Abstract
Fresh loquat leaves have been used as folk health herb in Asian countries for long time, although the evidence supporting their functions is still minimal. This study aimed to clarify the chemopreventive effect of loquat tea extract (LTE) by investigating the inhibition on proliferation, and underlying mechanisms in human promyelocytic leukemia cells (HL-60). LTE inhibited proliferation of HL-60 in a dose-dependent manner. Molecular data showed that the isolated fraction of LTE induced apoptosis of HL-60 as characterized by DNA fragmentation; activation of caspase-3, -8, and -9; and inactivation of poly(ADP)ribose polymerase. Moreover, LTE fraction increased the ratio of pro-apoptotic Bcl-2-associated X protein (Bax)/anti-apoptotic myeloid cell leukemia 1 (Mcl-1) that caused mitochondrial membrane potential loss and cytochrome c released to cytosol. Thus, our data indicate that LTE might induce apoptosis in HL-60 cells through a mitochondrial dysfunction pathway. These findings enhance our understanding for chemopreventive function of loquat tea.
Graphical Abstract
Schematic molecular mechanism of loquat tea-induced apoptosis in HL-60 cells which involves a mitochondrial dysfunction pathway

Loquat (Eriobotrya japonica) is a plant of the Rosaceae family, and all parts of loquat such as fruits, leaves, and peels have been reported to have health benefits. In particular, the leaves have higher flavonoid content than the peel or fruits, with stronger radical scavenging activityCitation1) , and have been reported to have preventive effects against skin diseases, chronic bronchitis, and cancer.Citation2,3) Recently, fresh loquat leaves were processed into a beverage, called as loquat tea (Biwa Cha in Japanese), after the fresh leaves are roasted to 350 °C for 30 min. In our previous study, we found that the chemical compounds in loquat tea extract (LTE) are different from the original components in fresh leaves, and some new phenolic compounds were produced during the processes of loquat leaves upon roasting.Citation4,5) In vitro analysis showed that LTE possessed higher phenolic contents than that from fresh leaves extract.Citation4) Bioactive assay data from both cell and animal models showed that LTE inhibited the production of pro-inflammatory factors and mouse paws edema.Citation5) Several lines of data have suggested that the bioactive compounds, such as phenolic compounds, that possess antioxidant and anti-inflammatory activities, will also have anticancer activity.Citation6,7) Thus, in the present study, we investigated the chemopreventive activity and underlying mechanisms of LTE by investigating the anti-proliferation effect and mechanisms in human promyelocytic leukemia cell (HL-60), which is a valid model for testing antileukemic or general antitumoral compounds.Citation8–10)
Cancer chemopreventive compounds have been reported to play an important role in the elimination of seriously damaged cells or tumor cells by inducing apoptosis.Citation11,12) The cells that have undergone apoptosis are typically shown in chromatin condensation and DNA fragmentation.Citation13) They are rapidly recognized by macrophages before cell lysis and can be removed without induction of inflammation.Citation11,14) Cell survival or apoptosis is determined by the complex interplay between proapoptotic (e.g. Bax and Bak) and antiapoptotic Bcl-2 family proteins (e.g. Bcl-2, Bcl-xL, or Mcl-1).Citation15) The change in the ratio of Bax/Bcl-2 is known to initiate caspase signaling.Citation15) In human malignancies, the increased expression of antiapoptotic proteins commonly occurs and is associated with cancer progression and resistance to chemotherapy. Thus, designing small molecule Bcl-2 inhibitors has been developed as one of the cancer therapy strategies.Citation16) Bax is localized to the cytoplasm under normal conditions. Upon stimulation, activated Bax leads directly to mitochondrial membrane permeabilization,Citation17,18) which promotes the release of proapoptotic factors including cytochrome c.Citation19) Released cytochrome c can activate caspase-9, which in turn cleaves and activates executioner caspase-3.Citation20) After caspase-3 activation, some specific substrates for caspase-3 such as poly(ADP)ribose polymerase (PARP) are cleaved and eventually lead to apoptosis.Citation21) Several lines of studies also demonstrated that caspase-8 can directly activate caspase-3 and sequentially induces apoptosis.Citation22,23)
Based on the properties of LTE and the roles of mitochondria dysfunction in initiating apoptosis, in the present study, we used HL-60 cells as the target cells to investigate the anti-proliferation activity and molecular mechanisms of LTE, focusing on mitochondria dysfunction pathway.
Materials and methods
Extraction and characterization of loquat tea
Extraction and characterization of loquat tea was reported previously.Citation4,5) In brief, loquat leaves were roasted to 350 °C for 30 min in a ceramic vessel. Both fresh leaves and roasted loquat tea were then boiled at 100 °C for 15 min. The supernatant of loquat tea was further separated by MCI gel-CHP20P to obtain A (water extract), B (30% EtOH extraction), C (50% EtOH), and D fraction (100% acetone). The fraction C containing highest polyphenols was further separated by ODS gel column to obtain C1-C9 fractions eluted by 10–90% MeOH. The extracts were analyzed with HPLC to identify the compounds known in fresh leaves as described previously.Citation4) All extracts and fractions were evaporated and stored at −20 °C for less than two months.
Reagents and cell culture
The antibodies against caspase-8, caspase-9, caspase-3 and PARP were from Cell Signaling Technology (Beverly, MA, USA). The antibodies against Bax (B-9), cytochrome c, COX-4, and α-tubulin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mcl-1 antibody was from (BD Biosciences, CA, USA), 3,3′-Dihexyloxacarbocyanine iodide (DiOC6(3)) was from Molecular Probes (Eugene, OR, USA). 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) were form Sigma (St. Louis, MO, USA). HL-60 cell line was obtained from the Cancer Cell Repository, Tohoku University, Japan and cultured at 37 °C, 5% CO2 in RPMI 1640 medium containing 10% fetal bovine serum (FBS) from Equitech-Bio (Kerrville, TX, USA) and 1% penicillin streptomycin and glutamine (PSG).
Phenolic content determination and DPPH assay
The concentration of the total phenolic substances was measured according to the previous method with some modification.Citation24) In brief, LTE or its fractions (10 μL) was mixed with 2% Na2CO3 (200 μL) in 96-well plates. Half-diluted Folin-Ciocalteu reagent (10 μL) was added to each well. The absorbance was measured at 595 nm after the mixture was stood for 30 min at room temperature. The gallic acid was used as standard, and the total phenolic content was expressed as a gallic acid equivalent (GAE) in milligrams per gram of LTE or its fractions. The radical scavenging activity of LTE and its fractions were measured by the 1-diphenyl-2-picrylhydrazyl (DPPH) method.Citation24) Briefly, 10 μL of each extraction fraction was mixed with 100 μM DPPH (190 μL) in 96-well plate. The absorbance was then measured at 492 nm after the mixture was stood for 30 min at room temperature. 6-Hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid (Trolox) was used as a standard. The DPPH scavenging activity was described as IC50 value.
Cell viability assay
The cell survival rate was measured by a MTT assay according to previous authors.Citation25) Briefly, HL-60 cells (1.5 × 104 cells/100 μL/well) were seeded into each well of 96-well plates. After incubation for 24 h, the cells were treated with different concentrations of LTE for 24 h. Then, MTT solution (5 mg/mL) was added to each well. After incubating the cells for another 4 h, the resulting MTT-formazan product was dissolved by the addition of 0.04 M HCl–isopropanol solutions. The amount of formazan was determined by measuring the absorbance at 595 nm in a microplate reader (Thermo Scientific Multiskan™, version 1.00.79, Finland). The results were expressed as the optical density ratio of the treatment to control.
Extraction of whole cellular protein and subcellular fractionation of cytochrome c
HL-60 (7.5 × 105/5 mL) cells were precultured in 6 cm dish with RPMI containing 10% FBS and 1% PSG for 24 h. The cells were treated with LTE for the indicated 24 h. Whole cellular protein was prepared as described in detail previously.Citation26) In brief, the harvested pellets were lysed with cell lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 0.01% of a protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). The subcellular fractions of harvested pellets were prepared with Mitochondria/Cytosol Fractionation Kit (BioVision, CA, USA) as described in the manufacturer’s manual. In brief, the harvested pellets were lysed with cytosol extraction buffer and then homogenized with 40 strokes, following centrifugation at 700 × g for 10 min at 4 °C. The supernatant was further centrifuged at 10,000 × g for 30 min at 4 °C, and the supernatant was used as cytosolic fraction. The pellet was dissolved in mitochondria extraction buffer and used as mitochondrial fraction.
Western blot analysis
The lysate of whole cellular protein or subcellular protein was boiled for 5 min. Protein concentration was determined by dye-binding protein assay (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s manual. Equal amounts of lysate protein were run on 10% SDS-PAGE and electrophoretically transferred to PVDF membrane (GE Healthcare, Buckinghamshire, UK). After blotting, the membrane was incubated with specific primary antibody overnight at 4 °C and further incubated for 1 h with HRP-conjugated secondary antibody. Bound antibodies were detected by using ECL system (GE Healthcare, Buckinghamshire, UK) with a Lumi Vision PRO machine (TAITEC Co., Saitama, Japan). The relative amount of proteins associated with specific antibody was quantified by Lumi Vision Imager software (TAITEC Co., Saitama, Japan).
DNA fragmentation and morphological analysis of apoptotic cells
DNA fragmentation assay was carried out as described previously.Citation27) Briefly, HL-60 (7.5 × 105/5 mL/6 cm dish) cells were treated with different concentrations of LTE for 24 h. The harvested cells were suspended in 500 μL of Tail buffer (100 mM NaCl, 50 mM Tris-HCl at pH 8.0, 100 mM EDTA, and 1% SDS) plus 0.5 mg/mL proteinase K. After 3 h of incubation at 55 °C, DNA was precipitated by adding an equal volume of isopropanol. The dried DNA precipitate was dissolved in 50 μL of TE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, pH 8.0) containing 0.1 mg/mL RNase A, and further incubated at 37 °C for 30 min to degrade contaminating mRNA. After incubation, loading buffer (6× LB Orange G, Wako, Nippon Gene, Tokyo, Japan) was added. The 100 bp DNA Ladder One (Nacalai Tesque, Kyoto, Japan) served as a molecular marker. DNA fragments were separated on 2% agarose gel and digitally imaged after staining with ethidium bromide. Cell morphology was observed under an optical Biozero microscope (BZ-8000, Keyence, Osaka, Japan).
Flow cytometric detection of mitochondrial membrane potential (ΔΨm) loss
ΔΨm was assessed by a cell permeable marker DiOC6(3), which specifically accumulates into mitochondria depending on ΔΨm.Citation28) HL-60 (1 × 105/2 mL/6-well plate) cells were treated with LTE for various times and then incubated with 20 nM of DiOC6(3) for 30 min. The cells were washed with PBS and resuspended in 1 mL of PBS. The cells were analyzed at FL1 (530 nm) with a flow cytometer (Cyflow, Partec GmbH™, Münster, Germany).
Statistical analysis
All data were statistically analyzed by one way ANOVA test. Means with differently lettered superscripts differ significantly at the probability of p < 0.05.
Results
Phenolic contents and DPPH scavenging activities
Previous studies suggested that phenolic components present in loquat leaves may contribute the antioxidant and anticancer activities.Citation3,29,30) Thus, we guided the purification of LTE by phenolic content and DPPH scavenging activity. We prepared loquat tea water extract (T) by boiling water for 15 min according to folk custom. Then, we separated T fraction with MCI gel, and C fraction by ODS gel as described in our previous paper.Citation5) Loquat leaves water extract (L) was used as a control. Their values responding to the purification steps are shown in Table , respectively. LTE (T) possessed higher phenolic content (94.0 ± 13 GAE mg/g) and stronger DPPH scavenging activity (IC50 = 51.8 ± 5.9) than that of fresh leaves extract (L). When LTE (T) was fractionated by MCI gel, C Fraction obtained by 50% EtOH elution showed highest phenolic content (258.2 ± 22.0 GAE mg/g) and strongest DPPH scavenging activity (IC50 = 25.9 ± 6.7) among the fractions by water (A), 30% EtOH (B) and 100% acetone (D). C fraction was further separated by ODS gel with MeOH elusion. As shown in Table , the highest phenolic content (267.0 ± 21.0 GAE mg/g) and strongest DPPH scavenging activity (IC50 = 20.1 ± 6.2) were observed in C2 fraction among other fractions which have been estimated in our previous works.Citation4)
Table 1. Phenolic content and DPPH scavenging activity of loquat leaves and LTE.
LTE inhibits the proliferation of HL-60 cells
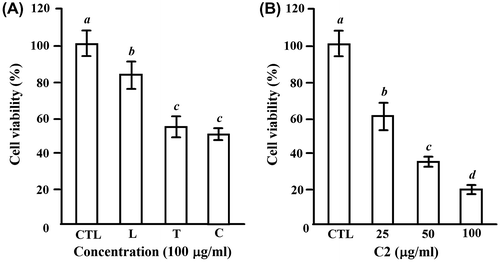
To test the effect of LTE on the proliferation of HL-60 cells, HL-60 cells were treated with 100 μg/mL of each extract for 24 h, and the survival fraction of HL-60 cells was investigated by a MTT assay. The cell viability of 82 ± 9.4% (L), 58 ± 7.2% (T), and 45 ± 3.8% (C) was observed, respectively (Fig. (A)). Since C fraction showed highest inhibition on the proliferation of HL-60 cells, we next performed a dose experiment for C2 fraction, which is separated from C fraction by ODS gel column and characterized as a group of similar phenolic compounds produced during the roasting process of fresh loquat leaves.Citation5) As shown in Fig. (B), C2 fraction showed a dose-dependent inhibition with the IC50 value of 40 μg/mL at 24 h treatment. Thus, we chose C2 fraction as a sample to investigate the mechanisms of LTE on the inhibition of the proliferation in HL-60 cells.
Fig. 1. The inhibitory effects of the extracts of loquat leaves and loquat tea on cell proliferation of HL-60 cells.
Notes: (A) The cells (1.5 × 104 cells/100 μL/well) were seeded into 96-well plates for 24 h, and then treated with 100 μg/mL of the loquat leaves water extract (L), loquat tea water extract (T), and C fraction separated from T by MCI gel with 50% EtOH (C) for another 24 h. (B) The cells were treated for 24 h with 25–100 μg/mL of C2 fraction, which was separated from C fraction by ODS gel with 20% MeOH. CTL cells were used as control cells without any treatment. MTT was added into medium for an additional 4 h. The survival of cells was detected by measuring the absorbance at a wavelength of 595 nm. The survival rate was expressed as the optical density ratio of the treatment to control. Each value represents the mean ± S.D. of triplicate cultures. Means with differently lettered superscripts differ significantly at the probability of p < 0.05.
LTE induces apoptosis
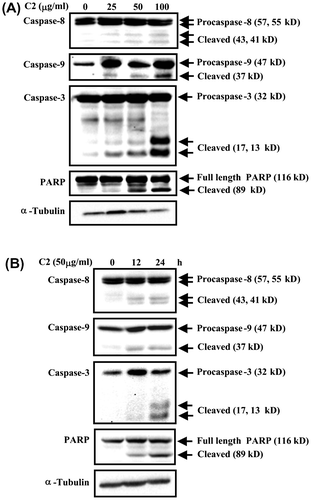
To elucidate whether C2 fraction inhibits the proliferation of HL-60 cells through apoptosis induction, we characterized the death cells by several aspects. First, an apoptotic morphology, including cell shrinkage and chromatin condensation, was observed under inverted microscopy after treatment with 25–100 μg/mL of C2 fraction for 24 h (Fig. (A)), and a dose-dependent DNA fragmentation was also detected in C2 fraction-treated cells (Fig. (B)). Moreover, other hallmarks of apoptosis including the proteolytic inactivation of PARP and the activations of caspase-3, -8, and -9 by cleaving the procaspases, were also detected in the indicated dose (Fig. (A)) and a time-dependent manner (Fig. (B)) in the fashion coinciding with morphology and DNA fragmentation (Fig. (B)). Taken together, we conclude that C2 fraction of LTE inhibits the proliferation of HL-60 cells through the induction of apoptosis.
Fig. 2. C2 fraction induces morphology changes and DNA fragmentation of HL-60 cells.
Notes: HL-60 cells were exposed to 0, 25, 50, and 100 μg/mL of C2 fraction for 24 h. Cell morphology was observed under an optical microscope (A). Then, the cells were harvested by centrifugation, and DNA was extracted as described in the Materials and methods section. The DNA fragments were separated on 2% agarose gel electrophoresis and visualized under ultraviolet light after staining with ethidium bromide. The 100 bp DNA Ladder One (Nacalai Tesque, Kyoto, Japan) served as a molecular marker (B).
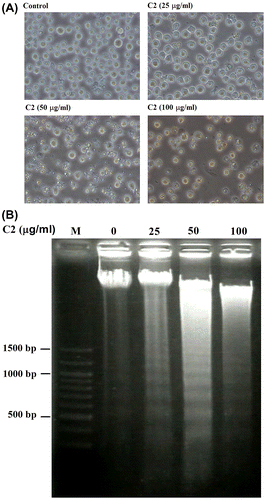
Fig. 3. C2 fraction-induced apoptosis involves the activation of caspase-8, caspase-9, and caspase-3 and inactivation of PARP.
Notes: (A) A dose-dependent experiment. HL-60 cells (7.5 × 105/5 mL/6 cm dish) were treated with 0, 25, 50, and 100 μg/mL of C2 fraction for 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. (B) A time-dependent experiment. HL-60 cells (7.5 × 105/5 mL/6 cm dish) were treated with 50 μg/mL of C2 fraction for 12 and 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. The result is a representative of triple experiments shown.
LTE induces apoptosis by upregulating the ratio of Bax and Mcl-1
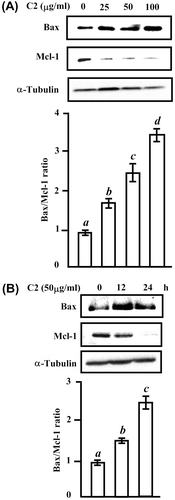
Cell survival is determined by the complex interplay between proapoptotic protein such as Bax, and antiapoptotic Bcl-2 family proteins including Mcl-1.Citation21) To know whether LTE influences these apoptotic regulatory proteins to induce apoptosis, we treated HL-60 cell for 24 h with indicated doses of C2 fraction (Fig. (A)) or with 50 μg/mL of C2 fraction for time-dependent (Fig. (B)). The increase in Bax protein and decrease in Mcl-1 protein were observed in both dose- and time-course experiments. The ratio of Bax and Mcl-1 was increased to 3.4-fold at 100 μg/mL of C2 fraction for 24 h (Fig. (A)), and to 1.5-fold at 12 h and 2.4-fold at 24 h by 50 μg/mL of C2 fraction (Fig. (B)). These data revealed the C2 downregulating antiapoptotic Mcl-1 protein and upregulating proapoptotic Bax protein.
Fig. 4. The change in the protein levels of proapoptogenic Bax and antiapoptogenic Mcl-1 by C2 fraction.
Notes: (A) A dose-dependent experiment. HL-60 cells (7.5 × 105/5 mL/6 cm dish) cells were treated with 0, 25, 50, and 100 μg/mL of C2 fraction for 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. (B) A time-dependent experiment. HL-60 (7.5 × 105/5 mL/6 cm dish) cells were treated with 50 μg/mL of C2 fraction for 12 and 24 h, and the whole-cell lysate was used for Western blotting analysis with the indicated specific antibodies, respectively. Each value represents the mean ± S.D. of triplicate cultures. Means with differently lettered superscripts differ significantly at the probability of p < 0.05.
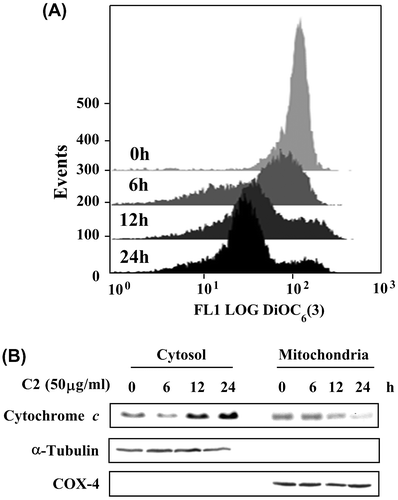
LTE-induced apoptosis involves ΔΨm loss and cytochrome c release
Accumulated data suggest that a mitochondria-initiated death pathway plays an important role in triggering apoptosis in response to chemicals.Citation15–17) To clarify whether apoptosis by C2 fraction of LTE is linked to mitochondria dysfunction, we examined the effect of C2 fraction on the ΔΨ m with a mitochondria-specific dye, DiOC6(3). As shown in Fig. (A), a time-dependent loss of ΔΨ m was observed after treatment with 50 μg/mL for 6, 12, and 24 h. The loss of ΔΨ m commonly leads to the release of cytochrome c from mitochondrial membrane to cytosol, which causes activations of caspases to induce apoptosis. Thus, we further analyze whether mitochondrial cytochrome c releases into cytosol. To elucidate whether mitochondrial cytochrome c releases into cytosol in LTE-induced apoptosis, we prepared mitochondrial and cytosolic fractions from the cells treated by C2 fraction, and then detected cytochrome c by Western blotting analysis. As shown in Fig. (B), a time-dependent accumulation of cytochrome c in the cytosol was detected. The integrity of the cytosolic and nuclear fractions was confirmed by the analysis of the compartment-specific cytosolic α-tubulin and mitochondria COX-4 proteins. These data indicate that apoptosis induced by C2 fraction of LTE involved a mitochondrial dysfunction pathway.
Fig. 5. The ΔΨm loss and cytochrome c release in HL-60 cells treated by C2 fraction.
Notes: (A) The ΔΨm loss. HL-60 (1 × 105/2 mL/6-wells plate) cells were treated with 50 μg/mL of C2 fraction for 0, 6, 12, and 24 h. The harvested cells were then incubated with 20 nM of DiOC6(3) for 30 min, followed by flow cytometric analysis as described under the Materials and methods section. (B) Cytochrome c release. HL-60 (7.5 × 105/5 mL/6 cm dish) cells were treated with 50 μg/mL of C2 fraction for 0, 6, 12, and 24 h. The harvested cells were fractionated into cytosolic and mitochondrial fractions as described under the Materials and methods section. Cytochrome c and the compartment-specific cytosolic α-tubulin or mitochondria COX-4 proteins were detected by Western blotting analysis with their respective antibodies.
Discussion
Mitochondrion has been reported to play a key role in the regulation of apoptosis.Citation31) Mitochondrial dysfunction including the loss of mitochondrial membrane potential (ΔΨm), permeability transition, and release of cytochrome c from the mitochondrion into the cytosol is associated with apoptosis.Citation32) Proapoptotic Bax can lead directly to mitochondrial membrane permeabilization to promote the release of cytochrome c.Citation17,19) Released cytochrome c activates downstream caspases to introduce apoptosis.Citation20) In this study, the upregulation of proapoptotic Bax protein and downregulation of antiapoptotic Mcl-1 protein were observed in the cells treated with C2 fraction of LTE (Fig. ). Moreover, the time-dependent loss of ΔΨm and cytochrome c release from mitochondrial to cytosol were observed in the cells with same treatment (Fig. ). These events might initiate the activation of caspases. Exactly, the activations of caspase-3, -8, and -9 were confirmed by detecting their cleaved peptides in both dose (Fig. (A)) and time experiments (Fig. (B)) in the fashion coinciding with apoptotic morphology and DNA fragmentation (Fig. (B)). These data demonstrated that a mitochondrial damage-dependent pathway might be involved in LTE-induced apoptosis in HL-60 cells.
Regarding the bioactive compounds contributing to the anticancer activities in LTE, we have characterized the bioactive compounds by HPLC, FT-IR and NMR methodologies in the previous study.Citation4) The strong and broad bands of O–H stretching were observed at 3252 cm−1, and one or more aromatic rings in a structure were normally readily determined from the C–H, C=C, and C–C rings. The peaks at 719.3 and 673.3 cm−1 were assigned as mono substitute benzene.Citation4) Furthermore, 1H-NMR spectra gave the signals for aromatic or olefinic protons and the signals for the methylene or alicyclic protons.Citation4) On other hand, we could not detect the bioactive flavonoids such as (-)-epicatechin, 3-caffeonylquinic acid, 5-caffeonylquinic acid and procyanidin B2 originally present in fresh loquat leaves.Citation4) Thus, we gathered that the new compounds in LTE might be composed of several kinds of similar phenolic compounds, which might be produced during the roasting process of fresh loquat leaves although these phenolic compounds need to be fully identified in a future study.Citation4,5) In the present study, we found that these phenolic-like compounds have the activity of anti-proliferation and apoptosis induction in HL-60 cells. Several lines of studies have showed that phenolic compound can induce apoptosis in HL-60 cells by generating reactive oxygen species,Citation10,28,33) which damage mitochondrial membrane to become dysfunction status including the loss of mitochondrial membrane potential (ΔΨm) and release of proapoptotic protein from the mitochondrion into the cytosol to initiate apoptosis. We have clarified the C2 fraction of LTE as a kind of similar phenolic compounds; thus, we considered that LTE might have same mechanisms as other phenolic compounds reported to induce apoptosis in HL-60 cells, although we will confirm these in our coming study.
In conclusion, LTE suppressed the proliferation of HL-60 cell by inducing mitochondrial dysfunction pathway. The results from cell models will help to understand the chemopreventive effects and molecular mechanisms of loquat tea.
Funding
This work was supported by the fund of Scholar Research of Kagoshima University, Japan.
Notes
Abbreviations: LTE, Loquat tea extract; Bax, Bcl-2-associated X protein: Mcl-1, anti-apoptotic myeloid cell leukemia 1; DiOC6(3), 3,3′-Dihexyloxacarbocyanine iodide; MTT, 3-(4,5-dimethylthiazol-2,5-diphenyltetrazolium bromide; DPPH, 1-diphenyl-2-picrylhydrazyl.
References
- Ferreres F, Gomes D, Valentão P, Gonçalves R, Pio R, Chagas EA, Seabra RM, Andrade PB. Improved loquat (Eriobotrya japonica Lindl.) cultivars: variation of phenolics and antioxidative potential. Food Chem. 2009;114:1019–1027.
- De Tommasi N, Aquino R, De Simone F, Pizza C. Plant metabolites. New sesquiterpene and ionone glycosides from Eriobotrya japonica. J. Nat. Prod. 1992;55:1025–1032.
- Ito H, Kobayashi E, Takamatsu Y, Li SH, Hatano T, Sakagami H, Kusama K, Satoh K, Sugita D, Shimura S, Itoh Y, Yoshida T. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem. Pharm. Bull. 2000;48:687–693.
- Zar PPK, Sakao K, Hashimoto F, Morishita A, Fujii M, Wada K, Hou DX. Antioxidant and anti-inflammatory activities of loquat (Eriobotrya japonica) tea. Funct. Food Health Dis. 2013;3:447–461.
- Zar PPK, Morishita A, Hashimoto F, Sakao K, Fujii M, Wada K, Hou DX. Anti-inflammatory effects and molecular mechanisms of loquat (Eriobotrya japonica) tea. J. Funct. Foods. 2014;6:523–533.
- Hu ML. Dietary polyphenols as antioxidants and anticancer agents: more questions than answers. Chang Gung Med. J. 2011;34:449–460.
- Ghiringhelli F, Rebe C, Hichami A, Delmas D. Immunomodulation and anti-inflammatory roles of polyphenols as anticancer agents. Anticancer Agents Med. Chem. 2012;12:852–873.
- Liu WK, Ho JCK, Che CT. Apoptotic activity of isomalabaricane triterpenes on human promyelocytic leukemia HL60 cells. Cancer Lett. 2005;230:102–110.
- Kim MK, Cho YH, Kim JM, Chun MW, Lee SK, Lim Y, Lee CH. Induction of apoptosis in human leukemia cells by MCS-C2 via caspase-dependent Bid cleavage and cytochrome c release. Cancer Lett. 2005;223:239–247.
- Hou DX, Ose T, Lin S, Harazoro K, Imamura I, Kubo M, Uto T, Terahara N, Yoshimoto M, Fujii M. Anthocyanidins induce apoptosis in human promyelocytic leukemia cells: structure-activity relationship and mechanisms involved. Int. J. Oncol. 2003;23:705–712.
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462.
- Galati G, Teng S, Moridani MY, Chan TS, O’Brien PJ. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metabol. Drug Interact. 2000;17:311–349.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257.
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449.
- Cory S, Adams JM. Killing cancer cells by flipping the Bcl-2/Bax switch. Cell. 2005;8:5–6.
- Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418.
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730.
- Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386.
- Lucken-Ardjomande S, Martinou JC. Newcomers in the process of mitochondrial permeabilization. J. Cell Sci. 2005;118:473–483.
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999;274:11549–11556.
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489.
- Kundu T, Dey S, Roy M, Siddiqi M, Bhattacharya RK. Induction of apoptosis in human leukemia cells by black tea and its polyphenol theaflavin. Cancer Lett. 2005;230:111–121.
- Schneider P, Tschopp. Apoptosis induced by death receptors. Pharm. Acta Helv. 2000;74:281–286.
- Rabah IO, Hou DX, Komine S, Shono M, Fujii M. Increase in antioxidant and cytotoxicity through apoptosis-induction on HL-60 of 60 sweet potato (Ipomoea batatas Lam. cv. Koganesengan) by subcritical water treatment. Food Sci. Technol. Res. 2005;11:122–126.
- Uto T, Fujii M, Hou DX. 6-(methylsulfinyl)hexyl isothiocyanate suppresses inducible nitric oxide synthase expression through the inhibition of Janus kinase 2-mediated JNK pathway in lipopolysaccharide-activated murine macrophages. Biochem. Pharmacol. 2005;70:1211–1221.
- Hou DX, Luo D, Tanigawa S, Hashimoto F, Uto T, Masuzaki S, Fujii M, Sakata Y. Prodelphinidin B-4 3′-O-gallate, a tea polyphenol, ins involved in the inhibition of COX-2 and iNOS via the downregulation of TAK-1-NF-κB pathway. Biochem. Pharmacol. 2007;74:742–751.
- Vakifahmetoglu H, Olsson M, Orrenius S, Zhivotovsky B. Functional connection between p53 and caspase-2 is essential for apoptosis induced by DNA damage. Oncogene. 2006;25:5683–5692.
- Lin S, Fujii M, Hou DX. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch. Biochem. Biophys. 2003;418:99–107.
- Ito H, Kobayashi E, Li SH, Hatano T, Sugita D, Kubo N, Shimura S, Itoh Y, Tokuda H, Nishino H, Yoshida T. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agric. Food Chem. 2002;50:2400–2403.
- Tanaka K, Tamaru S, Nishizono S, Miyata Y, Tamaya K, Matsui T, Tanaka T, Echizen Y, Ikeda I. Hypotriacylglycerolemic and antiobesity properties of a new fermented tea product obtained by tea-rolling processing of third-crop green tea (Camellia sinensis) leaves and loquat (Eriobotrya japonica) leaves. Biosci., Biotechnol., Biochem. 2010;74:1606–1612.
- Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377.
- Xia Z, Lundgren B, Bergstrand A, Depierre JW, Nässberger L. Changes in the generation of reactive oxygen species and in mitochondrial membrane potential during apoptosis induced by the antidepressants imipramine, clomipramine, and citalopram and the effects on these changes by Bcl-2 and Bcl-XL. Biochem. Pharmacol. 1999;57:1199–1208.
- Zunino SJ, Zhang Y, Seeram NP, Storms DH. Berry fruit extracts inhibit growth and induce apoptosis of high-risk acute lymphoblastic leukemia cells in vitro. J. Funct. Foods. 2010;2:187–195.
