points of view. We found that 2-O-α-d-glucopyranosyl-l-ascorbic acid (AA-2G), a stable ascorbic acid derivative, exerted radical-scavenging activity toward unnatural model radicals, including DPPH radicalCitation14−17) and ABTS•+.Citation16,18) The chemical properties of AA-2G as a radical scavenger were greatly different from those of ascorbic acid, in that the reaction rate with these model radicals of AA-2G was much slower than that of ascorbic acid, but the long-term radical scavenging ability per molecule of AA-2G was superior to that of ascorbic acid. We also found using a cell-based antioxidant assay system, oxidative hemolysis inhibition assay (OxHLIA), that the radical-scavenging activity of AA-2G was biologically relevant.Citation19) Recently, we reassessed the antioxidative activity of arbutin using five in vitro assay systems, although arbutin has been reported to possess weak antioxidant activity compared to that of its precursor, hydroquinone.Citation20) We found that arbutin exerted strong antioxidant activity comparable or even superior to that of hydroquinone in an ABTS•+-scavenging assay, an oxygen radical absorbance capacity (ORAC) assay, and two cell-based antioxidant assays. More recently, we systematically evaluated the antioxidant activity of vanillin and ethyl vanillin using multiple assay systems.Citation21,22) Vanillin and ethyl vanillin showed strong activity in the ABTS•+-scavenging assay but showed no activity in the DPPH radical- and galvinoxyl radical-scavenging assays. Vanillin and ethyl vanillin also showed much stronger antioxidant activity than that of Trolox in the ORAC assay and OxHLIA. Oral administration of vanillin and ethyl vanillin to mice increased the antioxidant activity in plasma.
Hence, in this study, we systematically reassessed the antioxidant properties of ascorbigen using multiple assay systems. We first performed assays using model radicals, DPPH radical, galvinoxyl radical, and ABTS•+. We then evaluated the antioxidant activity of ascorbigen by the ORAC assay and OxHLIA using physiologically relevant peroxyl radicals. We also identified a key product for the antioxidant activity of ascorbigen in the reaction with ABTS•+ and AAPH.
Materials and methods
Chemicals
Ascorbigen was purchased from Designed Nutritional Products (Orem, UT). Ascorbic acid and 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH) were from Wako Pure Chemical Industries (Osaka, Japan). 2-O-α-d-Glucopyranosyl-l-ascorbic acid (AA-2G) was a gift from Hayashibara Biochemical Laboratories, Inc. (Okayama, Japan). Galvinoxyl-free radical, indole-3-aldehyde, and 3-methylindole were from Tokyo Chemical Industry (Tokyo, Japan). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), H2O2 (3%), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were from Aldrich Chemical (Milwaukee, WI). 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), horseradish peroxidase (HRP; type VI-A, essentially a salt-free 1,310-units/mg solid), and sodium fluorescein were from Sigma Chemical (St. Louis, MO). Sheep erythrocytes were from Nippon Bio-Supp. Center (Tokyo, Japan). Reagents were used without further purification. All waters used were of Milli-Q grade.
DPPH radical-scavenging assay
DPPH radical-scavenging activities were assessed as described previously.Citation16) Briefly, DPPH radical (100 μM) was mixed with an antioxidant (20 μM) in 60% ethanol/40% citric acid-sodium citrate buffer (10 mM, pH 6). The reaction was carried out at room temperature for 2 h. The decrease in DPPH radical concentration was monitored by measuring the absorbance at 524 nm with a spectrophotometer (Hitachi U-1900, Tokyo, Japan).
Galvinoxyl radical-scavenging assay
Galvinoxyl radical-scavenging activities were assessed by a modification of a previous method.Citation16) Briefly, galvinoxyl radical (10 μM) was mixed with an antioxidant (2 μM) in 60% ethanol/40% citric acid–sodium citrate buffer (10 mM, pH 6). The reaction was carried out at room temperature for 2 h. The decrease in galvinoxyl radical concentration was monitored by measuring the absorbance at 432 nm with a spectrophotometer.
ABTS•+-scavenging assay
ABTS•+-scavenging activities were assessed as described previously.Citation18) Briefly, ABTS•+ (100 μM) generated with an ABTS/H2O2/HRP system was mixed with an antioxidant (20 μM) in citric acid–sodium citrate buffer (50 mM, pH 6). The reaction was carried out at room temperature for 2 h. The decrease in ABTS•+ concentration was monitored by measuring the absorbance at 730 nm with a spectrophotometer.
HPLC analysis of the reaction mixture of ascorbigen with ABTS•+
Ascorbigen (200 μM) was reacted with ABTS•+ (1.0 mM) generated with an ABTS/H2O2/HRP system in citric acid-sodium citrate buffer (50 mM, pH 6). The mixture (1 mL) was allowed to stand for 2 h at room temperature. An aliquot of the reaction mixture was periodically withdrawn and directly subjected to HPLC analysis. The HPLC analyses were carried out with a system consisting of a Shimadzu LC-10AD pump, SPD-10AV UV–vis spectrophotometric detector, CTO-6A column oven, and C-R7A chromatopac. The separation of ascorbigen and reaction products was achieved by isocratic elution from an Inertsil Ph-3 column (ϕ 4.6 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan) kept at 40 °C with methanol/H2O (45:55, v/v) at a flow rate of 0.7 mL/min. The absorbance at 280 nm was monitored.
Purification and structural determination of the reaction product of ascorbigen with ABTS•+
Ascorbigen (122 mg, 0.4 mmol) was dissolved in 900 mL of 50 mM citric acid–sodium citrate buffer (pH 6) and then mixed with 100 mL of 10 mM ABTS•+ (1.0 mmol). The solution was reacted for 1 h at 30 °C. The reaction mixture was chromatographed on a Diaion HP20 column (ϕ 4.2 × 36 cm, Mitsubishi Chemical, Tokyo, Japan) and eluted stepwise with water and 20, 40, 60, and 80% (v/v) aqueous acetone. The 60% acetone eluate was concentrated to dryness. The residue was dissolved in methanol/H2O (45/55, v/v) and was then repeatedly purified by semi-preparative HPLC to yield a reaction product (4.2 mg) under the following conditions: column, Inertsil Ph-3 (ϕ 10 × 250 mm, 5 μm, GL Sciences); column temperature, 40 °C; solvent, 45% methanol/H2O; flow rate, 4.73 mL/min; detection wavelength, 280 nm. NMR spectra were recorded on a Varian NMR System 600 MHz instrument. Electron spray ionization (ESI) high-resolution mass spectra were obtained on a Bruker Daltonics MicrOTOF II instrument using direct sample injection. 1H NMR (600 MHz, CD3OD) δH: 7.28 (1H, dd, J = 7.2, 7.8 Hz, H-5), 7.33 (1H, dd, J = 7.2, 8.4 Hz, H-6), 7.52 (2H, d, J = 8.4 Hz, H-7), 8.14 (1H, s, H-2), 8.20 (1H, d, J = 7.8 Hz, H-4), 9.93 (1H, s, CHO). 13C NMR (150 MHz, CD3OD) δC: 113.1 (C-7), 120.1 (C-3), 122.4 (C-4), 123.6 (C-5), 125.0 (C-6), 125.7 (C-9), 138.9 (C-8), 139.7 (C-2), 187.4 (CHO). ESI-HRMS m/z [M-H]−: Calcd. for C9H6NO: 144.0455, Found: 144.0456.
ORAC assay
ORAC assay was carried out as described previously.Citation23) Briefly, fluorescein (60 nM), an antioxidant (10 μM), and AAPH (18.75 mM) were incubated in 200 μL of KH2PO4-K2HPO4 buffer (75 mM, pH 7.4) containing 1% ethanol at 37 °C in a 96-well plate. Fluorescence (Ex, 485 nm; Em, 520 nm) was monitored every 2 min for 90 min by Varioskan Flash (Thermo Fisher Scientific, Waltham, MA).
Oxidative hemolysis inhibition assay
OxHLIA was carried out as described in a previous paper.Citation24) Briefly, sheep erythrocytes (50 μL) suspended at a concentration of 2.8% (v/v) in phosphate-buffered saline (PBS) and antioxidant solution (100 μL, 50 μM in PBS) or blank (100 μL, PBS) were added to a flat-bottom 96-well plate (Fukae Kasei, Kobe, Japan). The plate was pre-incubated with a lid at 37 °C in an air-heating shaking-incubator (M·BR-022, TAITEC, Saitama, Japan) with shaking at 1200 rpm for 10 min. Then AAPH (50 μL, 160 mM in PBS) was added to initiate the assay. The plate was promptly sealed with a sealing film and incubated at 37 °C in the incubator with shaking at 1200 rpm. The optical density at 650 nm was measured every 10 min by a microplate reader (Multiskan FC, Thermo Fisher Scientific). The percentage of erythrocytes that remained intact was calculated as follows:
where ODt is the optical density of the sample at t min, OD0 is the optical density of the sample at 0 min, and OD0,CH is the optical density of the complete hemolysis at 0 min.
HPLC analysis of the reaction mixture of ascorbigen with AAPH
Ascorbigen (200 μM) was reacted with AAPH (40 mM) in PBS. The mixture (1 mL) was placed in an air-heating shaking-incubator and was stirred at 1200 rpm for 2 h at 37 °C. An aliquot of the reaction mixture was periodically withdrawn and directly subjected to HPLC analysis. The separation of ascorbigen and reaction products was achieved by isocratic elution from an Inertsil Ph-3 column (ϕ 4.6 × 250 mm, 5 μm, GL Sciences) kept at 40 °C with methanol/H2O (45:55, v/v) at a flow rate of 0.7 mL/min. The absorbance at 280 nm was monitored.
Results
DPPH radical-, galvinoxyl radical-, and ABTS•+-scavenging activities
DPPH radical, galvinoxyl radical, and ABTS•+ are relatively stable radicals commonly used in antioxidant assays. Their characteristic absorption maxima in the visible region disappear when they are quenched, and so the decrease of these radicals can be easily monitored by a spectrometer.Citation25,26) We assessed the DPPH radical-, galvinoxyl radical-, and ABTS•+-scavenging activities of ascorbigen in buffered solutions at pH 6 for 120 min and compared them with those of ascorbic acid and 2-O-α-d-glucopyranosyl-l-ascorbic acid (AA-2G). The chemical properties of AA-2G as a radical scavenger were greatly different from those of ascorbic acid, in that the reaction rate with these model radicals of AA-2G was much slower than that of ascorbic acid, but the long-term radical scavenging ability per molecule of AA-2G was superior to that of ascorbic acid.Citation14−18) The chemical structures of these compounds are shown in Fig. . Ascorbic acid showed almost the same reaction profiles in the three assays (Fig. ), i.e. 2 and 20 μM of ascorbic acid rapidly quenched about 4 μM of galvinoxyl radical and about 40 μM of DPPH radical and ABTS•+ within 5 min, respectively. Thus, their reaction stoichiometries (number of radical molecules reduced by one molecule of antioxidant) were about 2. This is consistent with results of our previous studies.Citation16,21) Ascorbigen and AA-2G did not scavenge any DPPH radical or galvinoxyl radical (Fig. (A), (B)). In the ABTS•+ assay, ascorbigen as well as AA-2G showed significant radical-scavenging activity (Fig. (C)). The reaction between ascorbigen and ABTS•+ proceeded slowly and continuously for 120 min. The reaction profile of ascorbigen to ABTS•+ was almost the same as that of AA-2G. Ascorbigen (20 μM) had quenched about 40 μM of ABTS•+ at 15 min and about 70 μM at 120 min. Thus, their reaction stoichiometries were about 2 at 15 min and about 3.5 at 120 min. These results indicated that ascorbigen showed no activity in the DPPH radical- and galvinoxyl radical-scavenging assays but showed stronger activity than that of ascorbic acid in the ABTS•+-scavenging assay.
Fig. 2. Time courses of DPPH radical (A)-, galvinoxyl radical (B)-, and ABTS•+ (C)-scavenging reactions of ascorbigen, ascorbic acid, and AA-2G.
), ascorbic acid (○), and AA-2G (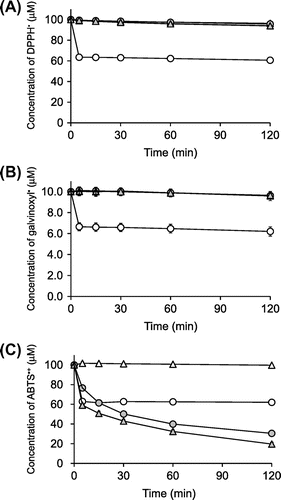
Reaction product of ascorbigen with ABTS•+
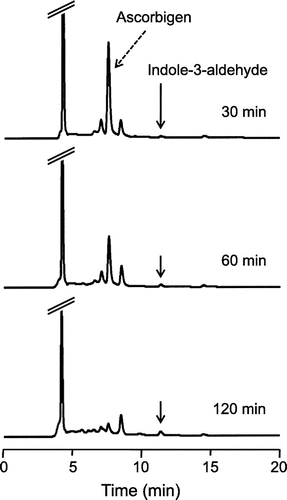
Ascorbigen slowly and continuously reacted with ABTS•+ over a period of 120 min (Fig. (C)). To investigate characteristics of the reaction, reaction mixtures of ascorbigen with ABTS•+ were analyzed by HPLC. The HPLC profiles of the reaction mixture of ascorbigen (200 μM) and ABTS•+ (1 mM) in citrate buffer (pH 6) at 30, 60, and 120 min of reaction are shown in Fig. . At 30 min, peak of a major reaction product, besides ascorbigen, was found. The peak of ascorbigen decreased with time, whereas the peak of the reaction product slowly increased. These results suggest that ascorbigen scavenged ABTS•+ to generate a reaction product during the whole experimental period.
Fig. 3. HPLC chromatograms of the reaction product of ascorbigen with ABTS•+.
Notes: Ascorbigen (200 μM) and ABTS•+ (1.0 mM) were incubated in citrate buffer (50 mM, pH 6). At 30, 60, and 120 min, aliquots of the reaction mixture were withdrawn and analyzed by HPLC.
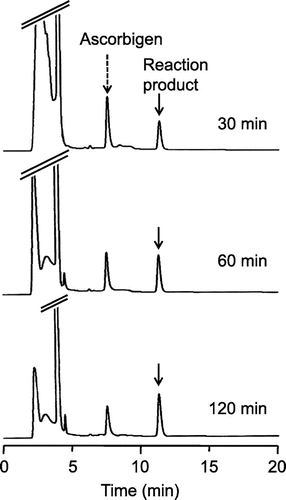
Clarification of the reaction product should show why the radical-scavenging activity of ascorbigen was higher than that of ascorbic acid at 120 min of reaction. We tried to isolate the reaction product from the reaction mixture of ascorbigen (0.4 mmol) and ABTS•+ (1.0 mmol) in citrate buffer (pH 6). The reaction mixture was chromatographed on a Diaion HP20 column. The 60% acetone eluate was concentrated to dryness and was then repeatedly purified by semi-preparative HPLC to yield a reaction product. The reaction product was fully characterized and identified to be indole-3-aldehyde by the mass spectrum and several NMR spectra (1H, 13C, HSQC, and HMBC) (Fig. ).
The formation of indole-3-aldehyde in the reaction mixture suggested that the indole moiety of ascorbigen contributed to the radical-scavenging activity. 3-Methylindole (Fig. ), an indole derivative without an ascorbic acid moiety, was used for comparison with ascorbigen, indole-3-aldehyde, and ascorbic acid. 3-Methylindole as well as ascorbigen showed significant radical-scavenging activity compared to that of ascorbic acid (Fig. ). The reaction between 3-methylindole and ABTS•+ proceeded slowly and continuously for 120 min. The reaction profile of 3-methylindole to ABTS•+ was almost the same as that of ascorbigen. The HPLC profile of the reaction mixture of 3-methylindole (200 μM) and ABTS•+ (1.0 mM) in citrate buffer (pH 6) containing 0.2% ethanol for 120 min showed the formation of indole-3-aldehyde as the major reaction product (data not shown). In contrast, indole-3-aldehyde scavenged very little ABTS•+.
Fig. 4. Time courses of ABTS•+-scavenging reactions of ascorbigen, 3-methylindole, indole-3-aldehyde, and ascorbic acid.
), ascorbic acid (○), 3-methylindole (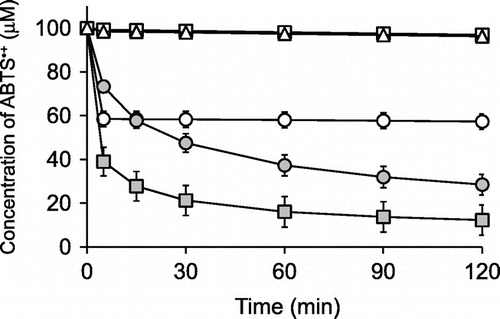
ORAC assay and OxHLIA
Ascorbigen showed stronger activity than that of ascorbic acid in the ABTS•+-scavenging assay but showed no activity in the DPPH radical- and galvinoxyl radical-scavenging assays. This discrepancy led us to assess the antioxidant efficacy of ascorbigen in more physiologically relevant assay systems, because DPPH radical, galvinoxyl radical and ABTS•+ are unnatural radical species that do not exist in the human body. The ORAC assay utilizes an AAPH-derived peroxyl radical, which mimics lipid peroxyl radicals involved in lipid peroxidation chain reaction in vivo. Inhibition of peroxyl radical-induced oxidations of a fluorescent probe, fluorescein, by antioxidants is serially monitored.Citation25) Ascorbigen had strong antioxidant activities in the ORAC assay. The order of inhibition was ascorbigen >> AA-2G > Trolox > ascorbic acid (Fig. (A)). Inhibition of fluorescence decay by ascorbigen and AA-2G showed slightly different profiles from those by ascorbic acid and Trolox. In the early phase of the reaction, ascorbigen and AA-2G partially suppressed the loss of fluorescence, whereas ascorbic acid and Trolox completely inhibited it. In the case of ascorbic acid and Trolox, fluorescence rapidly decayed after lag times of about 18 and 26 min, respectively. Ascorbigen and AA-2G continued partial inhibition for a longer period. In this way, the profiles of the fluorescence decay curves of ascorbigen and AA-2G and those of ascorbic acid and Trolox were slightly different.
Fig. 5. ORAC assay (A) and OxHLIA (B) for ascorbigen, ascorbic acid, AA-2G, and Trolox.
Notes: ORAC assay (A) reaction mixtures containing ascorbigen (
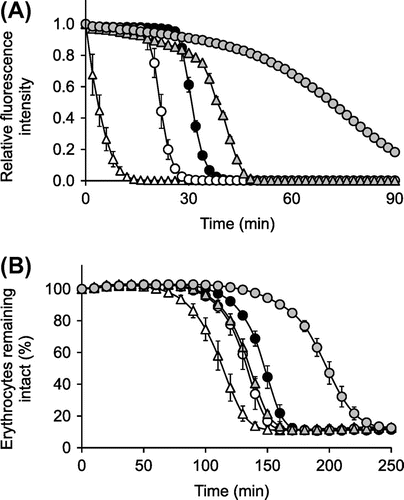
OxHLIA is a cell-based antioxidant assay using the same radical source as that used for the ORAC assay.Citation19,24,27) Oxidation of erythrocyte membranes by an AAPH-derived peroxyl radical induces oxidation of lipids and proteins and eventually causes hemolysis, and this hemolysis was inhibited by each antioxidant (Fig. (B)). The order of inhibition was ascorbigen >> Trolox > AA-2G ≈ ascorbic acid, which was slightly different from that observed in the ORAC assay (Fig. (A)). These results showed that the inhibitory effect of ascorbigen on hemolysis was far superior to that of the others. To investigate the characteristics of the reaction for the unexpectedly strong effect of ascorbigen, reaction mixtures of ascorbigen with AAPH-derived radicals were analyzed by HPLC. The HPLC profiles of the reaction mixture of ascrbigen (200 μM) and AAPH (40 mM) in PBS at 30, 60, and 120 min of reaction are shown in Fig. . At 30 min, a small amount of indole-3-aldehyde was observed. The peak of ascorbigen decreased with time, whereas the peak of indole-3-aldehyde slightly increased for 120 min. These results are quite different from those shown in Fig. .
Discussion
Ascorbigen showed stronger activity than that of ascorbic acid in the ABTS•+-scavenging assay but showed no activity in the DPPH radical- and galvinoxyl radical-scavenging assays (Fig. ). Ascorbigen slowly and continuously reacted with ABTS•+ over a period of 120 min (Fig. (C)). The reaction profile of ascorbigen to ABTS•+ was almost the same as that of AA-2G. It is estimated that one mole of ascorbigen scavenged about 3.5 mol of ABTS•+ in 120 min of reaction. It is thought that the ABTS•+-scavenging reaction will occur over a more extended time period, since a small amount of ascorbigen still remained in the reaction mixture after 120 min (Fig. ). AA-2G showed stronger radical-scavenging activity than that of ascorbic acid in the DPPH radical-scavenging assay under acidic conditionsCitation16) but showed no activity at pH 6.0 (Fig. (A)). AA-2G slowly and continuously reacted with DPPH over a period of 120 min under acidic conditions, and its long-term radical scavenging ability per molecule of AA-2G was superior to that of ascorbic acid. It has been suggested that AA-2G with only one oxidizable –OH group scavenged more than one equivalent of the DPPH radical via a covalent adduct formation with the radical.Citation17) Recently, we found that vanillin showed stronger activity than the activities of ascorbic acid and Trolox in the ABTS•+-scavenging assay, ORAC assay and OxHLIA.Citation21) The dimerization of vanillin contributed to a high reaction stoichiometry against ABTS•+ and AAPH-derived radicals to result in the strong effect of vanillin. Therefore, we were interested in investigating the reaction products when ascorbigen reacts with ABTS•+.
To predict the radical-scavenging mechanism of ascorbigen, we tried to isolate a reaction product from the reaction mixture of ascorbigen and ABTS•+ (Fig. ). The reaction product was purified and identified to be indole-3-aldehyde. The formation of indole-3-aldehyde suggested that the indole moiety of ascorbigen contributed to the radical-scavenging activity. 3-Methylindole, an indole derivative without an ascorbic acid moiety, was used for comparison with ascorbigen, indole-3-aldehyde and ascorbic acid, and it showed significant radical-scavenging activity compared to that of ascorbic acid (Fig. ). The reaction between 3-methylindole and ABTS•+ proceeded slowly and continuously for 120 min. The reaction profile of 3-methylindole to ABTS•+ was almost the same as that of ascorbigen. The HPLC profile of the reaction mixture of 3-methylindole and ABTS•+ for 120 min showed the formation of indole-3-aldehyde as the major reaction product (data not shown). Furthermore, HPLC analysis showed that the consumed amount of ascorbigen at 120 min was about 80%, while 3-methylindole was almost consumed. Ascorbigen (20 μM) and 3-methylindole (20 μM) had quenched 68 and 84 μM of ABTS•+ at 120 min, respectively (Fig. ). Thus, the stoichiometry of ascorbigen was 3.4 ± 0.2 in ABTS•+ scavenging reactions and that of 3-methylindole was 4.2 ± 0.3. Considering the difference in consumption amounts of antioxidants shown by HPLC analysis, the stoichiometry of ascorbigen and that of 3-methylindole against ABTS•+ seemed to be almost the same. These results indicate that the indole moiety of ascorbigen contributed to the radical-scavenging activity. Several peaks besides that of indole-3-aldehyde were observed from 8 to 10 min of retention time by HPLC analysis of the reaction mixture at 30 min (Fig. ). These peaks as well as the peak of ascorbigen decreased with time, whereas the peak of indole-3-aldehyde slowly increased (Fig. ). It was thought that the indole moiety of ascorbigen scavenged the radicals to produce indole-3-aldehyde via several intermediates, although it is unknown how the ascorbic acid moiety was released from ascorbigen. Indole-3-aldehyde is considered to be the final reaction product since indole-3-aldehyde scavenged very little ABTS•+ (Fig. ). It was reported that indole-3-carbinol (Fig. ), a well-known antioxidant, has two reaction pathways in ABTS•+ scavenging reactions: the first one is that indole-3-carbinol reacts with ABTS•+ and the intermediate is stabilized to indole-3-aldehyde, and the second one is that indole-3-carbinol scavenges ABTS•+ to form a covalent adduct (a pink compound) with the radical.Citation28) In our experiments, since color formation was not observed, it is thought that a covalent adduct with the radical was not formed. HPLC analysis showed that about 10% of the consumed ascorbigen was converted into indole-3-aldehyde in 120 min (data not shown). Therefore, these results indicate that ascorbigen scavenged ABTS•+ to generate indole-3-aldehyde as one of the final reaction products and that the radical-scavenging activity of ascorbigen was caused by the indole moiety of the molecule.
In the ORAC assay and OxHLIA, which utilizes a similar kind of peroxyl radical actually generated in vivo, ascorbigen showed much stronger antioxidant activity than did ascorbic acid and Trolox (Fig. ). In the early phase of the reaction of the ORAC assay, ascorbigen and AA-2G partially suppressed the loss of fluorescence, whereas ascorbic acid and Trolox completely inhibited it (Fig. (A)). Ascorbigen and AA-2G continued partial inhibition for a longer period. The results seem to be caused by the slow and long-lasting radical-scavenging reaction of ascorbigen and AA-2G. The order of inhibition in the ORAC assay was ascorbigen >> AA-2G > Trolox > ascorbic acid, and the order of inhibition in the OxHLIA was ascorbigen >> Trolox > AA-2G ≈ ascorbic acid. The antioxidant activity of Trolox was stronger than that of AA-2G in the OxHLIA but was weaker in the ORAC assay. The ORAC assay and OxHLIA utilize the same “hydrophilic” peroxyl radicals derived from AAPH but different oxidizable targets, i.e. a “hydrophilic” fluorescein or “lipophilic” biomembrane of erythrocytes. The microlocalization of each antioxidant in OxHLIA might have reflected the result that relatively lipophilic Trolox was superior to relatively hydrophilic AA-2G in protection against free radical-induced membrane damage. These results showed that the antioxidative effect of ascorbigen was far superior to that of the others under both assay conditions. Indole-3-aldehyde was hardly observed in the reaction of ascorbigen with AAPH-derived radicals (Fig. ), suggesting that the radical-scavenging mechanism of ascorbigen against AAPH-derived radicals differed from that of ascorbigen against ABTS•+. Ascorbigen was reported to prevent tert-buthylhydroperoxide-induced cytotoxicity more effectively than ascorbic acid in cultured human keratinocytes.Citation9) We have also found that AA-2G exerts a protective effect on AAPH-induced cytotoxicity more effectively than dose ascorbic acid in human dermal fibroblasts incubated for 24 h.Citation29) It was shown that AA-2G, which slowly and continuously reacts with radicals, exerted a protective effect in the latter part of the experimental period and that AA, which quickly reacts with radicals, exerted an effect in only the initial part of the experimental period. These results suggested that the slow and long-lasting radical-scavenging reaction of ascorbigen against unnatural model radicals as mentioned above is physiologically relevant to the prevention of oxidative stress.
In this study, we systematically reevaluated the antioxidant properties of ascorbigen using multiple assay systems. Ascorbigen showed stronger activity than that of ascorbic acid in the ABTS•+-scavenging assay but showed no activity in the DPPH radical- and galvinoxyl radical-scavenging assays. Ascorbigen slowly and continuously reacted with ABTS•+ over the experimental period. In the ABTS•+-scavenging assay, it was shown that ascorbigen scavenged ABTS•+ to generate indole-3-aldehyde as one of the final reaction products and that the radical-scavenging activity of ascorbigen was caused by the indole moiety of the molecule. Ascorbigen also showed much stronger antioxidant activity than that of ascorbic acid and Trolox in the ORAC assay and OxHLIA using physiologically relevant peroxyl radicals, AAPH-derived radicals. These results suggested that the slow and long-lasting radical-scavenging reaction of ascorbigen against unnatural model radicals is physiologically relevant to the prevention of oxidative stress. It was recently reported that ascorbigen, which is not present in intact plant tissues, was observed as a metabolite in human plasma and urine after dietary intake of broccoli.Citation30) Therefore, antioxidant activity of ascorbigen might be more beneficial than has been thought for daily health care.
Acknowledgments
We wish to thank Hayashibara Biochemical Laboratories, Inc., Okayama, Japan for the supply of AA-2G. The authors are grateful to the SC-NMR Laboratory of Okayama University and the MS Laboratory of Faculty of Agriculture, Okayama University.
References
- Hrnčiřík K, Valušek J, Velíšek J. A study on the formation and stability of ascorbigen in an aqueous system. Food Chem. 1998;63:349–355.10.1016/S0308-8146(98)00016-8
- Hrncirik K, Valusek J, Velisek J. Investigation of ascorbigen as a breakdown product of glucobrassicin autolysis in Brassica vegetables. Eur. Food Res. Technol. 2001;212:576–581.10.1007/s002170100291
- Wagner AE, Rimbach G. Ascorbigen: chemistry, occurrence, and biologic properties. Clin. Dermatol. 2009;27:217–224.10.1016/j.clindermatol.2008.01.012
- Agerbirk N, Vos M, Kim JH, Jander G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009;8:101–120.10.1007/s11101-008-9098-0
- Kiesvaara M, Virtanen AI. Synthetic ascorbigen as a source of vitamin C for guinea-pigs. II. Acta Chem. Scand. 1963;17:849–853.10.3891/acta.chem.scand.17-0849
- Matano K, Kato N. Studies on synthetic ascorbigen as a source of vitamin C for guinea pigs. Acta Chem. Scand. 1967;21:2886–2887.10.3891/acta.chem.scand.21-2886
- Preobrazhenskaya MN, Bukhman VM, Korolev AM, Efimov SA. Ascorbigen and other indole-derived compounds from Brassica vegetables and their analogs as anticarcinogenic and immunomodulating agents. Pharmacol. Ther. 1993;60:301–313.10.1016/0163-7258(93)90012-3
- Ge X, Karmansky I, Yannai S. Indole derivatives, 3,3′-di-indolylmethane and ascorbigen, inhibit oxidation of human low-density lipoproteins in-vitro. Pharm. Pharmacol. Commun. 1999;5:395–398.10.1211/146080899128735045
- Wagner AE, Huebbe P, Konishi T, Rahman MM, Nakahara M, Matsugo S, Rimbach G. Free radical scavenging and antioxidant activity of ascorbigen versus ascorbic acid: studies in vitro and in cultured human keratinocytes. J. Agric. Food. Chem. 2008;56:11694–11699.10.1021/jf802403d
- Niki E, Noguchi N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life. 2000;50:323–329.10.1080/15216540051081119
- Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radical Res. 2002;36:177–187.10.1080/10715760290006411
- Janaszewska A, Bartosz G. Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Invest. 2002;62:231–236.10.1080/003655102317475498
- Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods1. Free Radical Biol. Med. 1999;27:1173–1181.10.1016/S0891-5849(99)00203-8
- Fujinami Y, Tai A, Yamamoto I. Radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl of ascorbic acid 2-glucoside (AA-2G) and 6-Acyl-AA-2G. Chem. Pharm. Bull. 2001;49:642–644.10.1248/cpb.49.642
- Takebayashi J, Tai A, Yamamoto I. Long-term radical scavenging activity of AA-2G and 6-Acyl-AA-2G against 1,1-diphenyl-2-picrylhydrazyl. Biol. Pharm. Bull. 2002;25:1503–1505.10.1248/bpb.25.1503
- Takebayashi J, Tai A, Gohda E, Yamamoto I. Characterization of the radical-scavenging reaction of 2-O-substituted ascorbic acid derivatives, AA-2G, AA-2P, and AA-2S: a kinetic and stoichiometric study. Biol. Pharm. Bull. 2006;29:766–771.10.1248/bpb.29.766
- Takebayashi J, Asano R, Nakae Y, Saito M, Gohda E, Yamamoto I, Tai A. 2-O-α-d-Glucopyranosyl-l-ascorbic acid scavenges 1,1-diphenyl-2-picrylhydrazyl radicals via a covalent adduct formation. Biosci. Biotechnol. Biochem. 2007;71:754–760.10.1271/bbb.60602
- Takebayashi J, Tai A, Yamamoto I. pH-dependent long-term radical scavenging activity of AA-2G and 6-Octa-AA-2G against 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation. Biol. Pharm. Bull. 2003;26:1368–1370.10.1248/bpb.26.1368
- Takebayashi J, Kaji H, Ichiyama K, Makino K, Gohda E, Yamamoto I, Tai A. Inhibition of free radical-induced erythrocyte hemolysis by 2-O-substituted ascorbic acid derivatives. Free Radical Biol. Med. 2007;43:1156–1164.10.1016/j.freeradbiomed.2007.07.002
- Takebayashi J, Ishii R, Chen J, Matsumoto T, Ishimi Y, Tai A. Reassessment of antioxidant activity of arbutin: multifaceted evaluation using five antioxidant assay systems. Free Radical Res. 2010;44:473–478.10.3109/10715761003610760
- Tai A, Sawano T, Yazama F, Ito H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta, Gen. Subj. 2011;1810:170–177.10.1016/j.bbagen.2010.11.004
- Tai A, Sawano T, Yazama F. Antioxidant properties of ethyl vanillin in vitro and in vivo. Biosci. Biotechnol. Biochem. 2011;75:2346–2350.10.1271/bbb.110524
- Takebayashi J, Yagi Y, Ishii R, Abe S, Yamada K, Tai A. Antioxidant properties of 2-O-β-d-Glucopyranosyl-l-ascorbic acid. Biosci. Biotechnol. Biochem. 2008;72:1558–1563.10.1271/bbb.80063
- Takebayashi J, Iwahashi N, Ishimi Y, Tai A. Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA). Food Chem. 2012;134:606–610.10.1016/j.foodchem.2012.02.086
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food. Chem. 2005;53:4290–4302.10.1021/jf0502698
- Shi H, Noguchi N, Niki E. Galvinoxyl method for standardizing electron and proton donation activity. Methods Enzymol. 2001;335:157–166.10.1016/S0076-6879(01)35240-0
- Takebayashi J, Chen J, Tai A. A method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. Methods Mol. Biol. 2010;594:287–296.10.1007/978-1-60761-411-1
- Arnao MB, Sanchez-Bravo J, Acosta M. Indole-3-carbinol as a scavenger of free radicals. Biochem. Mol. Biol. Int. 1996;39:1125–1134.
- Hanada Y, Iomori A, Ishii R, Gohda E, Tai A. Protection of free radical-induced cytotoxicity by 2-O-α-d-Glucopyranosyl-l-ascorbic acid in human dermal fibroblasts. Biosci. Biotechnol. Biochem. 2014;78:301–306.
- Hauder J, Winkler S, Bub A, Rüfer CE, Pignitter M, Somoza V. LC-MS/MS quantification of sulforaphane and indole-3-carbinol metabolites in human plasma and urine after dietary intake of selenium-fortified broccoli. J. Agric. Food. Chem. 2011;59:8047–8057.10.1021/jf201501x