Abstract
We found that, under iron-limiting conditions, Aeromonas hydrophila ATCC 7966T could utilize the xenosiderophore desferrioxamine B (DFOB) for growth by inducing the expression of its own outer membrane receptor. Two consecutive genes, desR and desA, were selected as candidates involved in DFOB utilization. The presence of the ferric-uptake regulator boxes in their promoters suggested that these genes are under iron-dependent regulation. Mutation of desA, a gene that encodes the outer membrane receptor of ferrioxamine B, disrupted the growth of the amonabactin-deficient mutant in the presence of DFOB. β-Galactosidase reporter assays and reverse transcriptase-quantitative PCR demonstrated that desR, a gene that encodes an AraC-like regulator homolog is required for the induction of desA transcription in the presence of DFOB and under iron-limiting conditions. The functions of desA and desR were analyzed using complementation experiments. Our data provided evidence that DesA is powered primarily by the TonB2 system.
Graphical Abstract
Proposed model for DesR (AraC-type transcriptional regulator)-mediated transcriptional regulation of desA (encoding the outer membrane receptor for ferrioxamine B) gene in Aeromonas hydrophila.
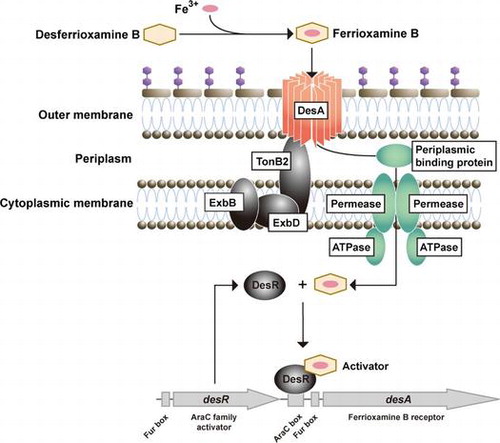
Iron is an essential element for the survival and growth of nearly all bacteria. However, in an aerobic environment at neutral pH, iron is highly insoluble and therefore scarcely available to bacteria.Citation1) To acquire iron from their environment, bacteria have developed specific strategies that permit them to scavenge the element. Most bacteria produce low-molecular mass chelators, siderophores that have a high affinity for ferric iron, allowing bacteria to scavenge iron from their surroundings.Citation1) In addition to their own siderophores, some bacteria have evolved the ability to utilize heterologous siderophores (xenosiderophores) of bacterial or fungal origin. In Gram-negative bacteria, iron-bound siderophores (ferric siderophores) are transported across the outer and inner membranes by specific iron-repressible outer membrane protein (IROMP) receptors and ATP-binding cassette transport systems, respectively.Citation1–3) Transport of ferric siderophores by using specific IROMP receptors is an energy-dependent process. Proton-motive force created by the inner membrane is transduced to IROMP receptors through the TonB system, which generally consists of the integral inner membrane proteins, TonB, ExbB, and ExbD. The TonB system activates by altering their structure to one that recognizes ferric siderophores.Citation2,3) Therefore, these IROMPs are referred to as TonB-dependent receptors. When the cytoplasmic Fe2+ concentration increases, the ferric uptake regulator protein (Fur) binds to Fe2+ as a cofactor, repressing the expression of iron acquisition genes including those involved in siderophore biosynthesis, ferric-siderophore transport, and virulence.Citation4,5) The Fur–Fe2+ complex binds to the Fur box consensus sequence that is located near the −10 and −35 elements of Fur-targeted genes. Binding of the Fur–Fe2+ to the Fur box leads to the transcriptional repression of target genes. AraC-type regulators reportedly control the transcription of iron acquisition genes in bacteria including Pseudomonas aeruginosa,Citation6) Yersinia pestis,Citation7) Bordetella species,Citation8,9) Vibrio vulnificus,Citation10) and Vibrio furnissii.Citation11) In these species, the cognate siderophores or xenosiderophores can serve as co-activators of transcriptional regulators, and the transcription of the AraC-type regulator genes is generally under the control of Fur.
Aeromonas hydrophila is a facultative anaerobic invasive motile Gram-negative bacterium associated with a variety of human infections, including water-borne traumatic secondary wound infections, septicemia, and gastroenteritis,Citation12,13) In response to iron starvation, A. hydrophila typically secrete and utilize amonabactins, a group of four peptide-based catecholate siderophores with two 2,3-dihydroxybenzoic acid Fe3+-binding moieties.Citation14,15) Recently, we reported that entA, which encodes 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase, is essential for the production of amonabactin, and the 66.2 kDa IROMP encoded by irgA functions as the ferric enterobactin receptor, which is dependent solely on the TonB2 system.Citation16)
Here, we report that, under iron-limiting conditions, A. hydrophila ATCC 7966T can utilize the xenosiderophore, desferrioxamine B (DFOB) by regulating the expression of its own outer membrane receptor encoded by desA. In addition, we show the involvement of desR, which encodes an AraC-type regulator, in the transcriptional induction of desA in the presence of DFOB and under iron-limiting conditions. Furthermore, we demonstrate that DesA function depends on energy transduced by the TonB1 and, to a lesser extent, by TonB2 systems.
Materials and methods
Bacterial strains, plasmids, primers, and growth conditions
Bacterial strains and plasmids used in this study are listed in Table . A. hydrophila and Escherichia coli were grown at 30 and 37 °C, respectively, in Luria-Bertani (LB) media (with shaking) or grown on LB agar plates (1.5% agar) containing 0.5% NaCl. E. coli β2155, a diaminopimelic acid auxotroph, was grown in LB media containing 1 mM diaminopimelic acid. LB media with or without an iron chelator, ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDA; 250 μM; Sigma-Aldrich, St. Louis, MO, USA) were used as iron-limiting (−Fe) and iron-replete (+Fe) media, respectively. Appropriate antibiotics were added to the media as needed at the following concentrations: ampicillin (50 μg/mL), chloramphenicol (10 μg/mL), gentamicin (10 μg/mL), and tetracycline (10 μg/mL). PCR primers used in this study are listed in Table .
Table 1. Bacterial strains and plasmids used in this study.
Table 2. PCR primers used in this study.
Growth assay
To avoid the effect of the endogenous siderophore amonabactin on growth in −Fe medium, all deletion mutants used for this assay were generated from an entA deletion mutant (ΔentA) derived from A. hydrophila ATCC 7966T strain.Citation16) The mutant strains were grown overnight in LB medium. Fresh +Fe and −Fe media (5 mL) were inoculated with an aliquot of the preculture (OD600 of 0.005). While the cultures were shaking at 70 rpm at 30 °C, OD600 was automatically measured every 1 h by using an Advantec TVS062CA biophotorecorder (Advantec, Tokyo, Japan). When required, DFOB (Sigma-Aldrich) was added to −Fe medium at 20 μM (−Fe/+DFOB).
DNA manipulations
Standard DNA manipulations were carried out as described by Sambrook et al.Citation17) Chromosomal DNA and plasmid DNA were extracted using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) and High Pure Plasmid Isolation Kit (Roche Diagnostics, Mannheim, Germany), respectively. Restriction enzymes were purchased from Roche Diagnostics. The Ligation-Convenience Kit (Wako Pure Chemical Industries, Osaka, Japan) was used for DNA ligation. DNA fragments were purified from agarose gels by using the MagExtracter-PCR & Gel Clean-Up DNA Fragment Purification Kit (Toyobo, Osaka, Japan). E. coli was transformed by electroporation using a MicroPulser™ apparatus (Bio-Rad, Benicia, CA, USA). The oligonucleotide primers used in this study (Table ) were designed based on the A. hydrophila ATCC 7966T genome sequence.Citation18) Homology searches were performed using the National Center for Biotechnology Information Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/). A putative AraC-binding site (AraC box) sequence was identified using the Virtual Footprint promoter analysis software based on the PRODORIC Release 8.9 (http://www.prodoric.de/) online database.
IROMP analysis
A. hydrophila ATCC 7966T cells grown in the presence or absence of DFOB for 12 h in LB media under −Fe conditions were harvested. The outer membrane protein (OMP)-rich fractions were prepared and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE), according to a procedure described previously.Citation19) The resulting gel was stained with Coomassie Brilliant Blue R-250, and visualized using a Gel Doc XR system (Bio-Rad). The protein was electroblotted onto a pre-wetted polyvinylidene difluoride membrane (ProBlott®; Applied Biosystems, Carlsbad, CA, USA) by using a Trans-Blot® semi-dry electrophoretic transfer cell (Bio-Rad). Their N-terminal amino acid sequences were determined by the automated Edman degradation method using a Procise® Model 491 protein sequencer (Applied Biosystems).
Fur titration assay
The Fur titration assay (FURTA)Citation20) was used to assess whether Fur box sequences were present in the promoter regions of A. hydrophila desA and desR genes. PCR amplicons encoding the putative Fur boxes of desA and desR were generated using DesA-7/8, and DesR-7/8 primer pairs. The resulting HindIII-EcoRI desA (250 bp) and KpnI-SacI desR (418 bp) promoter inserts were used to construct pBC-desAfur and pBC-desRfur plasmids, respectively. The pBC-desAfur and pBC-desRfur plasmids were electroporated into E. coli H1717 bearing a Fur-repressible fhuF::lacZ fusion. Transformants were incubated for 15 h on MacConkey lactose agar plates (BD, Franklin Lakes, NJ, USA) containing ampicillin (50 μg/mL) and ferric chloride (100 μM), and the phenotype of the colonies was examined. The FURTA-positive phenotype, denoted by red colonies (Lac+), indicated that the Fur protein bound to the promoter regions contained within the pBC-desAfur and pBC-desRfur plasmids that had been transformed into the E. coli H1717.
Construction of A. hydrophila ATCC 7966T deletion mutants and complementing strains
Deletion mutants were generated by allelic exchange using the R6K-ori suicide vector pXAC623, as described previously.Citation16,21) DNA fragments carrying deletions in the A. hydrophila desA, desB, desR, and lacZ (AHA_4101) genes were prepared by PCR-driven overlap extensionCitation22,23) using two primer sets for each gene (DesA1–4, DesB1–4, DesR1–4, and LacZ1–4, respectively). The fragments were digested using XbaI and were ligated into XbaI-digested pXAC623. The resulting plasmids (Table ) were transformed into E. coli β2155,Citation24) and then mobilized into the appropriate A. hydrophila strains by filter mating. The resulting merodiploids were selected on LB agar plates containing chloramphenicol (10 μg/mL) in the absence of diaminopimelic acid and were then incubated at room temperature for 48 h on LB agar plates containing 10% sucrose in the absence of both NaCl and chloramphenicol. Sucrose-resistant and chloramphenicol-sensitive colonies were selected. The deletions were confirmed by PCR using chromosomal DNA as template and the following primer pairs: DesA-5/6, DesB-5/6, DesR-5/6, LacZ-5/6, TonB1-5/6, TonB2-5/6, and TonB3-5/6. To complement mutants carrying a deletion in desA or desR, PCR amplicons containing the full-length genes were prepared using the primer pairs DesA-comp-F/R and DesR-comp-F/R and ligated into appropriately digested pRK415.Citation25) The complementing plasmids were transformed into E. coli β2155 and then mobilized into the appropriate A. hydrophila mutants by filter mating.
Construction of lacZ fusions used in β-galactosidase reporter assays
Two varieties of DNA fragments containing the upstream regions of desA were amplified by PCR using two primer pairs, DesR-7/DesA-8 and DesA-1/8. PCR fragments were digested with XbaI-EcoRI and KpnI-XbaI, respectively, and ligated into the digested pHRP309.Citation26) The resulting promoter-lacZ reporter plasmids, pHRP-desA and pHRP-desAR, were individually introduced into A. hydrophila ΔdesRΔlacZ, which was constructed by deleting lacZ from ΔdesR (described above). The resulting strains, ΔdesRΔlacZ/pHRP-desA and ΔdesRΔlacZ/pHRP-desAR, were grown at 30 °C in +Fe, −Fe, and −Fe/+DFOB media for 12 h. The β-galactosidase activities in their cell lysates were measured using the Miller method.Citation27)
Reverse transcriptase (RT)-PCR and quantitative (q)-PCR
A. hydrophila ATCC 7966T and ΔentA were grown in LB medium to an OD600 of 0.3. Each culture was split into two aliquots. One aliquot was left untreated (+Fe medium) and the other was supplemented with 250 μM EDDA (−Fe medium). Both cultures were further incubated until they reached an OD600 of 0.5. Total RNA was extracted from each cell pellet using the RNeasy® Protect Bacteria Mini Kit (Qiagen, Valencia, CA, USA) and then treated with RNase-free DNase I (Ambion, Austin, TX, USA), following the manufacturer’s instruction to eliminate trace chromosomal DNA contamination. These DNase I-treated total RNA preparations were used for RT-PCR and RT-qPCR. To generate cDNA for RT-PCR, 1 μg of the total RNA preparation was incubated at 42 °C for 1 h with primers complementary to the internal sequence of desR and desB, desR-R and desB-R. PCR was performed using 1 μL of the RT reaction mixture and gene-specific primer pairs, desA-F/R for desA, desB-F/R for desB, and desR-F/R for desR. The PCR conditions were as follows: after initial denaturation at 94 °C for 2 min, DNA was amplified by 30 cycles, with each cycle consisting of denaturation at 98 °C for 10 s, annealing at 58 °C for 30 s, and extension at 68 °C for 1 min. Total RNA not treated with RT was used as a negative control in the PCR reaction to confirm the absence of genomic DNA contamination. 16S rRNA was used as an endogenous internal control. RT-PCR products were run on a 1.5% agarose gel stained with ethidium bromide and visualized using a Gel Doc XR.
To perform RT-qPCR, 0.5 μg of the total RNA preparation was used to generate cDNA using ReverTra Ace® RT (Toyobo) and a random hexamer primer (Takara Bio, Otsu, Japan). The qPCR was performed using a Chromo4 Real-Time PCR detection system (Bio-Rad) and Thunderbird® SYBR® qPCR Mix (Toyobo). The following primer pairs were used: desA-qF/qR for desA; desR-qF/qR for desR; and 16S-qF/qR for 16S rRNA. Values were quantified using the comparative threshold cycle method and were normalized to the levels of 16S rRNA.
Results and discussion
Utilization of DFOB as a xenosiderophore by A. hydrophila ATCC 7966T
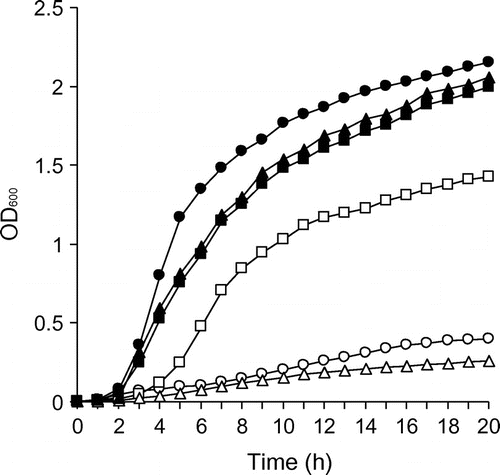
The amonabactin-deficient mutant, ΔentA, did not grow in −Fe medium; however, the addition of 20 μM DFOB to the same medium restored the growth of ΔentA (Fig. ). This indicates that A. hydrophila ATCC 7966T can utilize DFOB as a xenosiderophore.
Fig. 1. Growth assays of A. hydrophila ΔentA, ΔdesA, ΔdesR and complementing strains.
Notes: ΔentA, a mutant deficient in the cognate siderophore amonabactin, was grown in iron-limiting DFOB negative medium (−Fe/−DFOB; open circle) and iron-limiting medium containing DFOB (−Fe/+DFOB; filled circle). ΔentAΔdesA and ΔdesRΔentA, generated from the entA deletion mutant (ΔentA), and their complementing strains were grown in −Fe/+DFOB medium. ΔentAΔdesA/pRK415 (empty), open triangle; ΔentAΔdesA/pRK415-desA, filled triangle; ΔentAΔdesR/pRK415 (empty), open square; and ΔentAΔdesR/pRK415-desR, filled square. OD600 was measured every hour for 20 h. A representative example from three independent experiments is shown.
Identification of candidate genes involved in DFOB utilization in A. hydrophila ATCC 7966T
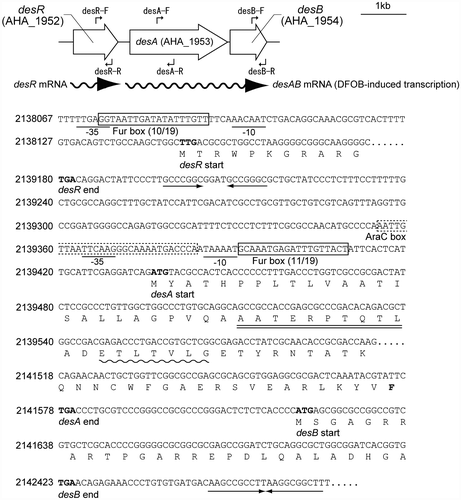
To identify candidate A. hydrophila ATCC 7966T genes involved in DFOB utilization, we performed BLAST searches on the A. hydrophila genome sequenceCitation18) using the amino acid sequence of the TonB-dependent ferrioxamine B (FOB; Fe3+-loaded form of DFOB) receptor DesA (VV2_1337) from V. vulnificus.Citation10) We identified a desA ortholog (AHA_1953) that encodes a 714 amino acid protein sharing 48% amino acid identity with V. vulnificus DesA. The product of AHA_1953 was annotated as a ferrichrome receptor.Citation18) An additional open reading frame (AHA_1954), desB, was found just downstream of desA. The predicted protein product of desB shared 33% amino acid identity with a V. vulnificus protein with unknown function (VV2_1339). A. hydrophila DesB was homologous to E. coli FhuF, which is reportedly involved in the removal of iron from cytoplasmic FOB through the reduction of Fe3+.Citation28) The protein product of desR (AHA_1952), an upstream gene adjacent to desA, shared 51% amino acid identity with V. vulnificus DesR (VV2_1338),Citation10) which encodes an AraC-type transcriptional activator of V. vulnificus DesA. A genetic map of the candidate genes in A. hydrophila ATCC 7966T involved in DFOB utilization is shown in Fig. (A).
Fig. 2. Genetic map and the partial nucleotide and deduced amino acid sequences of the desRAB region.
Notes: (A) The gene nomenclature annotated in the A. hydrophila ATCC 7966T genome sequence databaseCitation10) and the primers used for RT-PCR are illustrated. The wavy lines with arrowheads indicate the RNA messages. (B) The putative Fur boxes and AraC box are boxed with solid and dashed lines, respectively. The putative promoter sequences are labeled with −10 and −35. The predicted amino acid sequence of desA is double-underlined. This sequence is in accordance with the N-terminal amino acid sequence determined for the mature DesA. The putative TonB box sequence and the highly conserved C-terminal F residue in DesA are indicated with a wavy line and a bold letter, respectively. The terminator signal is indicated by converging arrows. Nucleotide numbers correspond to nucleotide sequence positions in the A. hydrophila Kyoto Encyclopedia of Genes and Genomes Database (http://www.genome.jp/kegg-bin/show_organism?org=aha).
Using homology searches, we revealed that Aeromonas salmonicida also possessed the DesR (ASA_2342), DesA (ASA_2341), and DesB (ASA_2340) orthologs that shared 80, 90, and 75% identity to their respective proteins in A. hydrophila. The desR, desA, and desB are also found in V. vulnificus. In A. hydrophila and A. salmonicida, the genes appear in the same order (desR-desA-desB) and each gene is in the same orientation. However, the genes in V. vulnificus were arranged in a different order (desB-desR-desA) and desB was in the reverse orientation. The DesA and DesR proteins in A. hydrophila also showed similarities to their V. furnissii counterparts. However, no orthologs of A. hydrophila and V. vulnificus desB were found in the V. furnissii genome.Citation29)
Characterization of DesA as an IROMP by SDS-PAGE and N-terminal amino acid sequence determination
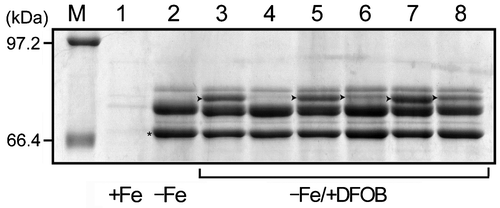
To analyze the OMP profiles of A. hydrophila ATCC 7966T grown in +Fe, −Fe, and −Fe/+DFOB media, sarkosyl-insoluble OMP fractions were preparedCitation19) and then separated by SDS-PAGE. In A. hydrophila grown in −Fe medium, we detected two major and one minor IROMP bands ranging in size from 66 to 80 kDa (Fig. , lane 2). These bands were not detected in A. hydrophila grown in +Fe medium (Fig. , lane 1). In contrast, when grown in −Fe/+DFOB medium, the strain robustly expressed a novel 76.7 kDa IROMP (Fig. , lane 3). This band was not detected under +Fe conditions, even in the presence of DFOB (data not shown). We determined the sequence of the first 10 N-terminal amino acids belonging to the 76.7 kDa IROMP AATERPTQTL. This sequence was consistent with the N-terminal region encoded by desA. These results indicate that a 76.7 kDa IROMP is induced by the presence of DFOB under −Fe conditions to serve as the receptor for FOB.
Fig. 3. Detection of DesA in A. hydrophila by SDS-PAGE.
Notes: A. hydrophila ATCC 7966T was grown in iron-replete (+Fe), −Fe, and −Fe/+DFOB media for 12 h. ΔdesA, ΔdesR, and their complementing strains were grown in −Fe/+DFOB media for 12 h. ΔentAΔdesR/pRK415 was also grown under the same conditions. SDS-PAGE was carried out by using a 7.5% polyacrylamide running gel (130 mm). The resulting gel was stained with Coomassie Brilliant Blue R-250. The amount of protein loaded in each lane was 25 μg. Only the relevant part of the SDS-PAGE gel is shown. Arrowheads in lanes 3, 5, 6, 7, and 8 indicate DesA. Lanes 1, 2, and 3, A. hydrophila ATCC 7966T; lanes 4, ΔdesA; lane 5, ΔdesA/pRK415-desA; lane 6, ΔdesR; lane 7, ΔdesR/pRK415-desR; lane 8, ΔentAΔdesR; and lane M, molecular mass marker proteins. *, ferric enterobactin receptor (IrgA).Citation16)
The desA and desB genes constitute a DFOB-induced operon
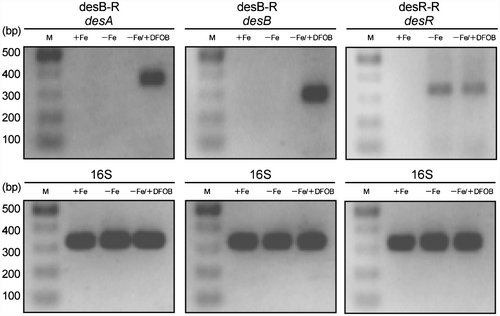
The consecutive location of desA and desB in the same orientation, and the single set of putative promoter elements and Rho-independent transcription terminator suggest that the two genes are transcriptionally linked (Fig. (B)). To determine whether desA and desB are arranged in an operon, RT-PCR was carried out. cDNA was generated from total RNA collected from cells grown in +Fe, −Fe, and −Fe/+DFOB media by using a primer complementary to the internal sequence of desB (Fig. (A)). The desA and desB amplicons from the cells grown in −Fe/+DFOB medium were of the expected size and had similar intensities (Fig. ), indicating that a polycistronic mRNA, composed of desA and desB, was expressed in response to DFOB under −Fe conditions (Fig. (A)). As expected, desR was expressed as a monocistronic message under −Fe conditions, but was not induced in the presence of DFOB (Fig. ). These data are consistent with the existence of a putative Fur box and a putative Rho-independent transcriptional terminator for desR in the intergenic region between desR and desA (Fig. (B)).
Fig. 4. Confirmation of desA and desB as a DFOB-induced operon by RT-PCR.
Notes: Total RNA was isolated from A. hydrophila ATCC 7966T cells grown in +Fe, −Fe, and −Fe/+DFOB media and was used for RT-PCR analysis. The primers desR-R and desB-R used for the preparation of cDNA are indicated in Fig. (A). PCR was carried out under the conditions described in the Materials and Methods and the amplicons were analyzed by agarose gel electrophoresis. The primers used for PCR are also indicated in Fig. (A). The predicted sizes of the amplicons derived from the representative genes are as follows: desA, 376 bp; desB, 304 bp; and desR, 345 bp. As an internal control, a 333 bp fragment of the 16S rRNA gene was included. Lane M, molecular size markers.
Features of des cluster genes and their protein products in A. hydrophila
The partial nucleotide sequences of the three des genes and their deduced amino acid sequences are shown in Fig. (B). Based on an in silico analysis, desR and desA promoters were shown to contain putative Fur boxes sharing 10/19 and 11/19 nucleotide matches, respectively, to the consensus Fur box of E. coli.Citation30) To unequivocally determine the functions of the Fur boxes, FURTA was carried out. The promoter regions of desR and desA, including the potential Fur boxes, were cloned into the FURTA indicator strain E. coli H1717.Citation20) Both of the cloned promoter regions showed a typical FURTA-positive phenotype as evidenced by the presence of Lac+ colonies on MacConkey agar plates (data not shown), indicating that the cloned regions harbor the binding sites of the E. coli Fur protein.
The molecular mass of the mature DesA protein was estimated to be 76,756 Da, consistent with the 76.7 kDa mass estimated by the electrophoretic mobility on SDS-PAGE (Fig. ). These data indicated that the N-terminal 25 amino acid residues of the premature DesA are indeed excluded as a signal peptide. Near its N terminus, DesA contains a putative TonB box sequence (38ETLTVLG44; highly conserved residues are underlined) (Fig. (B)).Citation31,32) In addition, DesA possesses a C-terminal phenylalanine residue characteristic of TonB-dependent siderophore receptors.Citation33) Members of the AraC-like family have been described as positive transcriptional regulators.Citation34) Therefore, we compared the promoter sequences of desA and desR to the PRODORIC database by using the Virtual Footprint promoter analysis software. This software has been used to predict the existence of a putative AraC-type regulator binding site in the V. anguillarum fvtA gene promoter.Citation35) A putative AraC-type regulator binding site (AraC box; AATTGTTAATTCAAGGGCAAAATGACCCA) was identified in the promoter of desA, overlapping with the −35 element (Fig. (B)), but was not found in the promoter of desR. However, it remains to be determined whether the predicted AraC box is functional and directly binds DesR. Using a homology search, we revealed that a putative helix-turn-helix DNA binding motif is present in the N-terminal portion of DesR. Because the majority of the proteins belonging to the AraC family have the helix-turn-helix DNA-binding motifs at the C-terminal portion,Citation34) this finding suggests that DesR belongs to a discrete subset of AraC family regulators, which includes Rob from E. coli.Citation36) Similar to Rob, the C-terminal segment of DesR is likely involved in binding with the co-activator, which is presumably FOB rather than DFOB. DesB was also found to contain a conserved Cys-Cys-Xaa10-Cys-Xaa2-Cys motif containing four consensus cysteine residues (underlined) in its C-terminal domain, as part of a 2Fe–2S cluster.Citation37) These data suggest that DesB exerts a reductase activity on Fe3+ bound to DFOB.
Phenotypic analysis and complementation of the desA and desR deletion mutants
To clarify the role of A. hydrophila DesA in DFOB utilization, we generated the ΔentAΔdesA deletion mutant from ΔentA.Citation16) In contrast to ΔentA, ΔentAΔdesA failed to grow in −Fe medium despite the presence of DFOB (Fig. ). In accordance with this finding, no protein band corresponding to DesA was detected for ΔdesA (Fig. , lane 4). The complementing strain, ΔentAΔdesA/pRK415-desA, showed restored growth to a level similar to that of ΔentA (Fig. ) and considerable expression of DesA (Fig. , lane 5). These results demonstrate that DesA is the outer membrane receptor for FOB. In −Fe medium, growth of ΔdesA (able to synthesize amonabactin) was indistinguishable from that of wild-type A. hydrophila ATCC 7966T (data not shown), indicating that DesA does not serve as a receptor for ferric amonabactin. DFOB-induced DesA expression was considerably lower in ΔdesR (Fig. , lane 6). The production of DesA was restored in the complementing strain ΔdesR/pRK415-desR (Fig. , lane 7). Taken together, these results suggest that DesR under iron-limiting conditions and in the presence of DFOB is involved in the induction of DesA expression. As shown in Fig. , ΔentAΔdesR/pRK415 showed reduced, but moderate growth in −Fe/+DFOB medium. The growth of ΔentAΔdesR/pRK415 correlated with the expression of DesA (Fig. , lane 8). The more prominent expression of DesA in ΔentAΔdesR compared to ΔdesR may be attributed to the background of entA deletion. We speculate that an alternative or redundant activator able to recognize FOB as a co-activator enhances DesA expression and allows cells to overcome severe iron deficiency. Although our data suggest that DesB is a Fe3+ reductase for FOB, DFOB caused no significant difference in growth promotion between ΔentAΔdesB and ΔentA (data not shown). This does not signify that desB is not involved in DFOB utilization. Instead, we speculate that an alternative or redundant reductase present in this species can compensate for the deletion of desB.
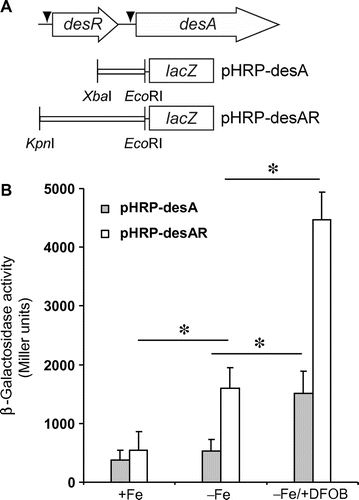
Assessment of the function of DesR by lacZ transcriptional fusions
A putative AraC box found in the promoter region of desA suggests that desA expression is positively regulated by DesR. To define the functions of DesR and Fur boxes in desR and desA expression, β-galactosidase reporter assays were performed in A. hydrophila ΔdesRΔlacZ grown in −Fe and −Fe/+DFOB media. A. hydrophila ΔdesRΔlacZ carried the following plasmids: pHRP-desA (encoding a lacZ fused to the promoter region of desA), and pHRP-desAR (encoding a lacZ fused to the promoter regions of desA and desR in addition to desR) (Fig. (A)). As shown in Fig. (B), there was no significant difference in β-galactosidase activity in ΔdesRΔlacZ/pHRP-desA cells grown in −Fe and +Fe media, suggesting that the promoter activity of desA might be very weak. When grown in −Fe/+DFOB medium, ΔdesRΔlacZ/pHRP-desA showed a significant increase in enzyme activity. This implied a possible contribution of an activator protein in addition to FOB. On the other hand, ΔdesRΔlacZ/pHRP-desAR had a more pronounced enzyme activity in −Fe medium than in +Fe medium. This may be due to the potential action of apo-DesR (DesR not bound with FOB) that is expressed by the cloned desR, because DFOB was not present in the medium. Furthermore, the enzyme activity of ΔdesRΔlacZ/pHRP-desAR grown in −Fe/+DFOB medium increased approximately three times compared with that grown in −Fe medium. This demonstrates that the lacZ fusion in ΔdesRΔlacZ/pHRP-desAR is activated by both the plasmid-derived DesR and the presence of DFOB.
Fig. 5. Effects of desR and DFOB on the expression of lacZ fusions.
Notes: (A) Schematic representation of desA and desR, and the plasmids pHRP-desA and pHRP-desAR that were constructed for the β-galactosidase reporter assay. The restriction enzyme sites used to insert the DNA fragments into pHRP309 are shown. An arrowhead indicates a putative Fur box. (B) The transcriptional levels of desA-lacZ fusions as measured by β-galactosidase activities. A. hydrophila ΔdesRΔlacZ carrying pHRP-desA and pHRP-desAR were grown in +Fe, −Fe, and −Fe/+DFOB media, and the β-galactosidase activities of their cell lysates were then assayed. ΔdesRΔlacZ/pHRP309 (empty plasmid) was grown in the same media. Its enzyme activity was subtracted from that obtained for strains carrying pHRP-desA and pHRP-desR. The mean β-galactosidase activities ± SD (n = 3) are shown in Miller units. p values were estimated using the Student’s t-test (*p < 0.05).
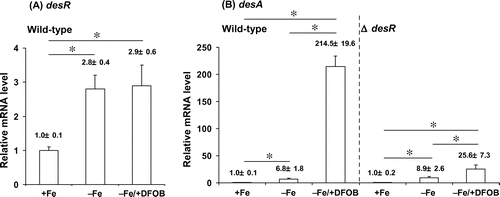
Analysis of the desR and desA transcriptional levels by RT-qPCR
RT-qPCR was performed to measure the transcript levels of desR and desA in wild-type A. hydrophila ATCC 7966T. desR transcript levels were increased by approximately threefold in A. hydrophila ATCC 7966T grown in both −Fe and −Fe/+DFOB media compared to those grown in +Fe medium (Fig. (A)). These data indicate that desR transcription is dependent on iron limitation, but is not affected by the presence of DFOB. The transcript levels of desA exhibited a pattern different from that of desR (Fig. (B)). Pronounced levels of desA transcript were observed in the wild-type cells grown in −Fe/+DFOB medium, indicating that desA is highly inducible in the presence of DFOB under −Fe conditions. RT-qPCR was also performed on ΔdesR grown in +Fe, −Fe, and −Fe/+DFOB media. When grown in −Fe/+DFOB medium, ΔdesR showed a drastic reduction in desA transcription. Thus, these results indicate that the transcriptional activation of desA under −Fe conditions is mediated through DesR and the co-activator DFOB (FOB). Notably, the wild-type strain showed an approximate sixfold increase in desA transcription when grown in −Fe medium vs. in +Fe medium; however, there was no detectable DesA protein (Fig. , lane 2). Therefore, low levels of DesA may play a role in the initial uptake of FOB prior to induction of desA expression. Moreover, desA transcription was more significantly activated when ΔdesR was grown in −Fe/+DFOB medium than in −Fe medium; this may be explained by the existence of an alternative or redundant DFOB-dependent activator. However, this potential alternative activator might be much less active than DesR. These data are consistent with the results for mutants carrying a desR deletion (Fig. ; Fig. , lanes 6 and 8; and Fig. (B)).
Fig. 6. RT-qPCR analysis of desA and desR transcript levels in A. hydrophila ATCC 7966T and ΔdesR.
Notes: The levels of desA and desR mRNAs were assessed by RT-qPCR for the total RNA samples extracted from wild-type A. hydrophila ATCC 7966T and ΔdesR grown in +Fe, −Fe, and −Fe/DFOB media. mRNA levels of desR and desA were normalized to 16S rRNA, and bars represent mean values ± SD (n = 5). p values were estimated using the Student’s t-test (*p < 0.05).
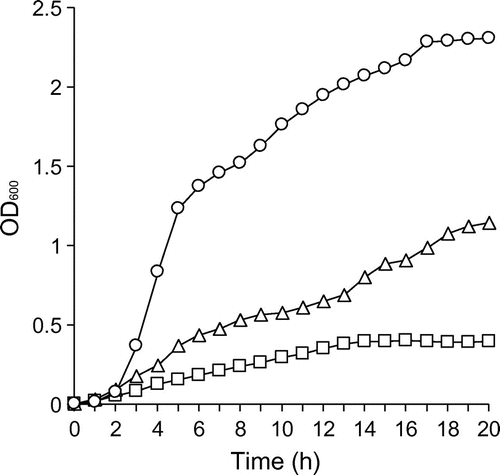
TonB specificity of DesA
Previously, the presence of three TonB clusters, TonB1 (AHA_1987-1985), TonB2 (AHA_4251-4248), and TonB3 (AHA_3435-3438), was identified in A. hydrophila ATCC 7966T.Citation16) To determine which TonB protein is involved in DesA-mediated transport of FOB, we generated a set of tonB double deletion mutants derived from ΔentA. The growth of the deletion mutants was then measured in −Fe/+DFOB medium (Fig. ). ΔentAΔtonB1ΔtonB3 cells grew effectively, whereas ΔentAΔtonB2ΔtonB3 showed moderate growth and ΔentAΔtonB1ΔtonB2 failed to grow. These results indicate that TonB2, and to a lesser extent, TonB1 supplied energy necessary for the DesA-mediated transport of FOB into the periplasmic space. TonB3, on the other hand, is not associated with this process. Therefore, DesA is distinct from the A. hydrophila ferric enterobactin receptor, IrgA, which is powered exclusively by the TonB2 system.Citation16)
Fig. 7. Determination of TonB specificity of DesA by performing growth assays of tonB double mutants.
Notes: ΔentAΔtonB1ΔtonB3 (circle), ΔentAΔtonB2ΔtonB3 (triangle), and ΔentAΔtonB1ΔtonB2 (square) were grown in the −Fe/+DFOB. OD600 was measured every hour for 20 h. A representative example from three independent experiments is shown.
In conclusion, we identified the desA and desR genes in A. hydrophila ATCC 7966T, which encode the outer membrane receptor for FOB and the AraC-type transcriptional regulator of desA, respectively. The function of desA was confirmed by growth assays of the desA deletion mutant and its complementing strain. Moreover, the IROMP expression profile and RT-qPCR analysis of the desR deletion mutant showed that DesR is responsible for the induction of desA under −Fe conditions. This occurs in the presence of DFOB, which serves both as a xenosiderophore and as a co-activator of its own IROMP receptor expression. Meanwhile, we found that unlike V. furnissii, A. hydrophila possesses desB which constitutes an operon with desA. Since DesB is homologous to E. coli FhuF that has been proposed to be involved in the reduction of ferric iron in cytoplasmic FOB,Citation37) an enzymatic study of DesB would give a clue for a better understanding of the subsequent utilization of FOB-bound iron in this species.
Bacteria typically express genes at considerable levels only when the encoded functions are beneficial for them to save energy. Considering that A. hydrophila commonly inhabits soil and freshwater, utilization of DFOB for iron acquisition may represent an important strategy that the bacterium has adopted to compete with neighboring micro-organisms for survival and proliferation within its various niches. In fact, different microbes that produce hydroxamate-type siderophores, including ferrioxamine species, are widely distributed in aquatic and terrestrial environments.Citation38–42) Future studies are required to characterize the inner membrane FOB transport system and elucidate the molecular mechanism by which desA transcription is induced by DesR in the presence of DFOB.
Acknowledgment
We thank Ms Y. Ido for assistance in the experiments.
Funding
This study was supported by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [20790124 to TF].
Notes
Abbreviations: DFOB, desferrioxamine B; EDDA, ethylenediamine-di(o-hydroxyphenylacetic acid); FOB, ferrioxamine B; Fur, ferric uptake regulator; FURTA, Fur titration assay; IROMP, iron-repressible outer membrane protein; OMP, outer membrane protein; LB, Luria-Bertani; PAGE, polyacrylamide gel electrophoresis; RT-qPCR, reverse transcriptase-quantitative PCR.
References
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000;54:881–941.10.1146/annurev.micro.54.1.881
- Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007;71:413–451.10.1128/MMBR.00012-07
- Krewulak KD, Vogel HJ. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta. 2008;1778:1781–1804.10.1016/j.bbamem.2007.07.026
- Escolar L, Pérez-Martin J, de Lorenzo V. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 1991;181:6223–6229.
- Carpenter BM, Whitmire JM, Merrell DS. This is not your mother’s repressor: the complex role of Fur in pathogenesis. Infect. Immun. 2009;77:2590–2601.10.1128/IAI.00116-09
- Heinrichs DE, Poole K. Cloning and sequence analysis of a gene (pchR) encoding an AraC family activator of pyochelin and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. J. Bacteriol. 1993;175:5882–5889.
- Fetherston JD, Bearden SW, Perry RD. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol. Microbiol. 1996;22:315–325.10.1046/j.1365-2958.1996.00118.x
- Beaumont FC, Kang HY, Brickman TJ, Armstrong SK. Identification and characterization of alcR, a gene encoding an AraC-like regulator of alcaligin siderophore biosynthesis and transport in Bordetella pertussis and Bordetella bronchiseptica. J. Bacteriol. 1998;180:862–870.
- Pradel E, Guiso N, Locht C. Identification of AlcR, an AraC-type regulator of alcaligin siderophore synthesis in Bordetella bronchiseptica and Bordetella pertussis. J. Bacteriol. 1998;180:871–880.
- Tanabe T, Takata N, Naka A, Moon Y-H, Nakao H, Inoue Y, Narimatsu S, Yamamoto S. Identification of an AraC-like regulator gene required for induction of the 78 kDa ferrioxamine B receptor in Vibrio vulnificus. FEMS Microbiol. Lett. 2005;249:309–314.10.1016/j.femsle.2005.06.025
- Tanabe T, Funahashi T, Miyamoto K, Tsujibo H, Yamamoto S. Identification of genes, desR and desA, required for utilization of desferrioxamine B as a xenosiderophore in Vibrio furnissii. Biol. Pharm. Bull. 2011;34:570–574.10.1248/bpb.34.570
- Semel JD, Trenholme G. Aeromonas hydrophila water-associated traumatic wound infections: a review. J. Trauma Injury Infect. Crit. Care. 1990;30:324–327.10.1097/00005373-199003000-00011
- Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010;23:35–73.10.1128/CMR.00039-09
- Barghouthi S, Young R, Olson MO, Arceneaux JE, Clem LW, Byers BR. Amonabactin, a novel tryptophan- or phenylalanine-containing phenolate siderophore in Aeromonas hydrophila. J. Bacteriol. 1989;171:1811–1816.
- Telford JR, Raymond KN. Amonabactin: a family of novel siderophores from a pathogenic bacterium. J. Biol. Inorg. Chem. 1997;2:750–761.10.1007/s007750050191
- Funahashi T, Tanabe T, Miyamoto K, Tsujibo H, Maki J, Yamamoto S. Biosci. Biotechnol. Biochem. 2013;77:353–360.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning, a laboratory manual. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1989.
- Seshadri R, Joseph SW, Chopra AK, Sha J, Shaw J, Graf J, Haft D, Wu M, Ren Q, Rosovitz MJ, Madupu R, Tallon L, Kim M, Jin S, Vuong H, Stine OC, Ali A, Horneman AJ, Heidelberg JF. Genome sequence of Aeromonas hydrophila ATCC 7966T: jack of all trades. J. Bacteriol. 2006;188:8272–8282.10.1128/JB.00621-06
- Yamamoto S, Akiyama T, Okujo N, Matsu-ura S, Shinoda S. Demonstration of a ferric vibrioferrin-binding protein in the outer membrane of Vibrio parahaemolyticus. Microbiol. Immunol. 1995;39:759–766.10.1111/mim.1995.39.issue-10
- Stojiljkovic I, Bäumler AJ, Hantke K. Fur regulon in Gram-negative bacteria. J. Mol. Biol. 1994;236:531–545.10.1006/jmbi.1994.1163
- Kuroda T, Mizushima T, Tsuchiya T. Physiological roles of three Na+/H+ antiporters in the halophilic bacterium Vibrio parahaemolyticus. Microbiol. Immunol. 2005;49:711–719.10.1111/mim.2005.49.issue-8
- Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2007;2:924–932.10.1038/nprot.2007.132
- Tanabe T, Funahashi T, Okajima N, Nakao H, Takeuchi Y, Miyamoto K, Tsujibo H, Yamamoto S. The Vibrio parahaemolyticus pvuA1 gene (formerly termed psuA) encodes a second ferric vibrioferrin receptor that requires tonB2. FEMS Microbiol. Lett. 2011;324:73–79.10.1111/fml.2011.324.issue-1
- Demarre G, Guérout AM, Matsumoto-Mashimo C, Rowe-Magnus DA, Marlière P, Mazel D. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPα) conjugative machineries and their cognate Escherichia coli host strains. Res. Microbiol. 2005;156:245–255.10.1016/j.resmic.2004.09.007
- Keen NT, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197.10.1016/0378-1119(88)90117-5
- Parales RE, Harwood CS. Construction and use of a new broad-host-range lacZ transcriptional fusion vector, pHRP309, for Gram− bacteria. Gene. 1993;133:23–30.10.1016/0378-1119(93)90220-W
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1972. p. 352–355.
- Matzanke BF, Anemüller S, Schünemann V, Trautwein AX, Hantke K. FhuF, part of a siderophore-reductase system. Biochemistry. 2004;43:1386–1392.10.1021/bi0357661
- Lux TM, Lee R, Love J. Complete genome sequence of a free-living Vibrio furnissii sp. nov. strain (NCTC 11218). J. Bacteriol. 2011;193:1487–1488.10.1128/JB.01512-10
- de Lorenzo V, Wee S, Herrero M, Neilands JB. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J. Bacteriol. 1987;169:2624–2630.
- Postle K, Larsen RA. TonB-dependent energy transduction between outer and cytoplasmic membranes. Biometals. 2007;20:453–465.10.1007/s10534-006-9071-6
- Sean Peacock R, Weljie AM, Peter Howard S, Price FD, Vogel HJ. The solution structure of the C-terminal domain of TonB and interaction studies with TonB box peptides. J. Mol. Biol. 2005;345:1185–1197.10.1016/j.jmb.2004.11.026
- Steuyvé M, Moons M, Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J. Mol. Biol. 1991;218:141–148.
- Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 1997;61:393–410.
- Balado M, Osorio CR, Lemos ML. Biosynthetic and regulatory elements involved in the production of the siderophore vanchrobactin in Vibrio anguillarum. Microbiology. 2008;154:1400–1413.10.1099/mic.0.2008/016618-0
- Kwon HJ, Bennik MHJ, Demple B, Ellenberger T. Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA. Nat. Struct. Biol. 2000;7:424–430.
- Muller K, Matzanke BF, Schunemann V, Trautwein AX, Hantke K. FhuF, an iron-regulated protein of Escherichia coli with a new type of [2Fe–2S] center. Eur. J. Biochem. 1998;258:1001–1008.10.1046/j.1432-1327.1998.2581001.x
- Tortell PD, Maldonado MT, Granger J, Price NM. Marine bacteria and biogeochemical cycling of iron in the oceans. FEMS Microbiol. Ecol. 1999;29:1–11.10.1111/fem.1999.29.issue-1
- Winkelmann G. Ecology of siderophores. In: Crosa JH, Mey AR, Payne SM, editors. Iron transport in bacteria. Washington (DC): ASM Press; 2004. p. 437–450.
- Essén SA, Bylund D, Holmström SJ, Moberg M, Lundström US. Quantification of hydroxamate siderophores in soil solutions of podzolic soil profiles in Sweden. BioMetals. 2006;19:269–282.10.1007/s10534-005-8418-8
- Mawji E, Gledhill M, Milton JA, Tarran GA, Ussher S, Thompson A, Wolff GA, Worsfold PJ, Achterberg EP. Hydroxamate siderophores: occurrence and importance in the Atlantic Ocean. Environ. Sci. Technol. 2008;42:8675–8680.10.1021/es801884r
- Gledhill M, Buck KN. The organic complexation of iron in the marine environment. Front. Microbiol. 2012;3:1–17.
