Abstract
The pro-oncogenic role of RhoA has been well identified in other cancers, but rarely in cervical cancer (CC), one of the main causes of cancer-related death in women. In the present study, we identified the overexpression of RhoA and its downstream effectors, ROCK-1 and ROCK-II, in CC specimens using western blotting. Then, we determined the effect of RhoA on the proliferation and migration of Hela cells, one of CC cell lines, by upregulating or downregulating the RhoA expression in Hela cells. We found that there was an overexpression of RhoA, ROCK-I/II in CC, which was associated with the progression of CC. And we confirmed that RhoA promoted the proliferation and migration of CC cells. In conclusion, we found a positive correlation among RhoA with the progression of CC by in vivo and in vitro evidences. A high RhoA expression in CC may predict a high metastatic potential of CC.
Graphical Abstract
Correlated Overexpression of RhoA and ROCK-I/II in part cervical cancer specimens
Key words:
Cervical cancer (CC) is one of the main causes of cancer-related death in womenCitation1) and the second most common cancer among women worldwide.Citation2) It is an important health issue in developing countries, although the incidence of this disease is slowly decreasing in developed countries due to early diagnosis. Human papilloma virus (HPV) infectionCitation3) is considered the greatest risk factor for CC.Citation4) While the presence of HPV is detected in 99% of all cases,Citation5) infection with this agent is not sufficient to cause CC. Identification of contributing factors associated with progression is still necessary to improve the survival or prognosis of CC patients.Citation6)
Rho family of small GTPases is within the Ras-like protein superfamily, which includes the Ras, Rab, Arf, and Ran families.Citation7) Rho proteins are highly conserved from lower eukaryotes to plants and mammals.Citation8) The Rho family comprises some 21 genes in humans, encoding at least 23 signaling molecules.Citation9) Once activated, Rho GTPases bind different effector molecules and trigger a signaling cascade to direct cellular responses, including microtubule cytoskeleton organization, cell division, motility, cell adhesion, vesicular trafficking, phagocytosis, and transcriptional regulation.Citation10–12) Rho GTPases have been reported to contribute to most steps of cancer initiation and progression including the acquisition of unlimited proliferation potential, survival and evasion from apoptosis, tissue invasion, and the establishment of metastasis. Several Rho GTPases are upregulated in some human tumors, including RhoA, RhoC, Rac1, and so on.Citation13–15) And the upregulated Rho GTPases play their pro-oncogenic roles via stimulating cell cycle progression and regulating gene transcription, for example, in promoting Ras-induced transformation.Citation16) The induction of tumor vascularization is essential for tumors to grow beyond a certain size, and malignant cells release factors that promote angiogenesis from nearby pre-existing blood vessels. Some Rho GTPases are thought to be able to regulate the release of pro-angiogenic factors to promote neovascularization.Citation17) To establish the metastasis in distant tissues, tumor cells have to enter the vascular or lymphatic system, then exit it and proliferate in the new tissue. The ability of Rho GTPase family members to regulate cytoskeletal dynamics, cell adhesion, and cell migrationCitation10) points to a central role in cancer cell invasion and metastasis. The pro-oncogenic role of RhoA has been well identified in breast cancer,Citation18,19) in lung cancer,Citation20,21) and has been rarely determined in CC.Citation22) The migration enhancement of CC cells by erythropoietin is in a Janus kinase- and RhoA-dependent manner.Citation22)
In the present study, we identified the overexpression of RhoA and its downstream effectors, ROCK-I and ROCK-II, in CC specimens using western blot assay. Then, we determined the effect of RhoA on the proliferation and migration of Hela cells, one of CC cell lines, by upregulating or downregulating the RhoA expression in Hela cells. This study has revealed a critical promoting role of RhoA in CC proliferation and migration.
Materials and methods
Tissue specimens
Forty-six specimens of human squamous CCs and 34 cervicitis tissues (as control) were selected from the archives of China-Japan Union Hospital, Jilin University from April 2007 to June 2011, complying with the principle of random and balance, and with informed consent and agreement. All fresh specimens were snap frozen in liquid nitrogen and stored at −80 °C post undergoing surgery. Clinical pathological data of each CC tissue were available from the clinical records and all information was re-assessed independently by two specialists. All enrolled participants have not been exposed to radiotherapy and chemotherapy before the samples were collected. The clinical stage of participants was classified according to the International Federation of Gynecology and Obstetrics criteria. The present study has been approved by the medical ethics committee of China-Japan Union Hospital, Jilin University.
Cell culture and construction of RhoA-2A-EGFP overexpressing Hela cells
Human cervical carcinoma cell line, Hela, was purchased from American Type Culture Collection, grown in RPMI-1640 (Invitrogen, Carlsbad, CA, USA), supplemented with 5% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), and incubated at 37 °C in a humidified chamber supplemented with 5% CO2. To generate a RhoA overexpressing Hela cell line, we constructed a co-expressing plasmid of RhoA and EGFP with a 2A peptide linker. RhoA (GenBank Accession NM_001664.2) and EGFP (GenBank Accession #U55762) cDNA were separately amplified and then overlapped with a 2A peptide coding sequence; then, the overlapping sequence was cloned into pcDNA3.1(+) vector and sequencingly confirmed. RhoA-2A-EGFP-pcDNA3.1(+) or control pcDNA3.1(+) vectors (Invitrogen, Carlsbad, CA, USA) were transfected into Hela cells, respectively. The positive clone, Hela (RhoA+), and Hela (Con) were selected in the presence of 1.5 mg/mL G418 (Thermo Scientific, Rockford, USA) and maintained in medium containing G418 at 1 mg/mL. To clarify the direct role of RhoA expression on the proliferation and migration of CC cells, we assessed the levels of RhoA expression in each cell line. siRNA-RhoA (targeted sequences were 5′-GAUUGUUGGUGAUGGAGCCUGUGGA-3′, nt 27–51 relative to the start codon; Genepharma, Shanghai, China) was used to block RhoA expression, with siRNA-Con as a negative control (targeted sequences: 5′-CAGUCAGGAGGAUCCAAAGTG-3′, without homology with any human genomic sequences).
Real-time quantitative PCR
Total mRNA from CC tissues or cells were extracted with the RNeasy plus mini kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions, and were reverse transcribed into cDNA (TaKaRa Bio Inc., Tokyo, Japan). The cDNA was then amplified by real-time quantitative TaqMan PCR with RhoA (former primer: 5′-CAG TTC CCA GAG GTG TAT GT-3′, latter primer: 5′-GGT GGA TGG AAA GCA GGT AG-3′, probe sequence: 5′-GCG CGG GGC CGC GAC C-3′) or β-actin (an internal control) (former primer: 5′-AAT CTG GCA CCA CAC CTT CTA CA-3′, latter primer: 5′-CTG ACC GAG GCC CCC CTG A-3′, probe seuquence: 5′-TGT GGC TCC CGA GGA GC-3′) specific primers and probes with the Lightcycler 480 II (Roche, Mannheim, Germany). The data were normalized to β-actin and expressed as the fold change over control and calculated with the ∆∆Ct method.Citation23)
Protein extraction and western blotting
Total proteins from CC tissues or Hela cells were extracted with cell lysis reagent (Promega, Madison, WI, USA), and supplemented with Complete Mini protease inhibitor cocktail (Roche Diagnostics, GmbH, Germany). The protein samples were loaded on and separated by SDS-PAGE, after being quantitatively determined by using Bradford Reagent (Bio-Rad, Hercules, CA, USA), and then were transferred to PVDF membranes, which were then blocked in 5% skimmed milk for 1 h at room temperature and probed with an antibody to RhoA (sc-179), ROCK-I (sc-5560), ROCK-II (sc-5561), or β-actin (sc-1616) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antibody binding was detected by using chemiluminescence (Thermo Scientific, Rockford, USA) according to the manufacturer’s instructions with a peroxidase-conjugated anti-mouse antibody. The housekeeping gene β-actin was used as an internal control. The data were expressed as percentage to β-actin.
Cell proliferation and migration assay
Cell Counting Kit-8 (CCK-8) was utilized to assay the influence of RhoA on the cell proliferation, Hela, Hela (RhoA+), or Hela (Con) cells with or without siRNA-RhoA or siRNA-Con transfection, and then were incubated in CCK-8 solution (DOJINDO, Kumamoto, Japan). The 450-nm absorbance of each well was detected after visual color occurrence at 24, 48, or 72 h. The capability of cell migration was examined by scratch assay. Cells were cultivated to 90% confluence on the 12-well plates and transfected with or without siRNA-RhoA or siRNA-Con. Then, a cell scratch was used to scratch the confluent cells after transfection for 24 h. Cellular growth at 0 and 48 h was observed.
Statistical analysis
Statistical analyses were performed using SPSS16.0 software (IBM SPSS, Armonk, NY, USA). The RhoA expression in mRNA or protein level and the ROCK-I/II expression in protein level between two groups were analyzed by Student’s t test. Correlations between the RhoA expression and the expression of ROCK-I or ROCK-II were analyzed using the Spearman rank correlation. p value < 0.05 or less was considered statistically significant.
Results
Overexpression of RhoA GTPases is associated with progression in CC
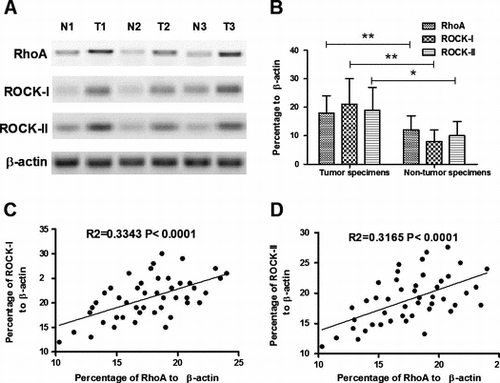
Protein expression of RhoA, ROCK-I and ROCK-II, two isoforms Citation24) of ROCK, one of the best characterized downstream effectors of RhoA, was determined in both tumor (n = 46) and non-tumor specimens (n = 34) of CCs using western blotting. β-actin was used as an internal reference molecule. Results demonstrated that the percentage of RhoA, ROCK-I, and ROCK-II in CC specimens were 18.23 ± 6.11, 20.97 ± 8.92, and 18.75 ± 8.20, respectively, while the percentage of the three molecules were 12.24 ± 5.09, 8.31 ± 3.88, and 10.25 ± 4.76 (Fig. (A) and (B)). There was a significant difference between the tumor and non-tumor specimens in the RhoA, ROCK-I, or ROCK-II expression (p < 0.01, p < 0.01, and p < 0.05, respectively, paired-samples t-test). To confirm the overexpression of RhoA, ROCK-I, and ROCK-II in CCs, correlation between RhoA overexpression and the ROCK-I/II overexpression was analyzed. It was shown that there were strong associations of RhoA expression with the ROCK-I/II expression (Fig. (C) and (D)); R2 = 0.3343 p < 0.0001 for ROCK-I and R2 = 0.3165 p < 0.0001 for ROCK-II).
Fig. 1. Overexpression of RhoA and ROCK-I/II in CC specimens.
Notes: (A) and (B) overexpression of RhoA and ROCK-I/II in part CC specimens; and (C) and (D) correlation of RhoA overexpression with the ROCK-I(C) or ROCK-II (D) expression. Statistical significance was considered with a p value < 0.05 or less.
Association of RhoA overexpression with the clinicopathological variants of CCs
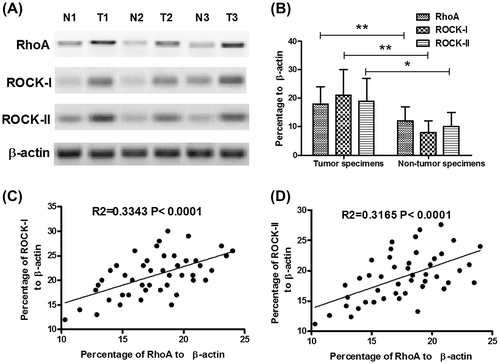
The clinical pathological features were classified according to age, tumor size, HPV infection, FIGO stage, FIGO histological grade, vascular invasion, lymph node metastasis, expression of estrogen receptor, and progesterone receptor. Firstly, we confirmed that there was no association of RhoA overexpression with the age and tumor size. Secondly, the relative RhoA level to β-actin varied from 14.21 ± 4.28 (n = 18) to 23.22 ± 5.21(n = 8); among the specimens of different FIGO stages (stage I–IV), there was a significant difference between the stage I and stage III or stage IV (either p < 0.05) (Fig. (A)). And the RhoA overexpression correlated with the vascular invasion and lymph node metastasis; there was a significantly high RhoA level in the specimens of patients with vascular invasion or lymph node metastasis (either p < 0.05) (Fig. (B) and (C)). In addition, the closely associated HPV infection, estrogen receptor, and progesterone receptor were also evaluated to confirm the correlation of them with the RhoA overexpression. And no significant difference was observed. All these findings suggested a promoting role of RhoA overexpression to the CC proliferation and invasion.
Fig. 2. Association of RhoA overexpression with the clinicopathological variants of CCs.
Notes: (A) RhoA expression of CC specimens from patients with different FIGO stages; (B) RhoA expression of CC specimens from patients with or without vascular invasion; and (C) RhoA expression of CC specimens from patients with or without lymph node metastasis. Statistical significance was considered with a p value < 0.05 or less.
Construction of a Hela cell line, overexpressing RhoA
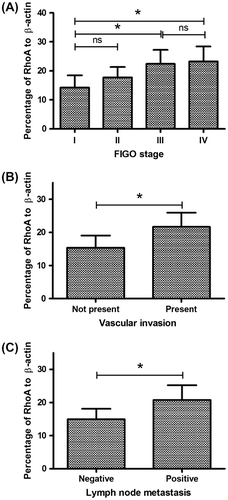
To generate a RhoA-overexpressed Hela cell line (a cervical adenocarcinoma cell line), RhoA and EGFP (enhanced green fluorescent protein) cDNA were separately amplified and then overlapped with a 2A peptide coding sequenceCitation25,26) between them (Fig. (A)). After the RhoA-2A-EGFP coding sequence were successfully cloned into the pcDNA3.1(+) vectors and sequencing confirmed (data not shown). After being transfected with the recombinant plasmid, Hela cells were serially cultured under 1.5 mg/mL G418. Hela cells, overexpressing RhoA and EGFP were selected according to the green fluorescence (Fig. (B)). Then, the overexpression of RhoA in both protein and mRNA levels was determined. The overexpression of the RhoA and EGFP co-overexpressed Hela cells, Hela (RhoA+) cells, and the RhoA upregulation in the protein level were confirmed by the western blot analysis (Fig. (C)) (p < 0.01). And the real-time quantitative PCR with the RhoA-specific primers also demonstrated that the RhoA mRNA was significantly upregulated in the Hela (RhoA+) cells (p < 0.01) (Fig. (D)). In addition, to reconfirm the pro-oncogenic role of Rho in CC, the loss-of-function approach was also applied in our study. RhoA-specific siRNA was used to block the RhoA overexpression in both Hela (Con) and Hela (RhoA+) cells. Fig. (E) and (F)) indicated that the siRNA transfection inhibited the RhoA expression in both mRNA and protein levels in both cells (p < 0.05 or p < 0.01).
Fig. 3. Construction of a Hela cell line, overexpressing RhoA.
Notes: (A) schematic diagram of RhoA and EGFP co-expression with a 2A peptide sequence; the RhoA and EGFP coding sequence linked by 2A peptide coding sequence was cloned into pcDNA3.1 vector; a single mRNA coding CTGF and RFP could translate RhoA and EGFP separately; (B) EGFP expression of Hela (RhoA+) cells; (C) RhoA expression in protein level in the Hela (RhoA+) cells; (D) RhoA expression in mRNA level in the Hela (RhoA+) cells; and (E) and (F) knockdown of RhoA in mRNA level (E) or in protein level (F) by siRNAs. All results are the average of three independent experiments, and statistical significance was considered with a p value < 0.05 or less.
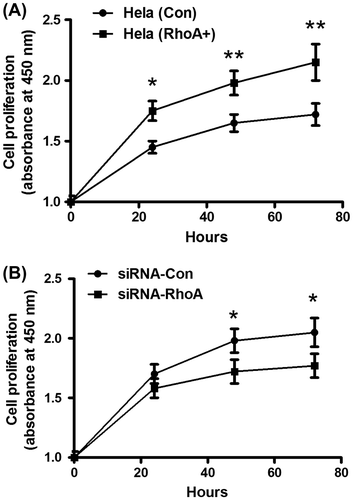
Rho promotes CC cell proliferation, invasion, and migration
Rho GTPases have shown to play pro-oncogenic roles via regulating gene transcription and stimulating cancer cell progression.Citation16) To explore the promotion by RhoA to the proliferation of CC cells, we test the cell proliferation of Hela (Con) and Hela (RhoA+) cells by CCK-8 assay. Fig. (A) demonstrated a significantly higher proliferation of Hela (RhoA+) than Hela (Con) cells, from 24-h post-planting (p < 0.05 for 24 h, p < 0.01 for both 48 and 72 h). Then, we blocked the RhoA overexpression in the Hela (RhoA+) cells with RhoA-specific siRNA and determined the proliferation of Hela (RhoA+) cells. Fig. (B) showed that the siRNA transfection reversed the promotion of the overexpressed RhoA to Hela cell proliferation, and 48 or 72 h post siRNA-RhoA transfection, Hela (RhoA+) cells proliferated significantly lower than being transfected with the control siRNA (either p < 0.05). Thus, evidence from both gain-of-function and loss-of-function experiments revealed the promotive role of RhoA in CC cell proliferation.
Fig. 4. Rho promotes CC cell proliferation.
Notes: (A) significantly higher proliferation of Hela (RhoA+) cells than Hela (Con) cells. The growth curve of both cells was evaluated by CCK-8 assay. (B) growth curve of Hela (RhoA+) cells after transfecting with siRNA-RhoA or siRNA-Con, examined by CCK-8 assay. The experiments were performed separately in triplicate. Statistical significance was shown as *p < 0.05, **p < 0.01.
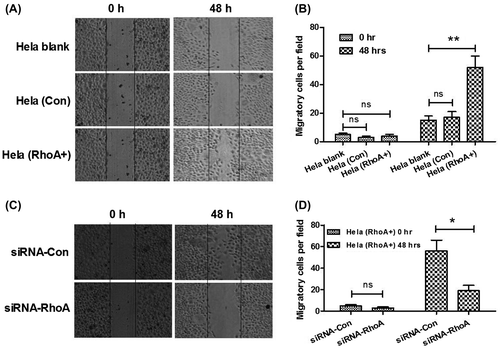
We have found that there was an association of RhoA overexpression with the vascular invasion and lymph node metastasis of CC, and it is well known that cell migration was responsible for tumor metastasis.Citation27) Therefore, we next determined the higher migration of Hela (RhoA+) than Hela (Con) cells with the scratch assay. Firstly, the differences in migration process between Hela (RhoA+) and Hela (Con) cells were showed in scratch assay (Fig. (A) and (B)). It was obvious that the RhoA-overexpressed Hela cells, Hela (RhoA+), were more metastatic than the Hela (Con) cells. Then, we adopted the RNAi technology to knockdown the RhoA overexpression in the Hela (RhoA+) cells. As shown in Fig. (C) and (D), the RhoA-specific siRNA, siRNA-RhoA, could significantly reverse the promotion of RhoA to the migration of Hela (RhoA+) cells, compared to the control siRNA, siRNA-Con. All these results indicated that overexpression of RhoA stimulated the migration of CC cells in vitro and possibly in vivo.
Fig. 5. Rho promotes CC cell migration.
Notes: (A) and (B) higher migration of Hela, Hela (RhoA+) cells than Hela (Con) cells. Migration of cells was depicted post inoculation at 0, or 48 h by cells scratch assay. Solid lines showed the edges at the start of experiments. (C) and (D) inhibition by siRNA-RhoA, of the promotion of RhoA to the Hela cell migration. Migration of Hela (RhoA+) cells was depicted post transfecting with siRNA-RhoA or siRNA-Con at 0, or 48 h by cells scratch assay. The experiments were performed separately in triplicate. Statistical significant was showed as *p < 0.05 and **p < 0.01, ns: not significant.
Discussion
This study examined the expression of RhoA and its two downstream effectors, ROCK-I and ROCK-II,Citation24) in clinical specimens of cervical carcinoma. Western blotting quantitatively indicated the overexpression of RhoA ROCK-I and ROCK-II in 46 cervical carcinoma tissues, and there was a significant correlation of the RhoA overexpression with the overexpression of ROCK-I and ROCK-II. And the RhoA expression in cervical carcinoma was associated with FIGO stage, the vascular invasion, and lymph node metastasis, but not with the patients’ age, tumor size, ER, PR, or HPV infection. High RhoA expression in CC was positively correlated with cancer clinic stage and lymphatic metastasis, suggesting that RhoA may play a substantial role in the development, invasion, and metastasis of cervical carcinoma. However, the precise mechanism by which RhoA induces adverse clinical characteristics in patients with cervical carcinoma is poorly understood.
Rho GTPases have shown to play pro-oncogenic roles via regulating gene transcription and stimulating cancer cell progression.Citation16) And the Rho GTPases have been reported to regulate the unlimited proliferation, invasion, and metastasis in some human tumors.Citation13,15) The RhoA signaling has been reported to be activated in the invasion or metastasis including, but not limited to, colorectal cancer, breast cancer, and prostate cancer.Citation28–30) In order to explore its pro-oncogenic role in cervical carcinoma, we manipulated the RhoA level in Hela cells by overexpressing or knockdowning RhoA. Firstly, we overexpressed RhoA and EGFP with the 2A peptide linker, which is a “self-cleavage” peptide of foot-and-mouth disease virus.Citation25) Coding sequences linked by the 2A peptide are within the same open reading frame and are transcribed into one mRNA molecule. And the mRNA molecule is translated into two different proteins, which are independent in function.Citation26,31) The overexpression of the RhoA and EGFP co-overexpressed Hela cells, Hela (RhoA+) cells, was confirmed by the western blot analysis in protein level and by real-time quantitative PCR in mRNA level. On the other side, the RhoA-specific siRNA was used to block the RhoA expression in both Hela (Con) and Hela (RhoA+) cells. Then, the Hela (Con) and Hela (RhoA+) cells were used to determine the promotion of RhoA to the cancer cell proliferation, invasion, and migration. A significantly higher proliferation and migration of Hela (RhoA+) than Hela (Con) cells was observed; and the blockage of the RhoA overexpression in the Hela (RhoA+) cells with RhoA-specific siRNA reversed the promotion of RhoA to the proliferation and migration of Hela cells. Thus, evidence from both gain-of-function and loss-of-function experiments revealed the promotive role of RhoA in CC cells.
In conclusion, the present study indicated that there was a positive correlation among RhoA with the FIGO stage, vascular invasion, and lymphatic metastasis of CC, reflecting its promotive role in the cancer invasion and metastasis. And the in vitro experiments demonstrated that RhoA promoted the proliferation and migration of CC cells. Since a high RhoA expression in CC may predict a high metastatic potential, RhoA would be an excellent biomarker in forecasting CC metastasis.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150.10.1200/JCO.2005.05.2308
- Movva S, Rodriguez L, Arias-Pulido H, Verschraegen C. Novel chemotherapy approaches for cervical cancer. Cancer. 2009;115:3166–3180.10.1002/cncr.v115:14
- Zur HH. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer. 2002;2:342–350.
- Tjalma WA, Fiander A, Reich O, Powell N, Nowakowski AM, Kirschner B, Koiss R, O’Leary J, Joura EA, Rosenlund M, Colau B, Schledermann D, Kukk K, Damaskou V, Repanti M, Vladareanu R, Kolomiets L, Savicheva A, Shipitsyna E, Ordi J, Molijn A, Quint W, Raillard A, Rosillon D, De Souza SC, Jenkins D, Holl K. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int. J. Cancer. 2013;132:854–867.10.1002/ijc.27713
- Ganguly N, Parihar SP. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J. Biosci. 2009;34:113–123.10.1007/s12038-009-0013-7
- Smith JS, Green J, Berrington DGA, Appleby P, Peto J, Plummer M, Franceschi S, Beral V. Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet. 2003;361:1159–1167.10.1016/S0140-6736(03)12949-2
- Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101.10.1016/j.febslet.2008.04.039
- Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol. Biol. Evol. 2007;24:203–216.
- Wherlock M, Mellor H. The Rho GTPase family: a Racs to Wrchs story. J. Cell Sci. 2002;115:239–240.
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269.10.1146/annurev.cellbio.21.020604.150721
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255.10.1042/0264-6021:3480241
- Wilkins A, Insall RH. Small GTPases in Dictyostelium: lessons from a social amoeba. Trends Genet. 2001;17:41–48.10.1016/S0168-9525(00)02181-8
- Aronheim A, Broder YC, Cohen A, Fritsch A, Belisle B, Abo A. Chp, a homologue of the GTPase Cdc42Hs, activates the JNK pathway and is implicated in reorganizing the actin cytoskeleton. Curr. Biol. 1998;8:1125–1128.10.1016/S0960-9822(98)70468-3
- Gomez DPT, Benitah SA, Valeron PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27:602–613.
- Gouw LG, Reading NS, Jenson SD, Lim MS, Elenitoba-Johnson KS. Expression of the Rho-family GTPase gene RHOF in lymphocyte subsets and malignant lymphomas. Br. J. Haematol. 2005;129:531–533.10.1111/bjh.2005.129.issue-4
- Benitah SA, Valeron PF, van Aelst L, Marshall CJ, Lacal JC. Rho GTPases in human cancer: an unresolved link to upstream and downstream transcriptional regulation. Biochim. Biophys. Acta. 2004;1705:121–132.
- Merajver SD, Usmani SZ. Multifaceted role of Rho proteins in angiogenesis. J. Mammary Gland Biol. Neoplasia. 2005;10:291–298.10.1007/s10911-006-9002-8
- Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc. Nat. Acad. Sci. USA. 2014;111:E384–E393.10.1073/pnas.1321510111
- Hauser AD, Bergom C, Schuld NJ, Chen X, Lorimer EL, Huang J, Mackinnon AC, Williams CL. The SmgGDS splice variant SmgGDS-558 is a key promoter of tumor growth and RhoA signaling in breast cancer. Mol. Cancer Res. 2014;12:130–142.10.1158/1541-7786.MCR-13-0362
- Patlolla JM, Qian L, Biddick L, Zhang Y, Desai D, Amin S, Lightfoot S, Rao CV. Beta-Escin inhibits NNK-induced lung adenocarcinoma and ALDH1A1 and RhoA/Rock expression in A/J mice and growth of H460 human lung cancer cells. Cancer Prev. Res (Phila). 2013;6:1140–1149.10.1158/1940-6207.CAPR-13-0216
- Carr HS, Zuo Y, Oh W, Frost JA. Regulation of focal adhesion kinase activation, breast cancer cell motility, and amoeboid invasion by the RhoA guanine nucleotide exchange factor Net1. Mol. Cell Biol. 2013;33:2773–2786.10.1128/MCB.00175-13
- Hamadmad SN, Hohl RJ. Erythropoietin stimulates cancer cell migration and activates RhoA protein through a mitogen-activated protein kinase/extracellular signal-regulated kinase-dependent mechanism. J. Pharmacol. Exp. Ther. 2008;324:1227–1233.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408.10.1006/meth.2001.1262
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193.10.1016/0014-5793(96)00811-3
- Ryan MD, King AM, Thomas GP. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J. Gen. Virol. 1991;72:2727–2732.10.1099/0022-1317-72-11-2727
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single “self-cleaving” 2A peptide-based retroviral vector. Nat. Biotechnol. 2004;22:589–594.10.1038/nbt957
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer J. Clin. 2005;55:74–108.10.3322/canjclin.55.2.74
- Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK. Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene. 2010;29:1293–1302.10.1038/onc.2009.420
- Ariake K, Ohtsuka H, Motoi F, Douchi D, Oikawa M, Rikiyama T, Fukase K, Katayose Y, Egawa S, Unno M. GCF2/LRRFIP1 promotes colorectal cancer metastasis and liver invasion through integrin-dependent RhoA activation. Cancer Lett. 2012;325:99–107.10.1016/j.canlet.2012.06.012
- Liao YC, Ruan JW, Lua I, Li MH, Chen WL, Wang JR, Kao RH, Chen JH. Overexpressed hPTTG1 promotes breast cancer cell invasion and metastasis by regulating GEF-H1/RhoA signalling. Oncogene. 2012;31:3086–3097.10.1038/onc.2011.476
- Szymczak-Workman AL, Vignali KM, Vignali DA. Verification of 2A peptide cleavage. Cold Spring Harb. Protoc. 2012;2012:255–257.
