Abstract
Cellular and organellar redox states, which are characterized by the balance between oxidant and antioxidant pool sizes, play signaling roles in the regulation of gene expression and protein function in a wide variety of plant physiological processes including stress acclimation. Reactive oxygen species (ROS) and ascorbic acid (AsA) are the most abundant oxidants and antioxidants, respectively, in plant cells; therefore, the metabolism of these redox compounds must be strictly and spatiotemporally controlled. In this review, we provided an overview of our previous studies as well as recent advances in (1) the molecular mechanisms and regulation of AsA biosynthesis, (2) the molecular and genetic properties of ascorbate peroxidases, and (3) stress acclimation via ROS-derived oxidative/redox signaling pathways, and discussed future perspectives in this field.
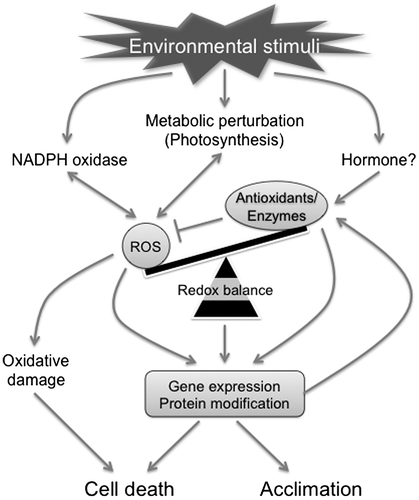
Graphical Abstract
Cellular redox states, which are characterized by the balance between oxidant and antioxidant pool sizes, are involved in plant stress acclimation.
Since a large amount of oxygen is produced during photosynthesis, plant (photosynthetic) cells can generate markedly higher amounts of reactive oxygen species (ROS), such as hydroxyl radical (HO•), superoxide radical (), singlet oxygen (1O2), and hydrogen peroxide (H2O2), as byproducts than animal and bacterial cells. Because of their high reactivity, the accumulation of ROS results in oxidative damage to all cellular components such as proteins, DNA, lipids, and sugars.Citation1,2) In addition, land plants cannot move away from unfavorable conditions, i.e. environmental stress, such as strong light, drought, salinity, heat, and cold, which have all been shown to enhance the production of ROS in plant cells. Photosynthesis, respiration, and photorespiration are the primary sources of ROS in chloroplasts, mitochondria, and peroxisomes, respectively.Citation1,2) Plants have developed sets of well-constructed antioxidative systems in various cellular compartments to tightly regulate ROS levels.Citation1–3) However, plants have also evolved to use ROS as signals to modulate gene expression and protein function, and this has consequently allowed plants to acclimate to stressful conditions.Citation1,4) Furthermore, it is now well established that in addition to ROS themselves, cellular and organellar redox states, which are characterized by the balance between oxidant and antioxidant pool sizes, are involved in the regulation of stress acclimation, programmed cell death (PCD), metabolism, hormonal response, cell cycle, growth and development, senescence, and morphogenesis (Fig. ).Citation1,5,6) Therefore, this is extremely important for determining the fate of cells, tissues, and whole plants.
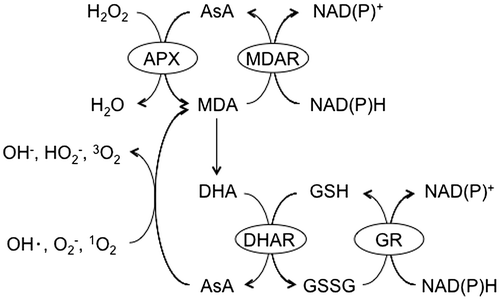
Ascorbic acid (AsA) is the most abundant soluble antioxidant in plant cells.Citation1,7) In addition to its high antioxidant activity, AsA serves as an electron donor for ascorbate peroxidase (APX) activity and the regeneration of α-tocopherol, the most abundant insoluble antioxidant.Citation1–3) Furthermore, AsA and APX are crucial components of the AsA-glutathione (GSH) cycle which is distributed in the cytosol and organelles (Fig. ).Citation1) AsA-deficient mutants have dwarf- and/or stress-sensitive phenotypes, and the complete inhibition of the major AsA biosynthetic pathway leads to seedling lethality without the exogenous application of AsA or its precursors,Citation8–10) demonstrating that the metabolism of AsA is the central modulator of cellular and organellar redox states. We previously studied (1) the molecular mechanisms and regulation of AsA biosynthesis, (2) the molecular properties and physiological roles of APX isoenzymes, and (3) redox signaling-mediated stress acclimation and its regulation through AsA metabolism. In this review, we described AsA-dependent cellular redox regulation and stress responses in plants, and discussed recent advances and future perspectives in this field.
I. Distribution and biosynthesis of AsA
High levels of AsA have been shown to accumulate in plant cells.Citation1) AsA levels in leaf cells often exceed those in chlorophylls and represent over 10% of soluble carbohydrates.Citation11) Although the subcellular distribution of this compound has been studied previously, difficulties have been associated with simultaneously quantifying AsA levels in all cellular compartments. Biochemical studies have indicated that high levels of AsA accumulate in chloroplasts as well as the cytosol (20–50 mM).Citation12,13) In contrast, vacuolar AsA concentrations were reported to be very low (0.6 mM).Citation12) Given that the main role of AsA is to regulate ROS levels, the highest accumulation of AsA in chloroplasts, which are one of the most significant sites of ROS production, is favorable for its action. In addition, AsA plays important roles in the photosynthetic electron transport (PET) chain and xanthophyll cycle.Citation14–17) Thus, we considered chloroplasts to be the most significant sink of AsA in plant cells. A recently developed immunocytochemical method using AsA antisera also demonstrated that AsA levels were high and low in the cytosol and vacuoles, respectively, in Arabidopsis and tobacco plants.Citation18) However, the estimated levels of AsA in chloroplasts by this method were not as high as those by biochemical studies, and were lower than those in the nucleus and peroxisomes.Citation18) Thus, the sub-cellular distribution of AsA remains controversial. Furthermore, no AsA transporter has been identified or characterized in plants until now.
The turnover rate of AsA was shown to be fast (13% of the total pool per hour) in pea embryonic axes even when the AsA pool size was low, and was enhanced when the pool size was high.Citation19) Therefore, the undisturbed and well-controlled biosynthesis of AsA is important for maintaining the pool size. Several pathways have been proposed for the biosynthesis of AsA (Fig. ).Citation20–29) Among them, the d-mannose/l-galactose (d-Man/l-Gal) pathway plays a predominant role, at least in plant leaves.Citation9,20,21) In this pathway, GDP-d-Man is synthesized from d-fructose-6-phosphate (Fru-6P) in three steps, which are catalyzed by phosphomannose isomerase (PMI), phosphomannomutase, and GDP-d-Man pyrophosphorylase (GMP).Citation30–32) Arabidopsis has two PMI genes, PMI1 and PMI2. Expressional and reverse genetic analyses revealed that only PMI1 was involved in the biosynthesis of AsA.Citation30) GDP-d-Man was shown to be successively converted to GDP-l-Gal, l-Gal-1-phosphate, l-Gal, l-galactono-1,4-lactone (l-GalL), and finally to AsA.Citation20,21,33) Five Arabidopsis vitamin C-deficient (vtc) mutants have been isolated and provided genetic evidence for the d-Man/l-Gal pathway.Citation8,20,21) All vtc mutations, except for vtc3, were found to be defective in the d-Man/l-Gal pathway: VTC1, VTC2/5, and VTC4 encode GMP, GDP-l-Gal phosphorylase, and l-Gal-1-phosphate phosphatase, respectively.Citation9,20,21,34–37) Double mutants lacking both VTC2 and VTC5 showed a seedling lethal phenotype, but normal growth when treated with l-Gal or AsA.Citation9) These findings demonstrated that AsA synthesized via the d-Man/l-Gal pathway is required for the development of Arabidopsis seedlings. In contrast, contribution of alternative pathways to regulation of the AsA pool size currently remains unclear because of the lack of genetic evidence.Citation24,26–29)
Fig. 3. Proposed ascorbate biosynthetic pathways in higher plants.
Notes: Thick arrows indicate the d-Man/l-Gal pathway. Parentheses indicate the names of ascorbate-deficient (VTC) Arabidopsis mutants. Enzymes: Enzymes: 1, phosphomannose isomerase; 2, phosphomannomutase; 3, GDP-d-Mannose pyrophosphorylase; 4, GDP-d-Mannose-3′,5′-epimerase; 5, GDP-l-Galactose phosphorylase; 6, l-Galactose-1P phosphatase; 7, l-Galactose dehydrogenase; 8, l-Galactono-1,4-lactone dehydrogenase; 9, l-Gulono-1,4-lactone dehydrogenase; 10, d-Galacturonate reductase; 11, aldonolactonase; 12, purple acid phosphatase; 13, myo-inositol oxygenase; 14, d-Glucuronate reductase.
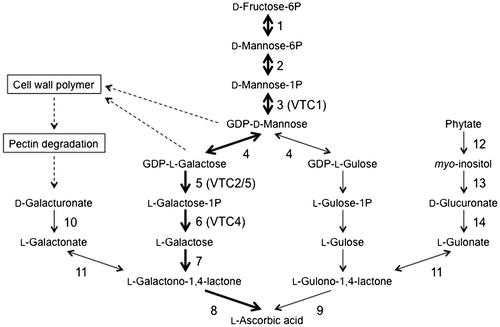
The rate-limiting step in the d-Man/l-Gal pathway as well as its regulation has been examined. Since the exogenous application of l-Gal and l-GalL markedly enhanced the AsA content in plant cells, neither l-Gal nor l-GalL dehydrogenases could catalyze the rate-limiting step.Citation23,33) Although one of the candidates for a rate-limiting enzyme was GME because of its low Vmax,Citation9,29) the transient overexpression of this enzyme had no effect on the AsA content in Arabidopsis or tobacco plants.Citation38,39) Since GDP-d-Man and GDP-l-Gal are substrates involved in the synthesis of polysaccharides as well as AsA,Citation30,31,40) Arabidopsis VTC2 and VTC5 catalyze the first committed step in the d-Man/l-Gal pathway (Fig. ). VTC2 is the predominant GDP-l-Gal phosphorylase.Citation9,41) Yoshimura et al.Citation39) recently reported that the estrogen-induced conditional overexpression of the VTC2 gene markedly increased the AsA content in Arabidopsis seedlings, while that of the other genes encoding GMP, GPP, GME, and PMI did not. In addition, the constitutive overexpression of the VTC2 gene enhanced the AsA content in tomato, strawberry, and potato plants.Citation42) The simultaneous overexpression of VTC2 and GME in tobacco leaves enhanced the AsA content more than the single overexpression of VTC2.Citation38) These findings suggested that VTC2 may catalyze the rate-limiting step in the d-Man/l-Gal pathway, and GME may limit the pathway in VTC2-overexpressing plants.
Light is the most important environmental cue for regulating the biosynthesis of AsA.Citation43) The content of AsA is known to increase under illumination and decrease in the dark.Citation43) Consistent with this finding, the expression of several genes involved in the d-Man/l-Gal pathway, including VTC2, was up- and down-regulated under light and in the dark, respectively.Citation43) The light activation of this pathway is known to be dependent on its intensity.Citation9,44) Exposure to strong light markedly enhanced the activity of GDP-l-Gal phosphorylase, but not the other enzymes in Arabidopsis, and resulted in the accumulation of AsA.Citation9) If VTC2 is considered to be the most feasible candidate for the rate-limiting enzyme, the light regulation of VTC2 expression appears to be a determinant of the AsA biosynthetic capacity. Treatments with inhibitors of the PET chain suppressed the expression of VTC2 and other genes as well as the AsA content under illumination.Citation43,45) Thus, the redox states of PET are crucial for the light-dependent activation of AsA biosynthesis. The photomorphogenic COP9 signalosome subunit 5B (CSN5B) was shown to interact with GMP and enhance its degradation at night in Arabidopsis.Citation46) CSN5B-disrupting plants accumulated high levels of AsA under light and dark conditions. Therefore, the COP9 signalosome negatively regulated the biosynthesis of AsA by degrading GMP in the dark. VTC3 was recently found to encode an unusual and novel polypeptide with an N-terminal protein kinase domain tethered covalently to a C-terminal protein phosphatase type 2C domain.Citation47) The VTC3 protein was localized in chloroplasts, and its knockout inhibited the accumulation of AsA in response to light, which suggested a role for this protein in the light- and PET-dependent regulation of the d-Man/l-Gal pathway. In addition to light, phytohormones, such as jasmonic acid and ethylene, are likely to regulate the biosynthesis of AsA; however, their contribution to the light regulation of AsA biosynthesis remains unclear.Citation48,49) The ethylene-dependent regulation of the d-Man/l-Gal pathway was found to require the Arabidopsis ethylene response factor 98.Citation49) Several lines of evidence have also been provided for the negative feedback regulation of AsA biosynthesis by AsA itself.Citation30,50,51)
II. Enzymatic properties, subcellular distribution, regulation, and physiological roles of APX isoenzymes
2.1. Enzymatic properties and subcellular distribution
Although H2O2 is relatively stable among ROS, and, thus, has lower cytotoxicity, it can give rise to HO•, which is the most cytotoxic ROS, through the Fenton reaction.Citation52) Considering its cytotoxicity and signaling function,Citation1,2) intracellular H2O2 levels should be adequately controlled. Several types of H2O2-scavenging enzymes have so far been identified. Glutathione peroxidase (GPX), catalase, and peroxiredoxin (Prx) were found to be crucial for scavenging H2O2 in animal cells.Citation53) GPX and Prx can remove H2O2 via GSH and thioredoxin (Trx) as electron donors, respectively.Citation1,53,54) Although higher plants possess GPX-like genes, the translational products of the genes use Trx, but not GSH as an electron donor for their peroxidase activity.Citation55) Higher plants exhibit no or little GPX activity in spite of their massive production of ROS during photosynthesis. In addition, plant catalase is localized exclusively in peroxisomes.Citation56) Euglena gracilis, a eukaryotic alga, also lacks catalase activity.Citation57) Therefore, these facts had suggested that unknown H2O2-scavenging enzyme(s) exist(s) in these photosynthetic organisms. The new H2O2-scavenging enzyme, APX, which uses AsA as an electron donor, was firstly purified from Euglena over 30 years ago.Citation58) This enzyme was shown to be extremely unstable in the absence of AsA.Citation59) Therefore, a high concentration of AsA was required for its purification from Euglena and plant cells.Citation58,60) This property may have delayed the discovery of this enzyme. Subsequent studies demonstrated that APX was widely distributed in photosynthetic eukaryotes, including higher plants, but not in prokaryotes.Citation3,21) Some parasitic protozoa, such as Trypanosome crusi and Leishmania major, also have a functional APX; however, its location in the endoplasmic reticulum is very different from that of plant APXs.Citation61,62) Although APX activity has also been detected in the bovine eye,Citation63) no APX-like gene was found in animal genomes. 1-Cys peroxiredoxin (1-Cys Prx) and cytochrome c are now known to exhibit APX activity,Citation64,65) which suggests that they may also do so in the bovine eye.
Euglena contains a sole APX that is localized exclusively in the cytosol,Citation58,66) whereas other eukaryotic algae, moss, fern, and higher plants conserve additional APX isoenzymes, which are located in organelles.Citation3,21,67,68) APX isoenzymes were shown to be distributed in the cytosol (cAPX), mitochondria (mitAPX), peroxisomes (pAPX), and chloroplasts (chlAPX) of higher plants.Citation3,21) chlAPXs have been further divided into two types: stromal (sAPX) and thylakoid membrane-bound (tAPX) isoenzymes.Citation3) APX isoenzymes, particularly chlAPXs, are highly specific to AsA as the electron donor.Citation60) Unlike Euglena APX,Citation58,66) plant APX isoenzymes cannot reduce lipid hydroperoxides.Citation3) APX isoenzymes have a high affinity for H2O2, with km values ranging between 10 and 130 μM and, consequently, play important roles in the regulation of cellular H2O2 levels. The catalytic mechanism of APX has been well characterized based on the three-dimensional structure of the substrate complex of cAPX and sAPX isoenzymes.Citation69–71) As described above, one of the atypical characteristics of APX is its instability in the absence of AsA. APX activity is rapidly lost under conditions in which the AsA concentration is lower than 20 μM in vitro.Citation59) chlAPXs are especially sensitive to inactivation; their half inactivation times are less than 30 s, while those of cAPX and mAPX are 1 h or more. The crystal structure of tobacco sAPX showed that it, but not cAPX, has a unique loop in the vicinity of the heme, and a chimera of Galdieria cAPX and spinach sAPX, in which the corresponding loop structure was removed from sAPX, was more stable under AsA depleted conditions, which revealed a causal relationship between the loop structure and stability of chloroplastic APX.Citation70,72)
The model plant Arabidopsis possesses seven isoenzymes of APX: three cytosolic (APX1, APX2, and APX6), two chloroplastic (sAPX and tAPX), and two peroxisomal (APX3 and APX5) isoenzymes.Citation2) Arabidopsis sAPX is a dual-targeting enzyme that is located in both chloroplasts and mitochondria.Citation73) Thus, there is no APX that is located in the mitochondria of this plant only. A previous study demonstrated that although Physcomitrella patens APX (PpAPX1), which is the most orthologous to Arabidopsis sAPX, only targeted chloroplasts, Picea glauca APX (PgAPX1) targeted both chloroplasts and mitochondria.Citation67) Rice sAPX-like enzymes (OsAPX5~7) are not dual-targeting enzymes: OsAPX5 and OsAPX6 are mitochondrial, whereas OsAPX7 is a chloroplastic isoenzyme.Citation67) These findings suggested that the dual-targeting ability of APX developed after the split between P. patens and P. glauca, and was subsequently lost in rice following monocot divergence.Citation67) Thus, the construction of chloroplastic and mitochondrial APX genes differs among plant species. The thylakoid lumen protein, Arabidopsis TL29, has sequence homology to APXs; however, this protein did not have APX function.Citation74)
2.2. Expression
APX activity is markedly increased under environmental stress conditions.Citation75) Although cAPX is highly responsive to photooxidative stress, such as high light (HL), the organellar types of APX were found to hardly respond.Citation75) Thus, elevations in total APX activity are dependent on the induction of cAPX during stress. The HL-response of Arabidopsis APX2 was inhibited by treatments with catalase but not with superoxide dismutase.Citation76) In addition, a treatment with H2O2 and methyl viologen (MV), a ROS-producing reagent, markedly enhanced the expression of APX2.Citation76–78) A treatment with 3-(3,4-dichlorophenyl)-l,l-dimethylurea (DCMU), an inhibitor of the reduction of the plastoquinone (PQ) pool, blocked the HL-response of APX2. On the other hand, a treatment with 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB), an inhibitor of the oxidation of this pool, enhanced the expression of APX2 even under normal light conditions.Citation77) Expression of cAPX gene in tobacco was shown to be regulated in a similar manner.Citation78) Thus, the expression of cAPX genes appears to be regulated by ROS and the redox states of the PQ pool under photooxidative stress conditions. The overexpression of tAPX markedly suppressed the MV treatment-induced expression of cAPX in tobacco plants, which suggests that H2O2 produced by chloroplasts can also induce the expression of cAPX.Citation78) However, the overexpression of tAPX had no effect on the expression of cAPX at the early stage of HL illumination.Citation78) Furthermore, the induction of Arabidopsis APX2 by HL was significantly inhibited in single knockout mutants lacking sAPX or tAPX.Citation79) These findings suggested the existence of a complicated interaction between chloroplastic H2O2 and HL signaling pathways for regulating the expression of cAPX. Further expressional analyses and forward genetic approaches have revealed that the phytohormone abscisic acid (ABA), GSH, and 3′-phosphoadenosine 5′-phosphate (PAP), which acts as retrograde signal from chloroplasts to the nucleus, are also involved in regulating the expression of APX2 in Arabidopsis.Citation80–83) The photoelectrophysiological signaling was also shown to regulate the gene expression systemically.Citation84) It will be important to clarify how these signals interact with one another to regulate the expression of APX2 in future studies.
Although sAPX and tAPX generally respond poorly to environmental stress, their expression is significantly affected by light intensity.Citation85) When low light-acclimated plants were transferred to 100-fold excess light, sAPX and tAPX transcripts were found to be markedly increased. However, the protein levels of both APXs did not reflect their transcript levels under the same conditions.Citation85) It is possible that light intensity regulates both the transcript levels and turnover of chlAPXs. sAPX and tAPX are encoded by a single gene in spinach, pumpkin, and tobacco plants, and this gene generates both isoenzymes by alternative splicing.Citation3,21,86–89) Alternative splicing occurs in the 3′-terminal region of chlAPX pre-mRNA and is regulated in a tissue-specific manner. The ratio of sAPX mRNA to tAPX mRNA was found to be close to 1 in leaves, and was markedly elevated in roots. The deletion of the splicing regulatory cis element (SRE) sequence, which is highly conserved in spinach and tobacco chlAPX genes, diminished the splicing efficiency of these genes.Citation89) Although the findings of a gel-shift assay suggested that SRE may interact with the nuclear proteins extracted from leaves, but not roots, these proteins have not yet been identified. When the tobacco chlAPX gene was introduced and expressed in Arabidopsis plants, tissue-specific alternative splicing occurred (our unpublished data), which suggested that the molecular mechanism involved in this tissue-specific event may be widely conserved in various types of plant species.
2.3. Physiological roles
A large number of reverse genetic approaches using Arabidopsis and rice plants have been performed to evaluate the physiological roles of APX isoenzymes.Citation79,90–107) Although chloroplasts, mitochondria, and peroxisomes are the significant sources of ROS, Arabidopsis knockout mutants lacking organellar APX isoenzymes showed very weak phenotypes against oxidative stress.Citation79,102–104,107) cAPX is known to be more important for stress tolerance than other APX isoenzymes even though the cytosol is not a significant source of ROS.Citation90–95) Thus, the lack of APX1 in Arabidopsis enhanced its sensitivity to various types of stresses such as HL, treatment with MV, and a combination of drought and heat.Citation90,91,95) In the APX1-knockout mutants (KO-APX1), the oxidation of cytosolic, plastidic, and extracellular proteins was enhanced.Citation91) The expression of APX1 was found to be enhanced in knockout Arabidopsis mutants lacking sAPX (KO-sAPX) under normal conditions.Citation91) These findings suggested cross-compartment protection against oxidative stress by cAPX. To clarify this cross-compartment protection in more detail, the effects of a lack of APX1 on the cytotoxic effects of ROS induced by wounding and a methyl jasmonic acid (MeJA) treatment were analyzed.Citation92) Plants temporarily produced ROS including and H2O2 via NADPH oxidases, which were activated by JA and MeJA in response to the damage, soon after wounding occurred to induce the expression of a set of defense genes.Citation108–110) Thus, the site of ROS production was restricted to apoplast. KO-APX1 plants were very sensitive to wounding and the MeJA treatment.Citation92) H2O2 only accumulated in the vicinity of the wound in the leaves of wild-type plants, but accumulated extensively in both damaged and undamaged regions in leaves of KO-APX1 plants. Photosynthesis and nuclear proteins were damaged more in KO-APX1 plants during wounding and the MeJA treatment. These findings demonstrated that Arabidopsis APX1 protected chloroplasts and nuclei from oxidative damage caused by wounding, and, thus, clearly support the cross-compartment protection afforded by APX1.Citation92) In the case of responses to wounding and MeJA, ROS should only act as signaling molecules, not as toxic compounds. The cytosol is a cellular compartment across organelles, such as chloroplasts, mitochondria, peroxisomes, and the nucleus as well as the intracellular and extracellular space. Spatial control by cAPX is likely to prevent the cytotoxic effects of ROS. Therefore, cAPX is a determinant of the redox states of entire cells.
Chloroplasts are one of the most significant sources of ROS in photosynthetic tissues.Citation1,111) The production of ROS in chloroplasts is dependent of the redox states of the PET.Citation1,111) When the over-reduction of PET occurs because of HL illumination and a reduced capacity of CO2 assimilation, the photoreduction of O2 to is promoted.
is spontaneously or enzymatically reduced to H2O2, which is then reduced to water molecules via the activity of APX.Citation1,111) Because of their extreme instabilities, chloroplastic APX isoenzymes were previously considered to be crucial for plant tolerance to stress and even for growth and development under normal conditions. The expression of Escherichia coli catalase in tobacco chloroplasts resulted in an increased capacity to protect the photosynthetic apparatus against photooxidative stress caused by HL under drought or by a treatment with MV, whereas chlAPXs were significantly inactivated with the depletion of AsA.Citation112,113) In addition, we found that transgenic tobacco plants overexpressing tAPX showed enhanced tolerance to photooxidative stress.Citation114) However, Arabidopsis KO-tAPX and KO-sAPX single mutants and even the double mutants exhibited no visible symptoms of stress after long-term HL exposure and a MV treatment.Citation103,104) Kangasjärvi et al.Citation103) reported that the double mutants only exhibited visible symptoms under very severe photooxidative stress (50 μM MV under HL). On the other hand, in a wheat mutant line, a 40% reduction in tAPX activity resulted in a reduction in photosynthetic carbon assimilation as well as decreased growth and seed production.Citation115) Thus, the physiological significance of chloroplastic APXs during photooxidative stress may differ among plant species.
To understand the role of chloroplastic APXs under photooxidative stress in more detail, the effects of the knockout of sAPX and tAPX on cellular redox states were examined.Citation79) Exposure to HL or a treatment with MV under illumination led to the accumulation of higher levels of H2O2 and oxidized proteins in KO-sAPX and KO-tAPX plants than in wild-type plants. The most prominent effect of photooxidative stress on oxidative damage was observed in KO-tAPX plants, not in KO-sAPX plants.Citation79) These findings suggested that chloroplastic APXs, particularly tAPX, are important for photoprotection under photooxidative stress in Arabidopsis leaves. The lack of chloroplastic APXs markedly enhanced GSH levels under photooxidative stress.Citation79) Similar to ROS, GSH has been found to act as a signal that regulates stress responses.Citation81) Thus, the GSH-dependent response may compensate for the lack of chloroplastic APXs under photooxidative stress.
The physiological significance of mitAPX and pAPX is still largely unknown. Because Arabidopsis sAPX is dual-targeted to both chloroplasts and mitochondria, it is difficult to evaluate the role of mitAPX using a reverse genetic approach. Arabidopsis knockout mutants of peroxisomal APX3 did not show any stress-sensitive phenotype.Citation107) On the other hand, an Arabidopsis peroxisomal membrane-bound monodehydroascorbate reductase (MDAR4) was found to be essential for the development of seedlings under autotrophic conditions.Citation116) These findings demonstrated the physiological importance of AsA metabolism in peroxisomes. Another pAPX, APX5, may compensate for the disruption of APX3 in Arabidopsis.
III. Redox signaling and stress responses
3.1. Common and source-/kind-specific ROS signaling pathways
Attention has recently been focused on the molecular mechanisms involved in ROS and redox signaling. Transcriptome analyses using ROS-generating agents or mutants lacking antioxidative enzymes have contributed to the identification of genes involved in ROS and redox signaling pathways.Citation1,2,4,90,91,117) Thousands of genes are now known to be responsive to ROS in Arabidopsis. Although several important proteins involved in signaling, such as protein kinases/phosphatases and transcription factors, have been identified,Citation118,119) the core mechanism remains unknown in plants. In other words, ROS- and/or redox-sensing mechanisms in plants have yet to be elucidated in detail. In contrast to other stable signaling molecules, ROS are unlikely to be bound by a protein because of their instability. One possible mechanism by which H2O2 could function as a signaling molecule is by interacting with Cys within proteins. This may change the conformation of the protein and activate (or inactivate) its function, as has been demonstrated for the yAP1 and oxyR transcription factors in yeast and E. coli, respectively.Citation120) Several transcription factors and co-activators, including NONEXPRESSER OF PR GENES1 (NPR1), which is a master regulator for salicylic acid signaling, were shown to be post-translationally regulated by ROS in higher plants,Citation121) even though their role in the oxidative stress response remains unclear.
Comparative analyses of ROS-responsive transcriptome data have demonstrated that the response of a number of genes to ROS is irrespective to the kind and production site of the compounds, whereas the response of another set of genes is highly specific.Citation117) This finding indicates that there are common and source-/kind-specific pathways for ROS signaling.Citation117) The synergistic and antagonistic interactions of multiple signaling pathways are thought to be important in the fine-tuning of plant responses to abiotic and biotic stress. For example, in Arabidopsis, a DNA damage response was specifically activated in a double mutant lacking both cAPX and catalase genes, in which H2O2 accumulated in the cytosol and peroxisomes, but not in single mutants for the respective genes, and this resulted in an enhanced tolerance to oxidative stress and an inhibition of PCD in the double mutants.Citation117)
In the context of a common pathway, ROS derived from different cellular compartments should be detected in the cytosol and/or nucleus. Unlike other ROS, H2O2 can easily permeate biological membranes, and, thus, is able to activate a common pathway. Redox-sensitive protein kinases/phosphatases and transcription factors may be involved in this pathway. Alternatively, the oxidative signaling pathways evoked from different cellular compartments may be integrated in the cytosol and/or nucleus. Arabidopsis topoisomerase IV was reported to bind to the promoter of ROS-responsive genes and also integrated 1O2- and H2O2-responsive genes in a distinct manner.Citation122) Arabidopsis transcription factors, such as the zinc-finger protein, Zat12, and heat shock factor A2 (HsfA2), which are both highly responsive to ROS irrespective of the kind and source of the compounds, have been identified using comparative transcriptome analysis, which suggests that these transcription factors may be involved in the common pathway.Citation117) Zat12 and HsfA2 regulate the expression of APX1 and APX2, respectively, through distinct routes, suggesting multiple routes even for the common pathway.Citation119,123,124)
The expression of HsfA2 as well as its target genes, APX2 and heat shock protein 18.1-C1 (Hsp18.1-C1), is markedly low under non-stressed conditions, while it is rapidly and strongly induced by heat shock, HL, and ROS in Arabidopsis.Citation123) We recently found that the expression of HsfA2 was predominantly and directly regulated by two class A1 Hsf (HsfA1) isoforms, HsfA1d and HsfA1e, under heat shock and oxidative stress conditions.Citation125) HsfA1 isoforms are master regulators for the heat shock response at least in Arabidopsis and tomato.Citation126–128) Animal and yeast Hsfs have been found to act as direct ROS sensors.Citation129) Although yeast Hsf was found to be post-translationally activated by ,Citation130) human and Drosophila Hsfs directly sensed H2O2 and assembled into a homotrimer as an active form in a reversible and redox-regulated manner.Citation131,132) Two Cys residues, located within and near the DNA-binding domain, were shown to be essential for the formation of intermolecular disulfide bonds, trimerization, and the nuclear localization of human Hsf in response to heat and H2O2.Citation133) Arabidopsis HsfA1s conserve two Cys residues; one is located near the DNA binding domain, while the other is in the C-terminus region. The substitution of these Cys residues for Ser slightly inhibited the nuclear localization of Arabidopsis HsfA1d, but markedly suppressed the expression of its target gene.Citation134) These findings demonstrated that this transcription factor could be a candidate for H2O2 sensors. All Arabidopsis HsfA1s are evenly distributed in the cytosol and nucleus, and these proteins, except for HsfA1e, are expressed constitutively.Citation135) These properties may be important for their function as H2O2 sensors.
3.2. Chloroplast-derived ROS signaling pathways
Chloroplasts are one of the most significant sites of ROS production in plant cells, and, therefore, are crucial sources of ROS signals. All types of ROS can be produced easily in organelles during illumination.Citation1,111) The identification of a conditional fluorescent (flu) mutant of Arabidopsis has provided genetic evidence that the release of 1O2 is involved in the regulation of PCD.Citation136–138) FLU acts as a negative regulator of the biosynthesis of chlorophyll, and, therefore, its defect leads to an accumulation of the intermediates of metabolism, which are photosensitizers, even during night.Citation136) Thus, the flu mutant allows the rapid production of 1O2 within plastids in a controlled manner by a dark-to-light shift. Immediately, after the shift, flu mutants stopped growing and developed necrotic lesions because of PCD. The onset of 1O2 production was rapidly followed by a loss in chloroplast integrity that preceded the rupture of the central vacuole and final collapse of the cell.Citation139) The lack of the two plastid proteins, EXECUTER (EX1) and EX2, in the mutant had no effect on the levels of 1O2, but completely abrogated PCD, which indicated that this response was due to an EX-dependent pathway rather than 1O2 directly.Citation138–140) Within the first 15 min of re-illumination, distinct sets of genes were activated in flu mutants that were different from those induced by the MV treatment.Citation137) These findings suggested that 1O2 may not primarily act as a toxin, but rather as a signal that activates several stress-response pathways, and also that the signaling function of 1O2 is distinct from that of H2O2/.
As described above, we found that the HL-induced expression of HsfA2 and APX2 was reduced in Arabidopsis KO-tAPX and KO-sAPX plants.Citation79) A reduction was also observed in the expression of APX2 in Arabidopsis knockdown mutants of chloroplastic copper zinc superoxide dismutase (CSD2).Citation141) In contrast, 1O2 strongly induced the expression of HsfA2.Citation117) These findings support the distinct signaling functions of 1O2 and H2O2/, and suggest that chloroplastic H2O2/
plays a negative role in the HL response of the common pathway. Furthermore, the overexpression of tAPX was found to enhance the chloroplastic 1O2-evoked PCD response in flu mutants, suggesting an antagonistic interaction between the 1O2- and H2O2-mediated pathways.Citation142)
A conditional system for producing H2O2 (similar to the flu mutants producing 1O2), but not a knockout or constitutive knockdown method, was needed to clarify the signaling function of H2O2 derived from chloroplasts in more detail because plants may acclimate to the knockout or constitutive knockdown of a target gene during growth and development. Furthermore, this system should not require any application of stress to plants because other signaling molecules, including hormones, are produced by stress and may act synergistically or antagonistically. To achieve this objective, we created a novel system for producing H2O2 in chloroplasts by chemical-dependent tAPX silencing using an estrogen-inducible RNAi method.Citation102) In the IS-tAPX plants having RNAi construct, tAPX transcript levels were almost completely suppressed 20 h after the treatment with estrogen, and tAPX protein levels were decreased after 48 h. Concomitant with tAPX silencing, chloroplastic-oxidized protein levels were increased even under normal light conditions, which suggested that the generation and accumulation of H2O2 were enhanced in chloroplasts during tAPX silencing. On the other hand, tAPX silencing had no effect on levels of oxidized proteins in the whole cell, photosynthesis, or plant growth and development. Thus, it is likely that the increases that occurred in H2O2 and oxidized protein levels in the chloroplasts of IS-tAPX plants were not high enough to have a negative effect on photosynthesis and plant viability.Citation102) This may be important for identifying chloroplast-specific signaling events because H2O2 can easily permeate biological membranes through aquaporin.Citation143) We identified 774 RTS (Responsive to tAPX silencing) genes as candidate genes responsive to the H2O2 produced by chloroplasts using microarray analysis with the tAPX silencing system.Citation102) The induction of RTS genes by tAPX silencing was inhibited by the application of AsA and dark, which suggested that this induction could be attributed to an increase in H2O2 levels. Among the RTS genes identified, only a few genes were known to be responsive to ROS. Furthermore, the RTS genes hardly overlapped with genes whose expression was affected by the lack of CSD2, catalase, and/or APX1.Citation102,144) Consequently, the majority of RTS genes were found to be novel ROS-responsive genes, indicating that H2O2 derived from chloroplasts may act as a signaling molecule through a specific pathway.
RTS genes overlapped, at least partially, with ABA- and PAP-responsive genes, which suggested an interaction between chloroplastic H2O2 and these signals.Citation102) In addition, we found that some RTS genes were involved in salicylic acid (SA)-mediated pathogen response/resistance,Citation102,145) suggesting that the H2O2 produced by chloroplasts may play a role in the SA response. SA levels and the expression of SA-responsive genes were enhanced in tAPX-silenced plants. In tobacco plants, the mitogen-activated protein kinase cascade, which plays a role in pathogen resistance, rapidly inhibited CO2 fixation in chloroplasts under illumination, which led to the generation of ROS in chloroplasts due to excess excitation energy in PET.Citation146) ROS accumulated in tobacco chloroplasts in a light-dependent manner following an infection with the tobacco mosaic virus, which is known to activate the mitogen-activated protein kinase cascade.Citation81) This finding indicated that the source of ROS is photosynthesis during virus infection. Furthermore, the overexpression of tAPX inhibited the hypersensitive response in Arabidopsis plants.Citation146) Taken together, these findings suggested that chloroplast-generated ROS are prominently involved in plant defense and responses to pathogens (Fig. ). Importantly, transcriptomic changes were only observed in estrogen-treated IS-tAPX plants, and not in KO-tAPX plants.Citation102) Signaling events were transient in many cases. Thus, acclimation to the lack of tAPX may occur during the developmental process in KO-tAPX plants, and the effects of the expression of tAPX on whole gene expression consequently differed between KO-tAPX plants and estrogen-treated tAPX-silenced plants. Accordingly, these findings demonstrated that chemical-inducible RNAi was more useful than knockout or constitutive knockdown for identifying source-specific oxidative signaling.
Fig. 4. Proposed model for chloroplastic H2O2-mediated signaling.
Notes: CAS, chloroplast calcium sensor; MVR, methylviologen-resistant; MVS, methylviologen-susceptible; PS, photosystem; SA, salicylic acid.
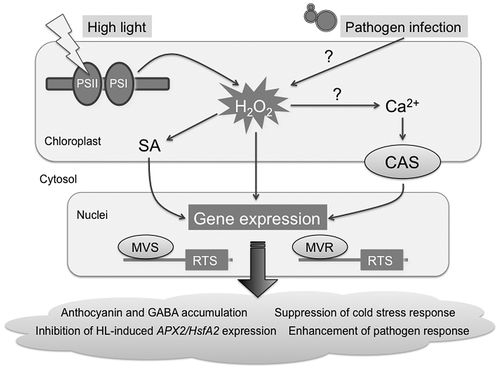
An alternative approach using Arabidopsis plants overexpressing glycolate oxidase (GO) in plastids (GO plants) was performed to elucidate chloroplastic H2O2-evoked signaling events.Citation147) In GO plants, the oxidation of glycolate occurred within chloroplasts, resulting in the enhanced production of H2O2 in the organelles. Because photorespiration can be blocked at high CO2 concentrations, transferring GO plants from high to ambient CO2 concentrations conditionally induced the production of H2O2 in chloroplasts. Unfortunately, no transcriptomic data has yet been obtained from GO plants. However, Balazadeh et al.Citation148) recently examined the expression of 187 major ROS-responsive genes in GO plants using quantitative RT-PCR (q-PCR), and reported that 58 of these genes, including HsfA2, were clearly up-regulated. Thus, the H2O2 produced by chloroplasts could activate the expression of major ROS-responsive genes, i.e. the common pathway, in GO plants. This finding completely differed from the transcriptome and q-PCR data we obtained from IS-tAPX plants,Citation102) and may have been due to different levels of H2O2 in GO and IS-tAPX plants. As described above, tAPX silencing had no effect on photosynthesis or growth and development under normal growth conditions.Citation102) In contrast, photosynthesis was markedly impaired in GO plants under ambient CO2 concentrations, which caused the dwarf phenotype and bleached leaves.Citation147) These differences indicated that very high levels of H2O2 are accumulated in the leaves of GO plants, unlike those of tAPX-silenced plants. It is likely that chloroplastic H2O2 has multiple functions in a dose-dependent manner. Alternatively, it may be possible that accumulation of glyoxylate or other glycolate breakdown products affect the gene expression in GO plants.
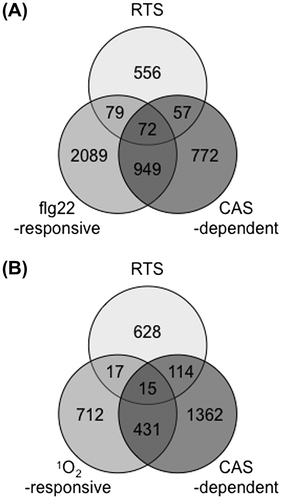
A chloroplast-localized Ca2+ sensor (CAS) protein was recently identified in Arabidopsis and was shown to play a role in immune responses.Citation149,150) Pathogen-associated molecular pattern (PAMP) signals such as flg22 were quickly relayed to chloroplasts and evoked the accumulation of Ca2+, which in turn activated the expression of defense genes through the CAS protein.Citation150) Genes, whose expression was regulated by CAS during the flg22 treatment markedly overlapped with 1O2-responsive genes, and this finding indicated the existence of an interaction between chloroplastic 1O2- and Ca2+ signaling pathways.Citation150) We, here, compared RTS genes and flg22-responsive and CAS-dependent genes.Citation150,151) As shown in Fig. , approximately 27% of RTS genes (208 genes) overlapped with flg22-responsive and/or CAS-dependent genes, which suggested that chloroplastic H2O2 as well as 1O2 may interact with CAS-dependent calcium signaling within chloroplasts. Only 15 of the overlapped genes overlapped with 1O2-responsive and CAS-dependent genes (Fig. ). Thus, it appears that chloroplastic H2O2 and 1O2 activated the CAS-dependent calcium signaling in a different manner (Fig. ). It is of interest to clarify how and why 1O2-, H2O2-, and Ca2+-evoked signaling pathways interact with one another to control the PAMP-induced immune response.
Fig. 5. The Relationship between chloroplastic ROS- and CAS-dependent responses.
Notes: (A) Overlap between chloroplastic H2O2-responsive,Citation102) flg22-responsive,Citation151) and CAS-dependent genes.Citation150) (B) Overlap between chloroplastic H2O2-responsive, 1O2-responsive,Citation137) and CAS-dependent genes.
Unlike 1O2-mediated retrograde signaling, the molecular mechanism underlying the chloroplastic H2O2-mediated pathway remains largely unknown. We have been conducting comprehensive reverse genetic analyses of RTS genes to clarify the molecular mechanism and further physiological function of the chloroplastic H2O2 signal.Citation152,153) By comparing the sensitivity of knockout and/or dominant-negative mutants for RTS genes and wild-type plants to MV-induced oxidative stress, we have screened some methylviologen-susceptible and -resistant (mvs and mvr) mutants.Citation152,153) The MVR2 gene encoded glutamate decarboxylase 1 (GAD1), which produced γ-aminobutyrate (GABA) at the early stage of the development of Arabidopsis seedlings.Citation152) tAPX silencing enhanced the expression of GAD1 and GABA in Arabidopsis leaves. These findings implied the existence of a relationship between chloroplastic H2O2 and GABA metabolism in the oxidative stress response. Among the mvs and mvr mutants, the mvs8 mutant was the sole anthocyanin-defective mutant under the MV treatment and HL exposure.Citation153) Ferulate 5-hydroxylase 1 (FAH1), which is involved in the phenylpropanoid pathway, was knocked out in this mutant. The expression of anthocyanin biosynthesis-associated genes was markedly reduced in the mvs8 mutant under oxidative stress, demonstrating that FAH1 is essential for the accumulation of anthocyanin and expression of anthocyanin biosynthesis-associated genes. tAPX silencing as well as the MV treatment and HL exposure enhanced the expression of FAH1 and accumulation of anthocyanin.Citation153) These findings suggested that chloroplastic H2O2 may activate the expression of FAH1 to induce the accumulation of anthocyanin in order to protect cells from photooxidative stress (Fig. ). In contrast, the HL-induced accumulation of anthocyanin was found to be inhibited in GO plants under ambient CO2 concentrations.Citation147) Thus, it is likely that a delicate balance in H2O2 levels in chloroplasts is one of the crucial determinants of the biosynthetic capacity of anthocyanin. A large number of MVS and MVR genes encode transcription factors (data not shown). Functional analyses of these transcription factors will reveal the molecular mechanism and further physiological role of chloroplastic H2O2-mediated oxidative signaling.
IV. Perspectives
Cellular and organellar redox states have been shown to modulate stress acclimation, hormonal responses, PCD, metabolism, and growth and development. Since ROS and AsA are most significant oxidants and antioxidant, respectively, in plant cells, the tight and spatiotemporal control of the levels of these redox compounds is essential for modulating physiological processes. Although AsA is an old antioxidant, even the subcellular distribution and transport system of the compound remain unclear. The d-Man/l-Gal pathway, the predominant pathway of AsA biosynthesis, has just recently been characterized. There is no genetic evidence that the pathway predominates the biosynthesis of AsA in all tissues of these plant species. Alternative pathways also need to be clarified by genetic analysis if they are operating in plant cells. APX isoenzymes act as redox modulators. Even the finding that the lack of organellar types of APX had little effect on plant growth and development under both normal and stressful conditions prompted us to consider enzymes as redox and signaling regulators. How these isoenzymes are coupled with one another or other antioxidative enzymes for the fine-tuning of the redox states is an issue that warrants further study.
The combined analyses of omics and reverse (or forward) genetics have recently revealed that there are common and source-/kind-specific pathways for ROS signaling. Their synergistic and antagonistic interactions are thought to be important for the fine-tuning of plant responses to abiotic and biotic stresses. Interactions between multiple pathways may be involved in recognizing the types and intensities of the actual environmental stresses to which plants are exposed to, because these affect the production rate of each kind of ROS in a different manner. The selective perception of ROS that are chemically distinct and produced preferentially under specific stress conditions is likely to enable plants to adjust their responses to the needs imposed by enhanced levels of a given ROS, for example, by increasing the levels of appropriate scavengers. Therefore, the molecular mechanism and physiological role of each pathway need to be elucidated to clarify how these signals interact and contribute to the fine-tuning of stress acclimation. Conditional and spatiotemporal ROS-producing systems such as flu mutants and IS-tAPX plants possess advantages for addressing these issues, as summarized above. On the other hand, the discovery of source-/kind-specific pathways leads to further questions regarding where and how ROS signals derived from organelles are sensed, even though general ROS and redox sensors have yet to be identified. Functional analyses of ROS-responsive genes, such as RTS and further forward genetic approaches, will assist in understanding ROS and redox-sensing mechanisms in higher plants.
Acknowledgments
We are deeply grateful for the continuous guidance and encouragement of Prof. Shozaburo Kitaoka, Prof. Yoshihisa Nakano, and Prof. Akiho Yokota. We are also grateful for encouragement of the late Prof. Yoshitomi Iizuka, the late Prof. Toshio Onishi, Prof. Toshiro Mitsunaga, Prof. Osamu Hirayama, Prof. Akira Wadano, and Prof. Keiichiro Nishimura. We would like to thank the following contributors: Drs. Takahiro Ishikawa, Masahiro Tamoi, Kazuya Yoshimura, Fumio Watanabe, Yukinori Yabuta, Yoshiko Miyagawa-Tsukada, Takahisa Ogawa, Noriaki Tanabe, Ayako Nishizawa-Yokoi, Takahiro Mieda, Toru Takeda, Gaber Ahmed, Rapolu Madhusudhan, Aqib Iqbal, Megumi Murakami, Hiroyoshi Matsumura, Takashi Adachi, Kei Wada, Yuri Tanioka, Tomoki Tabuchi, Teruyuki Morishita, Kazuya Ishikawa, Daisuke Ito, Daniel Padilla-Chacon, Ms. Kumi Otori, and Ms. Harumi Sakuyama. Thanks are also due to all past and present members in our laboratory.
Notes
This review was written in response to the author’s receipt of the JSBBA Award in 2013.
References
- Foyer CH, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100.10.1104/pp.110.166181
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498.10.1016/j.tplants.2004.08.009
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002;53:1305–1319.10.1093/jexbot/53.372.1305
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309.10.1016/j.tplants.2011.03.007
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875.10.1105/tpc.105.033589
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18.10.1104/pp.110.167569
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000;3:229–235.10.1016/S1369-5266(00)80070-9
- Conklin PL, Saracco SA, Norris SR, Last RL. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics. 2000;154:847–856.
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–689.10.1111/j.1365-313X.2007.03266.x
- Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH. The transcription factor ABI4 Is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell. 2011;23:3319–3334.10.1105/tpc.111.090100
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279.10.1146/annurev.arplant.49.1.249
- Rautenkranz A, Li L, Machler F, Martinoia E, Oertli JJ. Transport of ascorbic and dehydroascorbic acids across protoplast and vacuole membranes isolated from barley (Hordeum vulgare L. cv Gerbel) leaves. Plant Physiol. 1994;106:187–193.
- Foyer C, Rowell J, Walker D. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244.10.1007/BF00405188
- Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:1455–1464.
- Muller-Moule P, Conklin PL, Niyogi KK. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002;128:970–977.10.1104/pp.010924
- Toth SZ, Nagy V, Puthur JT, Kovacs L, Garab G. The physiological role of ascorbate as photosystem II electron donor: protection against photoinactivation in heat-stressed leaves. Plant Physiol. 2011;156:382–392.10.1104/pp.110.171918
- Tóth SZ, Schansker G, Garab G. The physiological roles and metabolism of ascorbate in chloroplasts. Physiol. Plant. 2013;148:161–175.10.1111/ppl.2013.148.issue-2
- Zechmann B, Stumpe M, Mauch F. Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta. 2011;233:1–12.10.1007/s00425-010-1275-x
- Pallanca JE, Smirnoff N. The control of ascorbic acid synthesis and turnover in pea seedlings. J. Exp. Bot. 2000;51:669–674.10.1093/jexbot/51.345.669
- Ishikawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol. Plant. 2006;126:343–355.10.1111/ppl.2006.126.issue-3
- Ishikawa T, Shigeoka S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 2008;72:1143–1154.10.1271/bbb.80062
- Maruta T, Ichikawa Y, Mieda T, Takeda T, Tamoi M, Yabuta Y, Ishikawa T, Shigeoka S. The contribution of Arabidopsis homologs of L-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Biosci. Biotechnol. Biochem. 2010;74:1494–1497.10.1271/bbb.100157
- Badejo AA, Wada K, Gao Y, Maruta T, Sawa Y, Shigeoka S, Ishikawa T. Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway. J. Exp. Bot. 2012;63:229–239.10.1093/jxb/err275
- Lorence A, Chevone BI, Mendes P, Nessler CL. myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004;134:1200–1205.10.1104/pp.103.033936
- Zhang W, Gruszewski HA, Chevone BI, Nessler CL. An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008;146:431–440.
- Endres S, Tenhaken R. Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol. 2009;149:1042–1049.
- Kanter U, Usadel B, Guerineau F, Li Y, Pauly M, Tenhaken R. The inositol oxygenase gene family of Arabidopsis is involved in the biosynthesis of nucleotide sugar precursors for cell-wall matrix polysaccharides. Planta. 2005;221:243–254.10.1007/s00425-004-1441-0
- Agius F, González-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003;21:177–181.10.1038/nbt777
- Wolucka BA, Van Montagu M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003;278:47483–47490.10.1074/jbc.M309135200
- Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S. Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. J. Biol. Chem. 2008;283:28842–28851.10.1074/jbc.M805538200
- Hoeberichts FA, Vaeck E, Kiddle G, Coppens E, van de Cotte B, Adamantidis A, Ormenese S, Foyer CH, Zabeau M, Inzé D, Périlleux C, Van Breusegem F, Vuylsteke M. A Temperature-sensitive mutation in the Arabidopsis thaliana phosphomannomutase gene disrupts protein glycosylation and triggers cell death. J. Biol. Chem. 2008;283:5708–5718.
- Qian W, Yu C, Qin H, Liu X, Zhang A, Johansen IE, Wang D. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. Plant J. 2007;49:399–413.10.1111/tpj.2007.49.issue-3
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369.
- Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG. Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J. Biol. Chem. 2007;282:18879–18885.10.1074/jbc.M702094200
- Laing WA, Wright MA, Cooney J, Bulley SM. The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc. Natl. Acad. Sci. USA. 2007;104:9534–9539.10.1073/pnas.0701625104
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc. Natl. Acad. Sci. USA. 1999;96:4198–4203.
- Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N. Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J. Biol. Chem. 2006;281:15662–15670.10.1074/jbc.M601409200
- Bulley SM, Rassam M, Hoser D, Otto W, Schunemann N, Wright M, MacRae E, Gleave A, Laing W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 2009;60:765–778.10.1093/jxb/ern327
- Yoshimura K, Nakane T, Kume S, Shiomi Y, Maruta T, Ishikawa T, Shigeoka S. Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci. Biotechnol. Biochem. 2014;78:60–66.10.1080/09168451.2014.877831
- Gilbert L, Alhagdow M, Nunes-Nesi A, Quemener B, Guillon F, Bouchet B, Faurobert M, Gouble B, Page D, Garcia V, Petit J, Stevens R, Causse M, Fernie AR, Lahaye M, Rothan C, Baldet P. GDP-D-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J. 2009;60:499–508.10.1111/tpj.2009.60.issue-3
- Gao Y, Badejo AA, Shibata H, Sawa Y, Maruta T, Shigeoka S, Page M, Smirnoff N, Ishikawa T. Expression analysis of the VTC2 and VTC5 genes encoding GDP-L-galactose phosphorylase, an enzyme involved in ascorbate biosynthesis, in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2011;75:1783–1788.10.1271/bbb.110320
- Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Allan AC, Laing WA. Enhancing ascorbate in fruits and tubers through over-expression of the L-galactose pathway gene GDP-L-galactose phosphorylase. Plant Biotechnol. J. 2012;10:390–397.10.1111/pbi.2012.10.issue-4
- Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007;58:2661–2671.10.1093/jxb/erm124
- Gatzek S, Wheeler GL, Smirnoff N. Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant J. 2002;30:541–553.10.1046/j.1365-313X.2002.01315.x
- Yabuta Y, Maruta T, Nakamura A, Mieda T, Yoshimura K, Ishikawa T, Shigeoka S. Conversion of L-galactono-1,4-lactone to L-ascorbate is regulated by the photosynthetic electron transport chain in Arabidopsis. Biosci. Biotechnol. Biochem. 2008;72:2598–2607.10.1271/bbb.80284
- Wang J, Yu Y, Zhang Z, Quan R, Zhang H, Ma L, Deng XW, Huang R. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013;25:625–636.10.1105/tpc.112.106880
- Conklin PL, DePaolo D, Wintle B, Schatz C, Buckenmeyer G. Identification of Arabidopsis VTC3 as a putative and unique dual function protein kinase:protein phosphatase involved in the regulation of the ascorbic acid pool in plants. J. Exp. Bot. 2013;64:2793–2804.10.1093/jxb/ert140
- Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, Masuda T, Takamiya K, Shibata D, Ohta H. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 2005;44:653–668.10.1111/tpj.2005.44.issue-4
- Zhang Z, Wang J, Zhang R, Huang R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012;71:273–287.10.1111/tpj.2012.71.issue-2
- Wolucka BA, Persiau G, Van Doorsselaere J, Davey MW, Demol H, Vandekerckhove J, Van Montagu M, Zabeau M, Boerjan W. Partial purification and identification of GDP-mannose 3′,5′-epimerase of Arabidopsis thaliana, a key enzyme of the plant vitamin C pathway. Proc. Natl. Acad. Sci. USA. 2001;98:14843–14848.10.1073/pnas.011578198
- Mieda T, Yabuta Y, Rapolu M, Motoki T, Takeda T, Yoshimura K, Ishikawa T, Shigeoka S. Feedback inhibition of spinach L-galactose dehydrogenase by L-ascorbate. Plant Cell Physiol. 2004;45:1271–1279.10.1093/pcp/pch152
- Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007;58:459–481.10.1146/annurev.arplant.58.032806.103946
- Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H2O2: regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 2005;7:619–626.10.1089/ars.2005.7.619
- Ursini F, Maiorino M, Brigelius-Flohé R, Aumann KD, Roveri A, Schomburg D, Flohé L. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38–53.
- Iqbal A, Yabuta Y, Takeda T, Nakano Y, Shigeoka S. Hydroperoxide reduction by thioredoxin-specific glutathione peroxidase isoenzymes of Arabidopsis thaliana. FEBS J. 2006;273:5589–5597.10.1111/ejb.2006.273.issue-24
- Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G. Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010;61:4197–4220.10.1093/jxb/erq282
- Graves LB Jr, Hanzely L, Trelease RN. The occurrence and fine structural characterization of microbodies in Euglena gracilis. Protoplasma. 1971;72:141–152.10.1007/BF01279047
- Shigeoka S, Nakano Y, Kitaoka S. Metabolism of hydrogen peroxide in Euglena gracilis Z by L-ascorbic acid peroxidase. Biochem. J. 1980;186:377–380.
- Chen GX, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998.
- Yoshimura K, Ishikawa T, Nakamura Y, Tamoi M, Takeda T, Tada T, Nishimura K, Shigeoka S. Comparative study on recombinant chloroplastic and cytosolic ascorbate peroxidase isozymes of spinach. Arch. Biochem. Biophys. 1998;353:55–63.10.1006/abbi.1997.0612
- Wilkinson SR, Obado SO, Mauricio IL, Kelly JM. Trypanosoma cruzi expresses a plant-like ascorbate-dependent hemoperoxidase localized to the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2002;99:13453–13458.10.1073/pnas.202422899
- Adak S, Datta AK. Leishmania major encodes an unusual peroxidase that is a close homologue of plant ascorbate peroxidase: a novel role of the transmembrane domain. Biochem. J. 2005;390:465–474.
- Wada N, Kinoshita S, Matsuo M, Amako K, Miyake C, Asada K. Purification and molecular properties of ascorbate peroxidase from bovine eye. Biochem. Biophys. Res. Commun. 1998;242:256–261.10.1006/bbrc.1997.7946
- Monteiro G, Horta BB, Pimenta DC, Augusto O, Netto LE. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc. Natl. Acad. Sci. USA. 2007;104:4886–4891.10.1073/pnas.0700481104
- Bischin C, Deac F, Silaghi-Dumitrescu R, Worrall JA, Rajagopal BS, Damian G, Cooper CE. Ascorbate peroxidase activity of cytochrome c. Free Radic. Res. 2011;45:439–444.10.3109/10715762.2010.540575
- Ishikawa T, Tajima N, Nishikawa H, Gao Y, Rapolu M, Shibata H, Sawa Y, Shigeoka S. Euglena gracilis ascorbate peroxidase forms an intramolecular dimeric structure: its unique molecular characterization. Biochem. J. 2010;426:125–134.10.1042/BJ20091406
- Xu L, Carrie C, Law SR, Murcha MW, Whelan J. Acquisition, conservation, and loss of dual-targeted proteins in land plants. Plant Physiol. 2013;161:644–662.10.1104/pp.112.210997
- Pitsch NT, Witsch B, Baier M. Comparison of the chloroplast peroxidase system in the chlorophyte Chlamydomonas reinhardtii, the bryophyte Physcomitrella patens, the lycophyte Selaginella moellendorffii and the seed plant Arabidopsis thaliana. BMC Plant Biol. 2010;10:133.10.1186/1471-2229-10-133
- Sharp KH, Mewies M, Moody PC, Raven EL. Crystal structure of the ascorbate peroxidaseascorbate complex. Nat. Struct. Biol. 2003;10:303–307.10.1038/nsb913
- Wada K, Tada T, Nakamura Y, Ishikawa T, Yabuta Y, Yoshimura K, Shigeoka S, Nishimura K. Crystal structure of chloroplastic ascorbate peroxidase from tobacco plants and structural insights into its instability. J. Biochem. 2003;134:239–244.10.1093/jb/mvg136
- Macdonald IK, Badyal SK, Ghamsari L, Moody PC, Raven EL. Interaction of ascorbate peroxidase with substrates: a mechanistic and structural analysis. Biochemistry. 2006;45:7808–7817.10.1021/bi0606849
- Kitajima S, Tomizawa K, Shigeoka S, Yokota A. An inserted loop region of stromal ascorbate peroxidase is involved in its hydrogen peroxide-mediated inactivation. FEBS J. 2006;273:2704–2710.10.1111/ejb.2006.273.issue-12
- Chew O, Whelan J, Millar AH. Molecular definition of the ascorbate glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003;278:46869–46877.10.1074/jbc.M307525200
- Granlund I, Storm P, Schubert M, Garcia-Cerdan JG, Funk C, Schroder WP. The TL29 protein is lumen located, associated with PSII and not an ascorbate peroxidase. Plant Cell Physiol. 2009;50:1898–1910.10.1093/pcp/pcp134
- Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 2000;123:223–234.10.1104/pp.123.1.223
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657.10.1126/science.284.5414.654
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell. 1997;9:627–640.10.1105/tpc.9.4.627
- Yabuta Y, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S. Two distinct redox signaling pathways for cytosolic APX induction under photooxidative stress. Plant Cell Physiol. 2004;45:1586–1594.10.1093/pcp/pch181
- Maruta T, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010;51:190–200.10.1093/pcp/pcp177
- Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, Truman W, Smirnoff N, Asami T, Davies WJ, Jones AM, Baker NR, Mullineaux PM. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell. 2009;21:2143–2162.10.1105/tpc.108.061507
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, Karpinski S, Mullineaux PM. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell. 2004;16:2448–2462.10.1105/tpc.104.022608
- Rossel JB, Walter PB, Hendrickson L, Chow WS, Poole A, Mullineaux PM, Pogson BJ. A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant Cell Environ. 2006;29:269–281.10.1111/pce.2006.29.issue-2
- Estavillo GM, Crisp PA, Pornsiriwong W, Wirtz M, Collinge D, Carrie C, Giraud E, Whelan J, David P, Javot H, Brearley C, Hell R, Marin E, Pogson BJ. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell. 2011;23:3992–4012.10.1105/tpc.111.091033
- Szechynska-Hebda M, Kruk J, Gorecka M, Karpinska B, Karpinski S. Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell. 2010;22:2201–2218.10.1105/tpc.109.069302
- Oelze ML, Vogel MO, Alsharafa K, Kahmann U, Viehhauser A, Maurino VG, Dietz kJ. Efficient acclimation of the chloroplast antioxidant defence of Arabidopsis thaliana leaves in response to a 10- or 100-fold light increment and the possible involvement of retrograde signals. J. Exp. Bot. 2012;63:1297–1313.10.1093/jxb/err356
- Ishikawa T, Sakai K, Yoshimura K, Takeda T, Shigeoka S. cDNAs encoding spinach stromal and thylakoid-bound ascorbate peroxidase, differing in the presence or absence of their 3′-coding regions. FEBS Lett. 1996;384:289–293.10.1016/0014-5793(96)00332-8
- Ishikawa T, Yoshimura K, Tamoi M, Takeda T, Shigeoka S. Alternative mRNA splicing of 3′-terminal exons generates ascorbate peroxidase isoenzymes in spinach (Spinacia oleracea) chloroplasts. Biochem. J. 1997;328:795–800.
- Yoshimura K, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S. Alternatively spliced mRNA variants of chloroplast ascorbate peroxidase isoenzymes in spinach leaves. Biochem. J. 1999;338:41–48.10.1042/0264-6021:3380041
- Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S. Identification of a cis element for tissue-specific alternative splicing of chloroplast ascorbate peroxidase pre-mRNA in higher plants. J. Biol. Chem. 2002;277:40623–40632.10.1074/jbc.M201531200
- Pnueli L, Liang H, Rozenberg M, Mittler R. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 2003;34:187–203.10.1046/j.1365-313X.2003.01715.x
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281.10.1105/tpc.104.026971
- Maruta T, Inoue T, Noshi M, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. Cytosolic ascorbate peroxidase 1 protects organelles against oxidative stress by wounding- and jasmonate-induced H2O2 in Arabidopsis plants. Biochim. Biophys. Acta. 2012;1820:1901–1907.10.1016/j.bbagen.2012.08.003
- Suzuki N, Miller G, Sejima H, Harper J, Mittler R. Enhanced seed production under prolonged heat stress conditions in Arabidopsis thaliana plants deficient in cytosolic ascorbate peroxidase 2. J. Exp. Bot. 2013;64:253–263.10.1093/jxb/ers335
- Vanderauwera S, Suzuki N, Miller G, van de Cotte B, Morsa S, Ravanat JL, Hegie A, Triantaphylides C, Shulaev V, Van Montagu MC, Van Breusegem F, Mittler R. Extranuclear protection of chromosomal DNA from oxidative stress. Proc. Natl. Acad. Sci. USA. 2011;108:1711–1716.10.1073/pnas.1018359108
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008;283:34197–34203.10.1074/jbc.M806337200
- Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007;144:1777–1785.10.1104/pp.107.101436
- Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inzé D, Mittler R. Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J. 2002;32:329–342.10.1046/j.1365-313X.2002.01427.x
- Caverzan A, Bonifacio A, Carvalho FE, Andrade CM, Passaia G, Schünemann M, Maraschin Felipe dos Santos, Martins MO, Teixeira FK, Rauber R, Margis R, Silveira JA, Margis-Pinheiro M. The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice. Plant Sci. 2014;214:74–87.10.1016/j.plantsci.2013.10.001
- Zhang Z, Zhang Q, Wu J, Zheng X, Zheng S, Sun X, Qiu Q, Lu T. Gene knockout study reveals that cytosolic ascorbate peroxidase 2(OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS One. 2013;8:e57472.10.1371/journal.pone.0057472
- Bonifacio A, Martins MO, Ribeiro CW, Fontenele AV, Carvalho FE, Margis-Pinheiro M, Silveira JA. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ. 2011;34:1705–1722.10.1111/pce.2011.34.issue-10
- Rosa SB, Caverzan A, Teixeira FK, Lazzarotto F, Silveira JA, Ferreira-Silva SL, Abreu-Neto J, Margis R, Margis-Pinheiro M. Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry. 2010;71:548–558.10.1016/j.phytochem.2010.01.003
- Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012;287:11717–11729.10.1074/jbc.M111.292847
- Kangasjärvi S, Lepistö A, Hännikäinen K, Piippo M, Luomala EM, Aro EM, Rintamäki E. Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem. J. 2008;412:275–285.10.1042/BJ20080030
- Giacomelli L, Masi A, Ripoll DR, Lee MJ, Wijk kJ. Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol. Biol. 2007;65:627–644.10.1007/s11103-007-9227-y
- Tarantino D, Vannini C, Bracale M, Campa M, Soave C, Murgia I. Antisense reduction of thylakoidal ascorbate peroxidase in Arabidopsis enhances paraquat-induced photooxidative stress and nitric oxide-induced cell death. Planta. 2005;221:757–765.10.1007/s00425-005-1485-9
- Murgia I, Tarantino D, Vannini C, Bracale M, Carravieri S, Soave C. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to Paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004;38:940–953.10.1111/tpj.2004.38.issue-6
- Narendra S, Venkataramani S, Shen G, Wang J, Pasapula V, Lin Y, Kornyeyev D, Holaday AS, Zhang H. The Arabidopsis ascorbate peroxidase 3 is a peroxisomal membrane-bound antioxidant enzyme and is dispensable for Arabidopsis growth and development. J. Exp. Bot. 2006;57:3033–3042.10.1093/jxb/erl060
- Suzuki N, Mittler R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic. Biol. Med. 2012;53:2269–2276.10.1016/j.freeradbiomed.2012.10.538
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell. 2004;16:616–628.10.1105/tpc.019398
- Maruta T, Inoue T, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. Arabidopsis NADPH oxidases, AtrbohD and AtrbohF, are essential for jasmonic acid-induced expression of genes regulated by MYC2 transcription factor. Plant Sci. 2011;180:655–660.10.1016/j.plantsci.2011.01.014
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:601–639.10.1146/annurev.arplant.50.1.601
- Shikanai T, Takeda T, Yamauchi H, Sano S, Tomizawa KI, Yokota A, Shigeoka S. Inhibition of ascorbate peroxidase under oxidative stress in tobacco having bacterial catalase in chloroplasts. FEBS Lett. 1998;428:47–51.10.1016/S0014-5793(98)00483-9
- Miyagawa Y, Tamoi M, Shigeoka S. Evaluation of the defense system in chloroplasts to photooxidative stress caused by paraquat using transgenic tobacco plants expressing catalase from Escherichia coli. Plant Cell Physiol. 2000;41:311–320.10.1093/pcp/41.3.311
- Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S. Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 2002;32:915–925.10.1046/j.1365-313X.2002.01476.x
- Danna CH, Bartoli CG, Sacco F, Ingala LR, Santa-María GE, Guiamet JJ, Ugalde RA. Thylakoid-bound ascorbate peroxidase mutant exhibits impaired electron transport and photosynthetic activity. Plant Physiol. 2003;132:2116–2125.10.1104/pp.103.021717
- Eastmond PJ. MONODEHYROASCORBATE REDUCTASE4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell. 2007;19:1376–1387.10.1105/tpc.106.043992
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inzé D, Mittler R, Van Breusegem F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006;141:436–445.10.1104/pp.106.078717
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, Knight H, Peck SC, Grierson CS, Hirt H, Knight MR. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature. 2004;427:858–861.10.1038/nature02353
- Rizhsky L, Davletova S, Liang H, Mittler R. The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 2004;279:11736–11743.10.1074/jbc.M313350200
- Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562.10.1016/j.redox.2014.02.006
- Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 2013;64:839–863.10.1146/annurev-arplant-042811-105606
- Simkova K, Moreau F, Pawlak P, Vriet C, Baruah A, Alexandre C, Hennig L, Apel K, Laloi C. Integration of stress-related and reactive oxygen species-mediated signals by Topoisomerase VI in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2012;109:16360–16365.10.1073/pnas.1202041109
- Nishizawa A, Yabuta Y, Yoshida E, Maruta T, Yoshimura K, Shigeoka S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006;48:535–547.10.1111/tpj.2006.48.issue-4
- Nishizawa-Yokoi A, Yoshida E, Yabuta Y, Shigeoka S. Analysis of the regulation of target genes by an Arabidopsis heat shock transcription factor, HsfA2. Biosci. Biotechnol. Biochem. 2009;73:890–895.10.1271/bbb.80809
- Nishizawa-Yokoi A, Nosaka R, Hayashi H, Tainaka H, Maruta T, Tamoi M, Ikeda M, Ohme-Takagi M, Yoshimura K, Yabuta Y, Shigeoka S. HsfA1d and HsfA1e involved in the transcriptional regulation of HsfA2 function as key regulators for the Hsf signaling network in response to environmental stress. Plant Cell Physiol. 2011;52:933–945.10.1093/pcp/pcr045
- Liu HC, Liao HT, Charng YY. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011;34:738–751.10.1111/pce.2011.34.issue-5
- Hahn A, Bublak D, Schleiff E, Scharf KD. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell. 2011;23:741–755.10.1105/tpc.110.076018
- Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 2002;16:1555–1567.10.1101/gad.228802
- Miller G, Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006;98:279–288.10.1093/aob/mcl107
- Lee S, Carlson T, Christian N, Lea K, Kedzie J, Reilly JP, Bonner JJ. The yeast heat shock transcription factor changes conformation in response to superoxide and temperature. Mol. Biol. Cell. 2000;11:1753–1764.10.1091/mbc.11.5.1753
- Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol. Cell. 1998;2:101–108.10.1016/S1097-2765(00)80118-5
- Ahn SG, Thiele DJ. Redox regulation of mammalianheat shock factor1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528.10.1101/gad.1044503
- Hahn JS, Thiele DJ. Activation of the Saccharomyces cerevisiae heat shock transcription factor under glucose starvation conditions by Snf1 protein kinase. J. Biol. Chem. 2004;279:5169–5176.
- Jung HS, Crisp PA, Estavillo GM, Cole B, Hong F, Mockler TC, Pogson BJ, Chory J. Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl. Acad. Sci. USA. 2013;110:14474–14479.10.1073/pnas.1311632110
- Kotak S, Port M, Ganguli A, Bicker F, von Koskull-Döring P. Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J. 2004;39:98–112.10.1111/tpj.2004.39.issue-1
- Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci USA. 2001;98:12826–12831.
- op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, Nater M, Apel K. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332.10.1105/tpc.014662
- Wagner D, Przybyla D, Op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, Apel K. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185.10.1126/science.1103178
- Kim C, Meskauskiene R, Zhang S, Lee KP, Lakshmanan Ashok M, Blajecka K, Herrfurth C, Feussner I, Apel K. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell. 2012;24:3026–3039.10.1105/tpc.112.100479
- Lee KP, Kim C, Landgraf F, Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2007;104:10270–10275.10.1073/pnas.0702061104
- Bechtold U, Richard O, Zamboni A, Gapper C, Geisler M, Pogson B, Karpinski S, Mullineaux PM. Impact of chloroplastic- and extracellular-sourced ROS on high light-responsive gene expression in Arabidopsis. J. Exp. Bot. 2008;59:121–133.10.1093/jxb/erm289
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2007;104:672–677.10.1073/pnas.0609063103
- Bienert GP, Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta. 2014;1840:1596–1604.10.1016/j.bbagen.2013.09.017
- Queval G, Foyer CH. Redox regulation of photosynthetic gene expression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:3475–3485.10.1098/rstb.2012.0068
- Noshi M, Maruta T, Shigeoka S. Relationship between chloroplastic H2O2 and the salicylic acid response. Plant Signal. Behav. 2012;7:944–946.10.4161/psb
- Yao N, Greenberg JT. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell. 2006;18:397–411.10.1105/tpc.105.036251
- Fahnenstich H, Scarpeci TE, Valle EM, Flugge UI, Maurino VG. Generation of hydrogen peroxide in chloroplasts of Arabidopsis overexpressing glycolate oxidase as an inducible system to study oxidative stress. Plant Physiol. 2008;148:719–729.10.1104/pp.108.126789
- Balazadeh S, Jaspert N, Arif M, Mueller-Roeber B, Maurino VG. Expression of ROS-responsive genes and transcription factors after metabolic formation of H2O2 in chloroplasts. Front. Plant Sci. 2012;3:234.
- Nomura H, Komori T, Kobori M, Nakahira Y, Shiina T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 2008;53:988–998.
- Nomura H, Komori T, Uemura S, Kanda Y, Shimotani K, Nakai K, Furuichi T, Takebayashi K, Sugimoto T, Sano S, Suwastika IN, Fukusaki E, Yoshioka H, Nakahira Y, Shiina T. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012;3:926.10.1038/ncomms1926
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant. 2008;1:423–445.10.1093/mp/ssn019
- Maruta T, Ojiri M, Noshi M, Tamoi M, Ishikawa T, Shigeoka S. Activation of γ-aminobutyrate production by chloroplastic H2O2 is associated with the oxidative stress response. Biosci. Biotechnol. Biochem. 2013;77:422–425.
- Maruta T, Noshi M, Nakamura M, Matsuda S, Tamoi M, Ishikawa T, Shigeoka S. Ferulic acid 5-hydroxylase 1 is essential for expression of anthocyanin biosynthesis-associated genes and anthocyanin accumulation under photooxidative stress in Arabidopsis. Plant Sci. 2014;219-220:61–68.10.1016/j.plantsci.2014.01.003