Abstract
Caenorhabditis elegans is a versatile, whole-organism model for bioactivity screening. However, this worm has extensive defensive mechanisms against xenobiotics which limit its use for screening of pharmacologically active compounds. In this study, we report that knockdown of nhr-8, a gene involved in the xenobiotic response, increased the worm’s sensitivity to the lipid-reducing effects of some isoquinoline alkaloids, especially berberine. On the other hand, crude extract of rhizome and cultured cells showed enhanced biological activity compared to the pure alkaloids in wild type worm, but this enhanced activity was not detected in nhr-8 RNAi worm, suggesting that some components in cell extracts might interfere with the defense response in this worm. The possibility of using C. elegans as a model for screening bioactive chemicals is discussed.
Graphical Abstract
Knockdown of nhr-8, a gene involved in the xenobiotic response, increased the worm's sensitivity to the lipid-reducing effects of some isoquinoline alkaloids, especially berberine.
Nuclear receptors (NRs) are a diverse class of transcription factors that mediate hormonal signaling processes in vertebrates and insects, and are known to extend beyond the direct transduction of endocrine signals to include responses to a variety of signaling molecules (including xenobiotics), participation in multiple signal transduction pathways, and regulation of diverse physiological and developmental processes. While many of the mechanisms by which ligand-regulated, hormone-responsive NRs activate or repress the transcription of target genes have been well characterized, little is known about the cognate ligands of the remaining orphan NRs.Citation1) The Caenorhabditis elegans genome sequence contains 284 confirmed or predicted NR genes, which is over five-fold more than the number found in the human genome,Citation2) and a few of them exhibit conserved physiological functions across taxa.
Under natural conditions, C. elegans is exposed to a wide range of chemical assaults found in soil. As a result, it must be able to efficiently detoxify organic chemicals for survival. Recent studies have identified many of the genes that encode sensors and enzymes that the worms may use in their xenobiotic responses such as a large number of four main classes of detoxification enzymes including cytochrome P450 (CYP), short-chain dehydrogenases (SDR), UDP-glucuronosyl or glycosyl transferases (UGT), and glutathione-S-transferases (GST).Citation3) Most studies on the mechanism of detoxification in C. elegans have attempted to circumvent its xenobiotic resistance in screening for more effective potential nematicides among toxic compounds, including plant secondary metabolites.
nhr-8 is a gene that encodes a nuclear hormone receptor which is involved in the xenobiotic resistance response of C. elegans. Although the actual target genes of nhr-8 have not yet been identified, the precedent PXR (pregnane X receptor) and constitutive androstane receptor activities in vertebrates suggest that NHR-8 may regulate the expression of the cytochrome P450 genes in C. elegans. The toxin sensitivity of nhr-8 is specific, since nhr-8 RNAi worms were found to be more sensitive than wild-type worms to the toxins colchicine and chloroquine, but not to the pathogenic bacterium Pseudomonas aeruginosa.Citation4)
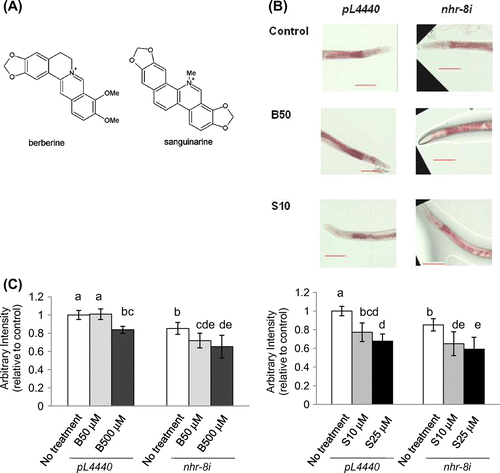
Plant alkaloids have been suggested to function in the defense against herbivores and pathogens. Therefore, they are likely to induce a xenobiotic response and consequently their bioavailability might be reduced in vivo. Accordingly, the isoquinoline alkaloids, berberine and sanguinarine (Fig. (A)), were also expected to induce a xenobiotic response in these worms. Previously, the genome-wide response of the worms to berberine and sanguinarine treatment was investigated by a microarray analysis and the results indicated that defense-response and detoxification genes including F08G5.6 (a defense-response gene), cyp-35C1, gst-5, ugt-21, and ugt-25 were up-regulated (data not shown).
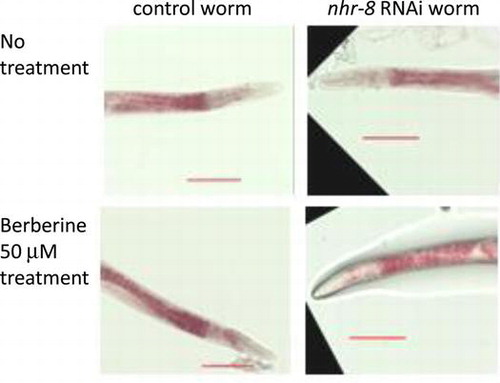
Fig. 1. Effects of berberine and sanguinarine on lipid accumulation in nhr-8 RNAi worms.
Notes: (A) Molecular structures of berberine and sanguinarine. (B) Effects of berberine and sanguinarine on lipid accumulation in RNAi control worm and nhr-8 RNAi worms. Images show typical results from Oil Red O staining of worms after treatment with berberine at 50 μM and sanguinarine at 10 μM. Images shown at 20× magnification (scale bar = 100 μm). B – berberine, S – sanguinarine. Images of worms treated with higher concentrations are shown in Supplementary Fig. S1. (C) Effects of berberine and sanguinarine on lipid accumulation in RNAi control worm and nhr-8 RNAi worms. Quantification of Oil Red O staining intensity using ImageJ. Values are the averages of 10–12 images for each compound tested and are normalized to the RNAi control sample; error bar = standard deviation; different letters indicate the statistic significance at p < 0.05 (ANOVA followed by Tukey’s test). B – berberine, S – sanguinarine.
Although nhr-8 RNAi worms have been used to investigate their sensitivity to toxic compounds that act as nematicides, there have been no studies on the response of nhr-8 RNAi worms to the bioactivities of the compounds being applied. In previous experiments with wild-type N2 worms, we found that treatment consistently yielded detectable lipid-reducing activity through Oil Red O staining at 500 μM for berberine and 25 μM for sanguinarine, but lower concentrations, such as 50 μM berberine and 10 μM sanguinarine, were less effective.Citation5) In an attempt to enhance the sensitivity of the worm screening system and to minimize the effective concentration of test compounds needed for bioactivity assays, the detoxification response in C. elegans was impeded by knocking down nhr-8 and treating nhr-8 RNAi worms with lower alkaloid concentrations to check if this could increase the alkaloid bioavailability in these worms. Next, we investigated if the increased sensitivity to alkaloid treatment could be due to higher bioavailability in the worms by analyzing the accumulation of alkaloids in the worms. Differences in the induction of detoxification genes between an alkaloid-treated control and nhr-8 RNAi worms were also analyzed.
Materials and methods
Chemicals and reagents
The following materials were used in the experiments: berberine sulfate (Tokyo Chemical Industry Co., Ltd), sanguinarine chloride (Sigma–Aldrich), palmatine chloride (Mitsui Petrochemical Industries), coptisine chloride (Wako Pure Chemicals, Osaka, Japan), magnoflorine (a gift from R. Nishida, Kyoto University), and columbamine (prepared in our laboratory).Citation6) Coptis rhizomes were purchased from a local market, and a Coptis japonica cell cultureCitation7) was established and maintained routinely in our laboratory. All other reagents were purchased from Wako Pure Chemicals.
Coptis rhizome and cultured C. japonica cell extracts
Ten g fresh weight of C. japonica (156-S) cells and 10.0 g of Coptis rhizome slices were soaked in 100 mL methanol for 48 h, respectively. The filtered extracts were concentrated using a rotary evaporator and dissolved in distilled water for a bioassay and analysis by LC-MS.
Nematode strains
Wild-type: N2 (Bristol) were maintained on nematode growth media at 20 °C according to standard culture methods.Citation8)
Oil Red O staining
Two-day-old worms were treated with and without alkaloids (as a control) for 24 h. About 200–300 worms were collected and washed three times with 1X PBS pH 7.4 buffer. Oil Red O staining was performed as previously reported.Citation9) The results were verified by reproducibility in at least two of three independent experiments.
RNA interference
An RNAi feeding methodCitation10) was used. Details are described in the Supplemental Information.
Quantitative RT–PCR
Total RNA was extracted with Sepasol-RNA I Super G (Nacalai Tesque), purified with an RNeasy Mini Kit (Qiagen), and reverse-transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen) with oligo(dT) primer. cDNA (final concentration of 500 pg μL−1) was subjected to qRT-PCR analysis using the CFX96 Real-Time PCR System (Bio-Rad Laboratories, Inc.) with IQ SYBR Green Super Mix (Bio-Rad). The conditions for the PCR reactions and the primer sequences are listed in the Supplemental Information.
LC-MS analysis of the accumulation of alkaloids and their metabolites in worms
Worms were washed with 0.1% SDS solution and then in M9 buffer to remove compounds stuck to the worm cuticle. After washing, worms were homogenized in 2X lysis solution (100 mM KCl, 20 mM Tris pH 8.3, 0.4% SDS, 120 μg/mL proteinase K) with coptisine chloride added as an internal standard. The details of the LC-MS analysis parameters and quantification are described in the Supplemental Information.
LC-MS analysis of Coptis rhizome and cultured cell extracts
The details of LCMS analysis parameters and quantification are described in the Supplemental Information.
Statistical analyses
The results are expressed as the mean ± standard deviation. Comparisons between groups were performed by analysis of variance (ANOVA) and Tukey test. p < 0.05 was considered statistically significant.
Results
RNAi knockdown of nhr-8 enhanced the lipid-reducing activity of berberine
In wild-type N2 worms, 500 μM berberine and 25 μM sanguinarine showed consistent lipid-reducing activity with Oil Red O staining, and lower concentrations such as 50 μM berberine and 10 μM sanguinarine were less effective.Citation5) Since the detoxification process might reduce the effectiveness of alkaloids, we knocked-down nhr-8, a nuclear hormone receptor gene that is involved in the xenobiotic resistance response, with RNAi and examined the worm’s susceptibility to alkaloid treatment (Supplemental Fig. 1). nhr-8 RNAi worms showed significantly greater lipid reduction (p < 0.05) at lower concentration of berberine (50 μM) than control worm without RNAi treatment after treatment for 24 h (Fig. (B) and (C), Supplemental Fig. 2), while control RNAi worms also showed lipid reduction with 10 μM sanguinarine. These results also indicated a difference in the enhancement of alkaloid sensitivity in nhr-8 RNAi worms.
nhr-8 RNAi worms accumulated more berberine and sanguinarine at low dosage
To clarify the mechanism of the enhanced lipid-reducing activity of alkaloids in nhr-8 RNAi worms, we investigated the metabolites of alkaloids in worms. Recent studies have reported that berberine is metabolized into four major compounds: berberrubine (m/z 322), thalifendine (m/z 322), demethyleneberberine (m/z 324) and jatrorhizine (m/z 338) in rat plasma, human liver microsomes, and rat and human urine.Citation11–14) While fewer metabolites have been reported for sanguinarine due to its cytotoxic property, dihydrosanguinarine (m/z 334), the main metabolite, and some metabolites such as m/z 334 after ring cleavage, m/z 320 after successive O-demethylation and m/z 336 after ring-cleavage of m/z 334 were also detected in human, rat and pig liver microsomes.Citation15,16)
Using these metabolic data for berberine and sanguinarine as a reference, we analyzed the alkaloid metabolites in treated worms using LC-MS (Fig. (A) and (B), Supplemental Fig. 3). Since preliminary experiments using wild-type worms did not show a distinct peak difference for cell extract or culture medium between 6 and 24 h-treatment, we analyzed nhr-8 RNAi worms after treatment for 24 h for the measurement of lipid reduction. In berberine treatment, only berberine was detected and no other molecular ion peak that corresponded to any of the four major metabolites (berberrubine, thalifendine, demethyleneberberine and jatrorhizine) or glucuronide conjugates was found. Thus, the change in berberine content was analyzed in an nhr-8 RNAi experiment. On the other hand, for sanguinarine treatment, sanguinarine was metabolized into several components, and the content of metabolites with m/z 334, m/z 336 were analyzed to estimate the alkaloid availability in worm.
Fig. 2. Accumulation of alkaloid metabolites in nhr-8 RNAi worms.
Notes: (A) Liquid chromatogram of extracts from RNAi vector control (pL4440) and nhr-8 RNAi (nhr-8i) worms treated with berberine at 50 μM and sanguinarine at 10 μM. B – berberine, S – sanguinarine. Chromatogram of the internal standard and worms treated with 500 μM berberine and 25 μM sanguinarine are shown in Supplemental Fig. S3. (B) Accumulation of berberine, sanguinarine and their metabolites in RNAi vector control and nhr-8 RNAi worms. Semi-quantitative values are presented here as μM equivalence of coptisine (internal standard). These values represent one of three independent experiments. Results were verified by reproducibility in at least two of three independent experiments. L4 – RNAi vector control, N8 – nhr-8 RNAi worm. B – berberine, S – sanguinarine. Accumulation of (C) berberine, (D) sanguinarine and their metabolites (m/z 334, 336) in RNAi control and nhr-8 RNAi worms. Values are presented relative to the RNAi vector control for each concentration tested. These values represent the average of three independent experiments. Error bar = standard deviation. L4 – RNAi vector control, N8 – nhr-8 RNAi worm. B – berberine, S – sanguinarine.
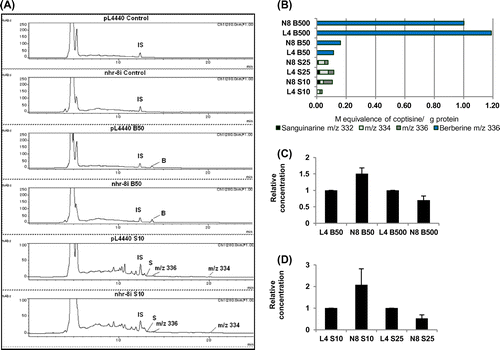
Although we expected that the nhr-8i worms would accumulate more alkaloids, cellular alkaloid contents considerably fluctuated by experiments and some increases only detected at low dosages, i.e. at 50 μM berberine and 10 μM sanguinarine (p-value about 0.3 by Student’s t-test). These increases were not detected at high dosages, i.e. at 500 μM berberine (Fig. (C)) and 25 μM sanguinarine (Fig. (D)). While it is unclear why nhr-8 RNAi worms accumulated less alkaloid than RNAi control worms at high dosage, other orphan receptor genes involved in xenobiotic metabolism in nhr-8 knockdown worm might enhance the detoxification pathways at high dosages.
The major detoxification response genes induced by berberine are mostly independent of NHR-8
To characterize the enhanced lipid-reducing effects of berberine in nhr-8 knockdown worms, we next analyzed the expressions of major detoxification genes that were up-regulated after berberine treatment in wild-type worms (data not shown). Unexpectedly, qRT-PCR results showed a similar high expression of detoxification genes after treatment with berberine in nhr-8 knockdown worms (Fig. ). Furthermore, the induction of detoxification defense genes was found to increase with an increased concentration of alkaloids in both control and nhr-8i worms. Sanguinarine had similar inducing effects on detoxification genes as berberine.
Fig. 3. Effects of alkaloid treatment on RNAi control and nhr-8 RNAi worms.
Notes: qRT-PCR showed the expression of detoxification genes. The mRNA abundance value represents the average of three independent experiments. pL4440 – RNAi vector control, nhr-8i – nhr-8 RNAi worm. B – berberine, S – sanguinarine.
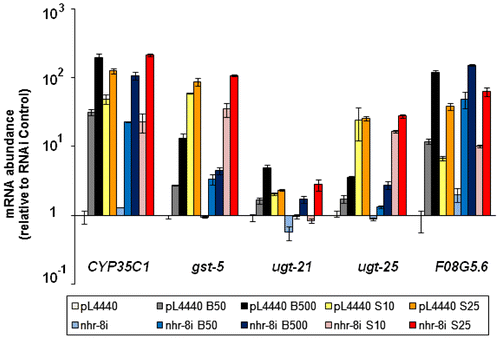
The C. elegans Mediator subunit MDT-15, a transcriptional coregulator in RNA polymerase II-dependent transcription, has been reported to play a role in integrating metabolic responses to ingested materials such as nutrients and xenobiotic compounds. Taubert et al.Citation17) reported that the expressions of CYP35C1, gst-5, and ugt-25, among several other detoxification genes, are MDT-15-dependent and nhr-8 was dispensable for the MDT-15-dependent expression of these genes. Their findings correlated with our qRT-PCR results in that the induction of major upregulated detoxification genes was independent of nhr-8.
nhr-8 RNAi worms survived during treatment for 24 h, while the lipid-reducing activity of alkaloids was more enhanced. Increased expression levels of detoxification genes after treatment with berberine and sanguinarine in nhr-8 RNAi worms could play a role in the worm’s defense against these alkaloids, while nhr-8 RNAi worms were more sensitive to some alkaloids such as colchicine and chloroquine.Citation4)
Effects of rhizome and cultured cell extracts on lipid accumulation in C. elegans
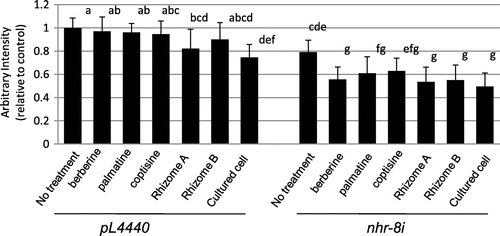
The greater sensitivity of nhr-8 RNAi worms to berberine suggested that we should investigate the effects of extracts of Coptis rhizomes and cultured C. japonica cells, which might mimic the results of berberine in nhr-8 RNAi worms. The cell extracts, which contain berberine, palmatine, coptisine, columbamine, magnoflorine and some unknown peaks with m/z 336, 322, 338 and 324 (Supplemental Fig. 4), were adjusted to contain about 100 μM berberine and fed to the worms. The rhizome and cultured cell extracts showed stronger lipid-reducing effects in the RNAi control worms than berberine, whereas the effects of the cell extract relative to that of berberine were weaker in nhr-8 RNAi worms than in RNAi control worms (Fig. (B)). Whereas extracts of rhizome and cultured cells contain several other isoquinoline alkaloids with berberine, these alkaloids such as palmatine and coptisine were also less effective on the lipid-reduction in control worm, suggesting that other type of metabolites in cell extracts would enhance the sensitivity. Measurement of nhr-8 expression by RT-PCR indicated that the effects of cell extracts were not due to a change in nhr-8 expression (data not shown). The investigation of chemicals that might mimic the suppression of nhr-8 expression is underway.
Fig. 4. Effects of berberine, palmatine, coptisine, cell extracts of Coptis rhizomes and cultured Coptis cells on lipid accumulation in RNAi control and nhr-8 RNAi worms.
Notes: Oil Red O staining intensity quantified as the average of 10–12 images for each compound tested and normalized to the RNAi control sample; error bar = standard deviation; different letters indicate the statistic significance at *p < 0.05 (ANOVA followed by Tukey’s test). Berberine, palmatine, and coptisine were treated at 100 μM. Cell extract of Coptis rhizome A contains 104 μM berberine, 30 μM palmatine, 23 μM coptisine, while that of rhizome B contains 95 μM berberine, 5 μM palmatine, 5 μM coptisine and that of cultured Coptis cell contains 105 μM berberine, 5 μM palmatine, 20 μM coptisine.
Discussion
Previously, we have shown that C. elegans is a versatile, whole-organism probe for screening the bioactivities of isoquinoline alkaloids.Citation5) However, this worm has extensive physical (such as a thick cuticle) and molecular (detoxification enzymes) defense mechanisms against xenobiotics. In many studies, the effective doses of test compounds in the worm were often several orders of magnitude higher than those in a mammalian cell culture.Citation18,19) These factors limit the usage of the worm for screening the bioactivity of pharmacologically active molecules.
In this study, nhr-8 knockdown worms were found to be more susceptible to the lipid-reducing effects of some isoquinoline alkaloids, such as berberine, palmatine and coptisine (Figs. and ). The increased sensitivity of nhr-8 RNAi worms to alkaloid treatment suggests that a detoxification mechanism may be impaired. Although we found that nhr-8 RNAi worms were susceptible to the lipid-reducing effects of several isoquinoline alkaloids, the degree of this sensitivity may vary due to the specificity of both the nhr-8 receptor and the alkaloid’s target. Berberine has been reported to be a substrate of the multidrug membrane transporter P-glycoprotein (P-gp) and is readily effluxed, which would result in low bioavailability.Citation20,21) nhr-8 knockdown could increase the accumulation of berberine (at 50 μM), suggesting that P-gp function may be impeded. On the other hand, nhr-8 knockdown showed less enhanced sensitivity to sanguinarine. Sanguinarine is known to act as a P-gp-mediated multidrug resistance reversal agent, and inhibits ABC transporter activity.Citation22) Whereas we could not confirm the high content of sanguinarine in worm, sanguinarine could have higher bioavailability and thus is effective at a lower concentration (25 μM) than berberine (500 μM), as found in our previous experiment.Citation5)
Many pharmaceutical drugs are isolated directly from plants or are semi-synthetic derivatives of natural products. However, the pipeline of drug discovery faces technical challenges in isolating new compounds with diverse structures and complex chemistries in sufficient quantities for screening.Citation23) Thus, the ability to detect bioactivity at lower dosages using nhr-8 RNAi worms (as observed with berberine) could be useful for future screening experiments in which the initial concentration of a bioactive compound may be too low to be effectively detected by high-throughput screening.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.940278.
Supplementary Material
Download MS Word (557.5 KB)Funding
This research was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) [grant number 21248013 to F.S.]. Y.L. Chow is the recipient of a MEXT Scholarship.
References
- Gissendanner CR, Crossgrove K, Kraus KA, Maina CV, Sluder AE. Expression and function of conserved nuclear receptor genes in Caenorhabditis elegans. Dev. Biol. 2004;266:399–416.10.1016/j.ydbio.2003.10.014
- Maglich JM, Sluder A, Guan X, Shi Y, McKee DD, Carrick K, Kamdar K, Willson TM, Moore JT. Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol. 2001;2: RESEARCH0029:1–7.
- Lindblom TH, Dodd AK. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J. Exp. Zool. A Comp. Exp. Biol. 2006;305A:720–730.10.1002/(ISSN)1552-499X
- Lindblom TH, Pierce GJ, Sluder AE. A C. elegans orphan nuclear receptor contributes to xenobiotic resistance. Curr. Biol. 2001;11:864–868.10.1016/S0960-9822(01)00236-6
- Chow YL, Sato F. Screening of isoquinoline alkaloids for potent lipid metabolism modulation with Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2013;77:409–416.
- Ikezawa N, Iwasa K, Sato F. Molecular cloning and characterization of methylenedioxy bridge-forming enzymes involved in stylopine biosynthesis in Eschscholzia californica. FEBS J. 2007;274:1019–1035.10.1111/j.1742-4658.2007.05652.x
- Sato F, Hashimoto T, Hachiya A, Tamura K, Choi KB, Morishige T, Fujimoto H, Yamada Y. Metabolic engineering of plant alkaloid biosynthesis. Proc. Nat. Acad. Sci. 2001;98:367–372.10.1073/pnas.98.1.367
- Stiernagle T. WormBook. ed. The C. elegans Research Community, WormBook, 2006. doi:/10.1895/wormbook.1.101.1. Available from: http://www.wormbook.org.
- O’ Rourke EJ, Soukas AA, Carr CE, Ruvkun G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 2009;10:430–435.10.1016/j.cmet.2009.10.002
- Lehner B, Tischler J, Fraser AG. RNAi screens in Caenorhabditis elegans in a 96-well liquid format and their application to the systematic identification of genetic interactions. Nat. Protoc. 2006;1:1617–1620.10.1038/nprot.2006.245
- Li Y, Ren G, Wang YX, Kong WJ, Yang P, Wang YM, Li YH, Yi H, Li ZR, Song DQ, Jiang JD. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J. Transl. Med. 2011;9:62.10.1186/1479-5876-9-62
- Ma JY, Feng R, Tan XS, Ma C, Shou JW, Fu J, Huang M, He CY, Chen SN, Zhao ZX, He WY, Wang Y, Jiang JD. Excretion of berberine and its metabolites in oral administration in rats. J. Pharm. Sci. 2013;102:4181–4192.
- Qiu F, Zhu Z, Kang N, Piao S, Qin G, Yao X. Isolation and identification of urinary metabolites of berberine in rats and humans. Drug Metab. Dispos. 2008;36:2159–2165.10.1124/dmd.108.021659
- Zuo F, Nakamura N, Akao T, Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Dispos. 2006;34:2064–2072.10.1124/dmd.106.011361
- Deroussent A, Ré M, Hoellinger H, Cresteil T. Metabolism of sanguinarine in human and in rat: characterization of oxidative metabolites produced by human CYP1A1 and CYP1A2 and rat liver microsomes using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010;52:391–397.10.1016/j.jpba.2009.09.014
- Zhang HH, Wu Y, Sun ZL, Liu ZY. Identification of sanguinarine metabolites in pig liver preparations by accurate mass measurements using electrospray ionization hybrid ion trap/time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2013;27:979–984.10.1002/rcm.6538
- Taubert S, Hansen M, Van Gilst MR, Cooper SB, Yamamoto KR. The Mediator subunit MDT-15 confers metabolic adaptation to ingested material. PLoS Genet. 2008;4: e1000021.
- Broeks A, Janssen HW, Calafat J, Plasterk RH. A P-glycoprotein protects Caenorhabditis elegans against natural toxins. EMBO J. 1995;14:1858–1866.
- Kwok TC, Ricker N, Fraser R, Chan AW, Burns A, Stanley EF, McCourt P, Cutler SR, Roy PJ. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–95.10.1038/nature04657
- Maeng HJ, Yoo HJ, Kim IW, Song IS, Chung SJ, Shim CK. P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J. Pharm. Sci. 2002;91:2614–2621.10.1002/(ISSN)1520-6017
- Wang YX, Wang YP, Zhang H, Kong WJ, Li YH, Liu F, Gao RM, Liu T, Jiang JD, Song DQ. Synthesis and biological evaluation of berberine analogues as novel up-regulators for both low-density-lipoprotein receptor and insulin receptor. Bioorg. Med. Chem. Lett. 2009;19:6004–6008.10.1016/j.bmcl.2009.09.059
- Eid SY, El-Readi MZ, Wink M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine. 2012;19:1288–1297.10.1016/j.phymed.2012.08.010
- Li JWH, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165.10.1126/science.1168243
