Abstract
Avenanthramides are characteristic constituents of oat seeds. We analyzed the methanol extract of oat seeds by HPLC and detected three compounds 1, 2, and 3 eluted at retention times similar to avenanthramides. The three compounds were purified by column chromatography and HPLC. Spectroscopic analyses of 1, 2, and 3 suggested that they are amides of 4,5-dihydroxyanthranilic acid with caffeic, p-coumaric, and ferulic acids, respectively. Their identities were confirmed by comparing spectra and chromatographic behavior with compounds synthesized from 4,5-dihydroxyanthranilic acid and N-hyrdroxysuccinimide esters of hydroxycinnamic acids. LC-MS/MS analysis with multiple reaction monitoring showed that the amounts of 1, 2, and 3 were 16.5–26.9% of corresponding avenanthamides with 5-hydroxyanthranilic acid. Compounds 1, 2, and 3 showed stronger 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity than the corresponding avenanthramides with 5-hydroxyanthranilic acid, indicating the involvement of 4,5-dihydroxyanthranilic acid moiety in the scavenging of DPPH radicals.
Graphical Abstract
New series of avenanthramides that contain a 4,5-dihydroxyanthranilate moiety were identified in oat seeds.
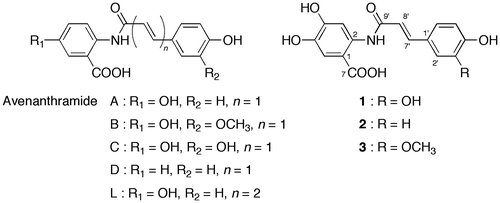
Plants have acquired biosynthetic pathways leading to various secondary metabolites in the relationships of the plants with environmental factors during the evolutionary process. Secondary metabolites have unique structures, some of which are useful to human life. Avenanthramides are secondary metabolites that have been found in oat (Avena sativa) groats and hulls.Citation1) Avenanthramides are hydroxycinnamic acid amides with hydroxyanthranilic acids (Fig. ). Collins described that at least 20 different phenolic conjugates with anthranilic acid derivatives are present in oats,Citation2) but their chemical structures have not been fully identified. The major avenanthramides in oat groats are avenanthramides A (N-p-coumaroyl-5-hydroxyanthranilic acid), B (N-feruloyl-5-hydroxyanthranilic acid), and C (N-caffeoyl-5-hydroxyanthranilic acid).
Avenanthramides play a role as phytoalexins in oat leaves because they exhibit anti-fungal activity and their synthesis is induced by infection with pathogensCitation3−5) and by treatment with elicitors.Citation6) In addition, avenanthramides have been shown to be incorporated into cell walls upon pathogen infection.Citation7) The incorporation of avenanthramides is considered to reinforce cell walls against cell wall-degrading enzymes secreted by pathogens. It has been demonstrated that the hydroxyanthranilate hydroxycinnamoyl-CoA hydroxycinnamoyltransferase (HHT) activity that catalyzes the final condensation reaction of avenanthramide synthesis is induced by elicitation.Citation8,9) Enzyme activity is also detected in the oat seed extract, indicating the involvement of the same enzyme in the synthesis of avenanthramides in seeds.Citation10) The genes encoding HHTs have been cloned by Yang et al.Citation11)
Oats are usually consumed as whole grains and contain the highest levels of protein and fat of any of the cereal grains.Citation12) In ancient Rome, oat flour was topically applied to treat various dermatologic conditions.Citation13) Recently, various bioactivities have been found in avenanthramides. Liu et al. demonstrated that avenanthramides are anti-inflammatory and anti-atherogenic by showing that they inhibit the adhesion of monocyte cells to aortic endothelial cell monolayers, the expression of adhesion molecules, and the production of proinflammatory cytokines.Citation14) Sur et al. indicated that avenanthramides exhibit anti-inflammatory and anti-itching activity of the skin.Citation15) They showed that topical application of avenanthramides mitigated inflammation in murine models of contact hypersensitivity and neurogenic inflammation and reduced pruritogen-induced scratching in a murine itch model. In addition, it is interesting that avenanthramides are very similar in their chemical structure to tranilast (N-3,4-dimethoxycinnamoylanthranilic acid, Rizaben®), which is an anti-allergy drug used to treat asthma, autoimmune diseases, and atopic and fibrotic pathologies.Citation16,17)
The anti-oxidant activity of avenanthramides has also been investigated. Peterson et al. measured anti-oxidant activity using two in vitro systems: inhibition of β-carotene bleaching and reaction with the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH). They indicated the order of activity was avenanthramide C > B > A in both assay systems.Citation18) Fagerlund et al. included synthesized analogs of avenanthramides in the analysis of the anti-oxidant activity of avenanthramides. They determined the activity by DPPH assay and by the linoleic acid hydroperoxide assay. In the scavenging DPPH radical, avenanthramides with feuloyl-, sinapoyl, and caffeoyl groups showed high activity.Citation19) In addition, they found that avenanthramides with the 4-methoxy-5-hydroxyanthranilic moiety showed greater activity than avenanthramides bearing anthranilic and 5-hydroxyanthranilic moieties. Thus, the 4-methoxy-5-hydroxyanthranilic moiety is involved in the radical scavenging reaction. In the linoleic acid assay, avenanthramides with one or more hydroxyl groups inhibited linoleic acid oxidation. However, the structure-activity relationship revealed by this system is not simple, and the order of activity was not explained in a rational way.
In oat groats, multiple uncharacterized avenanthramides are present. Even in the identified avenanthramides, usually only avenanthramides A, B, and C have been the target of the analysis. The uncharacterized avenanthramides did not attract attention probably because their amounts were small in comparison with known avenanthramides. However, the uncharacterized avenanthramides may have stronger or distinct activity. From this viewpoint, we searched for uncharacterized avenanthramides in oat seed and identified avenanthramides containing 4,5-dihydroxyanthranilic acid. Because they have an additional hydroxyl group that may contribute to anti-oxidant activity, we measured anti-oxidant activity using the DPPH radical scavenging assay. In addition, we developed a multiple reaction monitoring (MRM) method of liquid chromatography-tandem mass spectrometry (LC-MS/MS), and determined their contents in oat groats.
Materials and methods
General experimental procedures
1H and 13C NMR spectra were recorded on a Bruker Avance II 600 MHz spectrometer (Bruker, Billerica, MA, USA). Chemical shifts were referenced to CD3OD (δH 3.31) and (CD3)2SO (δH 2.50, δC 39.5). Positive and negative ESI MS were obtained using a Waters Quattro Micro mass spectrometer combined with a Waters PDA UPLC system equipped with an ODS column (Acquity UPLC BEH C18, 1.7 μm, 2.2 × 50 mm; Waters, Milford, MA, USA). The HRMS were obtained with an Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Preparative HPLC was performed using an ODS column (Wakosil-II, 5C18HG Prep, 20 × 250 mm; Wako Pure Chemical Industries, Osaka, Japan; Cosmosil Packed Column 5C18-AR-II, 20 × 250 mm; Nacalai Tesque, Kyoto, Japan) at a flow rate of 7.0 mL/min and a column temperature of 40 °C. Unless stated otherwise, a two-solvent system was used for HPLC to generate the mobile phase: solvent A, H2O-TFA (100:0.1); and solvent B, MeCN Analytical HPLC was performed using an ODS column (Cosmosil Packed Column 5C18-AR-II, 4.6 × 150 mm; Nacalai Tesque) at a flow rate of 0.8 mL/min and a column temperature of 40 °C. The same solvents were used as for preparative HPLC. A linear gradient 5–60% B/(A + B) within 60 min was applied.
Isolation of avenanthramides
Oat (Avena sativa L. cv. Shokan 1) seeds harvested from an experimental farm of the Faculty of Agriculture, Tottori University, Japan, in July 2010 and 2011 were used for extraction of avenanthramides. Oat seeds (515 g) without removal of hulls were powdered using a food processor. The powder was extracted three times with 4 L MeOH. The extracts were combined and evaporated. The residue was suspended in a mixture of 250 mL H2O and 50 mL MeOH. The suspension was washed with hexane three times to remove lipids, and was passed through an ODS column (Cosmosil 75C18-PREP, 100 g; Nacalai Tesque) equilibrated with MeOH H2O AcOH (80:10:1). The column was washed with 600 mL of the same solvent. The column through fraction and MeOH H2O AcOH (80:10:1) fraction were combined and evaporated. The obtained residue was dissolved in MeOH H2O–AcOH (5:90:1) and applied to an ODS column (Cosmosil 75C18-PREP, 100 g; Nacalai Tesque) equilibrated with MeOH H2O AcOH (5:95:1). The column was eluted with 750 mL each of a mixture of MeOH H2O AcOH (5:95:1, 20:80:1, 40:60:1, and 80:20:1) and MeOH. Avenanthramides were eluted in the MeOH H2O AcOH (60:40:1) fraction. The fraction was concentrated to dryness, and 0.70 g extract was obtained. This extract was applied to preparative HPLC with an ODS column (Wakosil-II, 5C18HG Prep, 20 × 250 mm; Wako Pure Chemical Industries). HPLC conditions were as follows: solvents: 0.1% TFA in H2O (A); acetonitrile (B); gradient: 28–35% B/(A + B) within 40 min; flow rate: 7 mL/min; column temperature: 40 °C; and detection: 340 nm. Peaks 1 and 3 were eluted at 12.2 and 17.3 min, respectively.
Since the fraction corresponding to peak 1 contained impurities, the fraction was further subjected to HPLC purification with a small column (Mightysil RP-18 GP, 150 × 4.6 mm (3 μm); Kanto Kagaku, Tokyo, Japan). HPLC conditions were as follows: solvents: 0.1% TFA (A), MeCN (B); elution: 20% A/(A + B); flow rate: 0.6 mL/min, column temperature: 40 °C, and detection 340 nm. The fraction corresponding to peak 1 (Rt. 9.6 min) was concentrated and subjected to a small ODS column (Cosmosil 75C18-PREP) to remove TFA. The column was washed with H2O and eluted with MeOH H2O (8:2) to give compound 1.
1 (1.0 mg). 1H NMR (600 MHz, CD3OD): δH (ppm) 8.23 (s, 1H, H-3), 7.51 (d, 1H, J = 15.6 Hz, H-7′), 7.50 (s, 1H, H-6), 7.07 (d, 1H, J = 2.0 Hz, H-2′), 6.98 (dd, 1H, J = 2.0 Hz and 8.1 Hz, H-6′), 6.79 (d, 1H, J = 8.1 Hz, H-5′), 6.46 (d, 1H, J = 15.6 Hz, H-8′). HR-ESI-MS: m/z 332.0764 ([M + H]+) (m/z 332.0765 calcd. for C16H14O7N).
The fraction corresponding to peak 3 also contained impurities. The fraction was subjected to preparative HPLC with a small column (Mightysil RP-18 GP, 150 × 4.6 mm (3 μm)). Peak 3 was eluted at 21.4 min. HPLC conditions were as follows: solvents: 0.1% TFA (A), MeCN (B); elution: 25% B/(A + B); flow rate: 0.6 mL/min, column temperature: 40 °C, and detection: 340 nm.
3 (2.5 mg). ¹H NMR (600 MHz, CD3OD): δH (ppm) 8.24 (s, 1H, H-3), 7.57 (d, 1H, J = 15.5 Hz, H-7′), 7.51 (s, 1H, H-6), 7.24 (d, 1H, J = 1.9 Hz, H-2′), 7.10 (dd, 1H, J = 1.9 Hz and 8.2 Hz, H-6′), 6.83 (d, 1H, J = 8.2 Hz, H-5′), 6.55 (d, 1H, J = 15.5 Hz, H-8′), 3.93 (s, 3H). HR-ESI-MS: m/z 346.0921 ([M + H]+) (m/z 346.0921 calcd. for C17H16O7N).
Oat seeds (150 g) were extracted with 1 L of MeOH three times, washed with hexane, and fractionated with an ODS column in a similar way to the isolation of 1 and 3. The MeOH-H2O-AcOH (60:40:1) fraction was subjected to preparative HPLC with an ODS column (Cosmosil Packed Column 5C18-ARII, 10 × 250 mm) with flow rate of 3 mL/min . The conditions for preparative HPLC other than the flow rate were the same as for the isolation of 1 and 3. The compound corresponding to peak 2 was eluted at 9.0 min. The fraction was concentrated to a small volume, and TFA was removed using a small ODS column to give compound 2.
2 (1.0 mg). 1H NMR (600 MHz, CD3OD): δH (ppm) 8.22 (s, 1H, H-3), 7.56 (d, 1H, J = 15.7 Hz, H-7′), 7.51 (s, 1H, H-6), 7.48 (d, 2H, J = 8.5 Hz, H-2′, 6′), 6.82 (d, 2H, J = 8.5 Hz, H-3′, 5′), 6.51 (d, 1H, J = 15.7 Hz, H-8′). HR-ESI-MS: m/z 316.0814 ([M + H]+) (m/z 316.0821 calcd. for C16H14O7 N).
Chemicals
N-hydroxysuccinimide esters of p-coumaric, ferulic, and caffeic acids were prepared by transesterification, according to the method described by Stöckigt and Zenk.Citation20) Syntheses of avenanthramides A, B, C, and D (N-4-hydroxycinnamoyl-5-hydroxyanthranilic acid) and L (N-[5-(4-hydroxyphenyl)-penta-2, 4-dienoyl]-5-hydroxyanthranilic acid) are described elsewhere.Citation1,21)
4,5-Dihydroxyanthranilic acid
4,5-dihydroxyanthranilic acid was prepared from 4,5-dimethoxyanthranilic acid according to Breuer et al.Citation22) 2-Amino-4,5-dimethoxybenzoic acid (0.99 g, 5.0 mmol) was mixed with hydrobromic acid (25 mL) and the mixture was refluxed for 3 h, and then evaporated in vacuo. After washing with ether, this material was dissolved in a small amount of ice water. The pH of the solution was adjusted to 4 with saturated NaHCO3 solution, precipitating 4,5-dihydroxyanthranilate.
Dark violet solid (0.50 g, 3.0 mmol, yield 60%). ¹H NMR (600 MHz, (CD3)2SO): δH (ppm) 9.42 (brs, 1H, OH), 8.23 (brs, 1H, OH), 8.02 (brs, 3H, NH3), 7.07 (s, 1H, H-6), 6.13 (s, 1H, H-3). 13C NMR (150 MHz, (CD3)2SO): δC (ppm) 169.5 (COOH), 152.4 (C-4), 147.2 (C-2), 135.9 (C-5), 116.7 (C-6), 102.5 (C-1), 101.1 (C-3).
N-Caffeoyl-4,5-dihydroxyanthranilic acid
4,5-Dihydroxyanthranilic acid (43.3 mg, 0.26 mmol) was dissolved in distilled H2O (5 mL). The pH of the solution was adjusted to 8.0 by adding NaHCO3 (12.5 mg, 0.15 mmol). Caffeic acid N-hydroxysuccinimide ester (12.5 mg, 0.049 mmol) was dissolved in 5 mL acetone and was added to the solution of 4,5-dihydroxyanthranilic acid. After standing for 24 h, acetone was removed by evaporation. The pH of the solution was neutralized by adding AcOH. The mixture was applied to an ODS column equilibrated with MeOH H2O AcOH (5:95:1). The column was eluted with 450 mL methanol H2O-AcOH (5:95:1, 60:40:1) and MeOH. The MeOH-H2O-AcOH (60:40:1) fraction was concentrated and subjected to preparative HPLC. HPLC conditions were the same as for isolation of 1 from oat grain extract.
Yellowish black solid (4.0 mg, 0.012 mmol, 24.5%). 1H NMR (600 MHz, CD3OD): δH (ppm) 8.23 (s, 1H, H-3), 7.50 (d, 1H, J = 15.6, H-7′), 7.50 (s, 1H, H-6), 7.07 (d, 1H, J = 2.0 Hz, H-2′), 6.98 (dd, 1H, J = 2.0 and 8.2 Hz, H-6′), 6.79 (d, 1H, J = 8.2 Hz, H-5′), 6.45 (d, 1H, J = 15.6 Hz, H-8′). ¹H NMR (600 MHz, (CD3)2SO): δH (ppm) 13.01 (brs, 1H, NH), 11.28 (brs, 1H, COOH), 10.07 (brs, 1H, OH), 9.52 (brs, 1H, OH), 9.14 (brs, 1H, OH), 9.11 (brs, 1H, OH), 8.19 (s, 1H, H-3), 7.39 (d, 1H, J = 15.8 Hz, H-7′), 7.37 (s, 1H, H-6), 7.07 (s, 1H, H-2′), 6.98 (d, 1H, J = 7.9 Hz, 1H, H-6′), 6.76 (d, 1H, J = 7.9 Hz, 1H, H-5′), 6.42 (d, 1H, J = 15.8 Hz, 1H, H-8′). 13C NMR (150 MHz, (CD3)2SO): δC (ppm) 169.6 (C-7), 163.5 (C-9′), 150.9 (C-4), 147.8 (C-4′), 145.6 (C-3′), 141.1 (C-7′), 140.4 (C-5), 135.5 (C-2), 126.0 (C-1′), 121.0 (C-6′), 118.9 (C-8′), 117.1 (C-6), 115.7 (C-5′), 114.4 (C-2′), 107.3 (C-3), 106.8 (C-1).
N-p-Coumaroy-4,5-dihydroxyanthranilic acid
N-p-Coumaroy-4,5-dihydroxyanthranilic acid was synthesized from 4,5-dihydroxyanthranilic acid (76.5 mg, 0.45 mmol) and p-coumaric acid N-hydroxysuccinimide ester (361.5 mg, 1.53 mmol) in a similar way to the synthesis of N-caffeoyl-4,5-dihydroxyanthranilic acid. Preparative HPLC gave N-coumaroyl-4,5-dihydroxyanthranilic acid. HPLC conditions were the same as for isolation of 2 from oat seed extract.
Pale brown solid (53.5 mg, 0.17 mmol, yield 38%). 1H NMR (600 MHz, CD3OD): δH (ppm) 8.22 (s, 1H, H-3), 7.56 (d, 1H, J = 15.6 Hz, H-7′), 7.51 (s, 1H, H-6), 7.48 (d, 2H, J = 8.6 Hz, H-2′, 6′), 6.82 (d, 2H, J = 8.6 Hz, H-3′, 5′), 6.51 (d, 1H, J = 15.6 Hz, H-8′). ¹H NMR (600 MHz, (CD3)2SO): δH (ppm) 13.01 (brs, 1H, NH), 11.29 (brs, 1H, COOH), 10.08 (brs, 1H, OH), 9.95 (brs, 1H, OH), 9.11 (brs, 1H, OH), 8.22 (s, 1H, H-3), 7.55 (d, 2H, J = 8.6 Hz, H-2′, 6′), 7.48 (d, 1H, J = 15.5 Hz, H-7′), 7.38 (s, 1H, H-6), 6.80 (d, 1H, J = 8.6 Hz, 1H, H-3′, 5′), 6.54 (d, 1H, J = 15.5 Hz, 1H, H-8′). 13C NMR (150 MHz, (CD3)2SO): δC (ppm) 169.6 (C-7), 163.6 (C-9′), 159.3 (C-4′), 150.9 (C-4), 140.8 (C-7′), 140.4 (C-5), 135.6 (C-2), 129.9 (C-2′, 6′), 125.5 (C-1′), 119.0 (C-8′), 117.1 (C-6), 115.7 (C-3′, 5′), 107.3 (C-3), 106.8 (C-1).
N-Feruloyl-4,5-dihydroxyanthranilic acid
N-Feruloyl-4,5-dihydroxyanthranilic acid was synthesized from 4,5-dihydroxyanthranilic acid (37.2 mg, 0.22 mmol) and ferulic acid N-hydroxysuccinimide (205.6 mg, 0.77 mmol) by a procedure similar to the synthesis of N-caffeoyl-4,5-dihydroxyanthranilic acid. The compound was purified by preparative HPLC. HPLC conditions were the same as for the isolation of 3.
Yellow solid (23.4 mg, 0.068 mmol, 31%) ¹H NMR (600 MHz, CD3OD): δH (ppm) 8.24 (s, 1H, H-3), 7.56 (d, 1H, J = 15.5 Hz, H-7′), 7.51 (s, 1H, H-6), 7.24 (d, 1H, J = 1.7 Hz, H-2′), 7.10 (dd, 1H, J = 1.7 Hz and 8.2 Hz, H-6′), 6.83 (d, 1H, J = 8.2 Hz, H-5′), 6.55 (d, 1H, J = 15.5 Hz, H-8′), 3.93 (s, 3H). ¹H NMR (600 MHz, (CD3)2SO): δH (ppm) 12.97 (brs, 1H, NH), 11.27 (brs, 1H, COOH), 10.08 (brs, 1H, OH), 9.54 (brs, 1H, OH), 9.12 (brs, 1H, OH), 8.23 (s, 1H, H-3), 7.47 (d, 1H, J = 15.5 Hz, H-7′), 7.38 (s, 1H, H-6), 7.32 (s, 1H, H-2′), 7.11 (d, 1H, J = 8.0 Hz, 1H, H-6′), 6.78 (d, 1H, J = 8.0 Hz, 1H, H-5′), 6.63 (d, 1H, J = 15.5 Hz, 1H, H-8′), 3.83 (s, 3H, OCH3). 13C NMR (150 MHz, (CD3)2SO): δC (ppm) 169.5 (C-7), 163.7 (C-9′), 150.9 (C-4), 148.8 (C-4′), 147.9 (C-3′), 141.2 (C-7′), 140.4 (C-5), 135.6 (C-2), 126.1 (C-1′), 122.6 (C-6′), 119.3 (C-8′), 117.1 (C-6), 115.6 (C-5′), 111.2 (C-2′), 107.4 (C-3), 106.7 (C-1), 55.7 (OCH3).
DPPH assay
Anti-oxidant activity of avenanthramides was evaluated by the DPPH assay according to the method described by Oki et al. with modifications.Citation23) Trolox (Wako Pure Chemical Industries) solutions (50, 100, 150, and 200 μM) in 80% EtOH were added to a mixture of 300 μL of 400 μM 1,1-diphenyl-2-picrylhydrazyl (DPPH; Wako Pure Chemical Industries) in ethanol, 300 μL of 200 mM MES buffer (pH6.0), and 200 μL of 20% EtOH, and vortexed. After 30 min incubation, the absorbance at 520 nm was measured with a Hitachi U-2001 spectrophotometer. In the same way, avenanthramide solutions in 80% EtOH at different concentrations were added to a mixture containing DPPH, and absorbance at 520 nm was measured 30 min after adding avenanthramide. From the slope of the graph generated by plotting absorbance values as a function of concentration, the Trolox equivalent of each avenanthramide was calculated. In this study, Trolox equivalent means Trolox amount (moles) that shows the same DPPH radical-scavenging activity with 1 mol of each avenanthramide.
Determination of avenanthramide contents in groats by MRM of LC-MS/MS analysis
Ten oat groats were homogenized with a mortar and pestle to a powder. The powder was extracted with 20 volumes of MeOH for 24 h. After filtration (Millex-LH, 0.45 μm; Millipore, Billerica, MA, USA), the extract was subjected to MRM of LC-MS/MS (Waters UPLC coupled with Quattro Micro). The amounts of avenanthramides in groats of oat cultivar Shokan 1 were calculated on the basis of calibration curve generated by analysis of external standards. UPLC conditions were as follows: column: Acquity UPLC BEH C18 1.7 μm (2.1 × 100 mm; Waters); solvents: A: 0.1% HCOOH in H2O and B: 0.1% HCOOH in MeCN; gradient: 15–55% B/ (A + B) within 10 min. MRM conditions were optimized with synthesized compounds (Table S1).
Results and discussion
Identification of avenanthramides
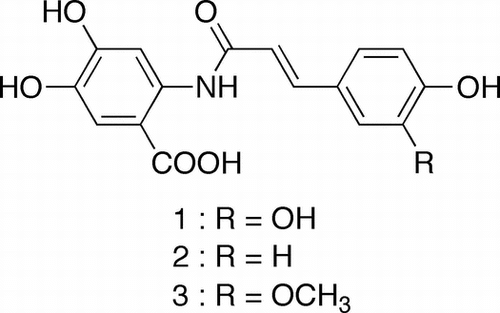
To find new avenanthramides, we analyzed a MeOH extract of oat seeds by HPLC (Fig. (A)). Detected peaks 1 and 3 were collected, concentrated, and subjected to LC-PDA-MS analysis. For a small-scale isolation of peak 2, a different gradient program with a larger column was applied (Fig. (B)). The respective positive ESI mass spectra of peaks 1, 2, and 3 showed ions at m/z 332, 316, and 346 as [M + H]+ ions and ions at m/z 163, 147, and 177 as fragment ions (Fig. ). In negative ESI mass spectra, peaks 1, 2, and 3 showed ions at m/z 330, 314, and 344 as [M − H]−, suggesting that their molecular masses are 331, 315, and 345, respectively. Because we recorded ESI mass spectra in MS scan mode, fragment ions are considered to be generated in ionization process. Ions at m/z 163, 147, and 177 are characteristic fragment ions of compounds bearing caffeoyl, p-coumaroyl, and feruloyl moieties. The subtraction of masses of the fragment ions from molecular masses was commonly 168. This suggested that the remaining part of the molecules is dihydroxyanthranilic acid moiety, with a mass of 168, because, in avenanthramides, fragment ions are generated by the cleavage of the amide bond between anthranilate and hydroxycinnamoyl moieties.
Fig. 2. HPLC analysis of avenanthramides in oat groats (A) and chromatogram for the isolation of 2 (B).
Notes: MeOH extract of oat groats was subjected to analytical HPLC. HPLC conditions were described in the part of general experimental procedures in materials and methods section. Sixty percent MeOH fraction obtained by ODS column chromatography of oat groat extract was subjected to preparative HPLC. HPLC conditions were described in the part of isolation of avenanthramides in materials and methods section.
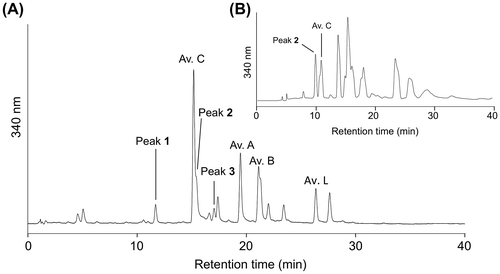
Fig. 3. Mass and absorption spectra of peak 1, 2, and 3.
Notes: Positive (A, D, and G) and negative (B, E, and H) ESI mass spectra, and absorption spectra (C, F, and I) of peak 1, 2, and 3.
Then, we performed large-scale extraction of oat seeds. The obtained MeOH extract was fractionated by ODS column chromatography, and the 60% MeOH fraction was subjected to preparative HPLC. Finally, we purified compounds 1, 2, and 3, which corresponded to peaks 1, 2, and 3.
The molecular formula of 1 was revealed to be C16H13O7N on the basis of HR-ESI-Orbitrap MS. This is compatible with the idea that 1 is avenanthramide composed of dihydroxyanthranilic acid and caffeic acid. 1H NMR spectrum exhibited signals corresponding to a trans double bond (δH 7.57 and 6.55) and to a 1,2,4-trisubstituted benzene ring (δH 7.07, 7.04, and 6.79). These signals were assigned to a caffeoyl moiety. The remaining signals were two singlets (δH 8.23, and 7.50), suggesting the presence of 4,5-dihydroxyanthranilic acid.
This compound was synthesized from 4,5-dihydroxyanthranilic acid and caffeic acid. First, 4,5-dihydroxyanthranilic acid was prepared by demethylation of 4,5-dimethoxyanthranilic acid by refluxing with HBr. Then, we tried direct condensation with 4,5-dihydroxyanthranilic acid and caffeic acid by dicyclohexylcarbodiimide (DCC) in various solvents. However, the formation of target compound was not detected by LC-MS analysis of the reaction mixture. Thus, we employed the condensation reaction with activated caffeic acid with 4,5-dihydroxyanthranilic acid. Caffeic acid was converted to N-hydroxysuccinimide ester by the condensation reaction with N-hydroxysuccinimide using DCC. Then, N-hydroxysuccinimide ester of caffeic acid was reacted with 4,5-dihydroxyanthranilic acid under alkaline conditions. The 1H NMR spectrum and chromatographic behavior of 1 and the synthesized compound were identical, indicating that 1 is N-caffeoyl-4,5-dihydroxyanthranilic acid.
The molecular formula of 2 was revealed to be C16H13O6N on the basis of HR-ESI-Orbitrap MS. The molecular formula agreed with that of N-p-coumarroyldihydroxyanthranilic acid. 1H NMR showed signals corresponding to a trans double bond (δH 7.56 and 6.51), and a 1,4-disubstituted benzene ring (δH 7.48 and 6.82). These signals were assigned to the p-coumaroyl moiety. The spectrum also showed two singlets (δH 8.22 and 7.51) corresponding to 4,5-dihydroxyanthranilic acid. The identity was confirmed by the synthesis of the compound from 4,5-dihydroxyanthranilic acid and p-coumaric acid in a similar way to the synthesis of N-caffeoyl-4,5-dihydroxy anthranilic acid. The 1H NMR and mass spectra and chromatographic behavior of 2 were identical to those of synthesized N-p-coumaroyl-4,5-dihydroxyanthranilic acid. Blaakmeer et al. synthesized this compound and reported its oviposition deterrent activity for Pieris brassicae.Citation24) The 1H NMR data was identical to that reported by Blaakmeer et al.Citation24)
The molecular formula of 3 was determined to be C17H15O6N on the basis of HR-ESI-Orbitrap MS. The molecular formula suggested that 3 is N-feruloyldihydroxyanthranilic acid. Supporting this, 1H NMR showed signals corresponding to a trans double bond (δH 7.56 and 6.51), a 1,3,4-substituted benzene ring (δH 7.24, 7.10 and 6.83), and a methoxy group (δH 3.83). These signals were assigned to a feruloyl moiety. The spectrum also showed two singlets (δH 8.22 and 7.51) corresponding to 4,5-dihydroxyanthranilic acid part. N-Feruloyl-4,5-dihydroxyanthranilic acid was synthesized from 4,5-dihydroxyanthranilic acid and ferulic acid in a similar way to the synthesis of 1 and 2. Their identical 1H NMR and ESI-mass spectra, and the same chromatographic behavior on reversed phase HPLC indicated that 3 is N-feruloyl-4,5-dihydroxyanthranilic acid.
Major avenanthramides in oat seeds are those composed of 5-hydroxyanthranilic acid. Avenanthramides with anthranilic, 4-hydroxyanthranilic, and 4-methoxyl-5-hydroxyanthranilic acids have also been identified. In addition, Wise described the detection of 2 as avenanthramide 5p by ESI-MS.Citation25) The same author also showed a chromatogram of avenanthramides in oat grain and indicated the peak of 3, but no spectroscopic data were reported.Citation26) Collins also described the presence of avenanthramides with 4,5-dihydroxyanthranilic acid, but without showing any spectroscopic data.Citation27)
Because of the presence of avenanthramide with 4-methoxyl-5-hydroxyanthranilic acid,Citation28) the occurrence of avenanthramides with 4,5-dihydroxyanthranilic acid is not surprising. Avenanthramides were shown to be synthesized by the condensation of anthranilic acids and hydroxycinnamoyl-CoAs.Citation8,9) Enzyme HHT accepted anthranilic acid and 4- and 5-hydroxyanthranilic acids as substrates, but not 3-hydroxyanthranilic acid. Thus, the enzyme may accept 4,5-dihydroxyanthranilic acid. On the other hand, in carnation, N-benzoyl-4-hydroxyanthranilic acid was indicated to be formed by the hydroxylation of N-benzoylanthranilic acid on the basis of the detection of hydroxylase activity.Citation29) Similar hydroxylation of anthranilate moiety may form 4- and 5-hydroxyanthranilic and 4,5-dihydroxyanthranilic acids in oats. In addition, it is plausible that the biosynthetic routes of avenanthramides may consist of a metabolic grid. To resolve this issue, extensive feeding studies with labeled avenanthramides are needed.
Determination of avenanthramide contents in oat groats
Conditions for MRM of avenanthramides were optimized with synthetic compounds (Table S1). Avenanthramides were detected by MRM of LC-MS/MS analysis, as shown in Fig. . The amounts of avenanthramides in groats of oat cultivar Shokan 1 were calculated on the basis of calibration curve generated by analysis of external standards. The results are summarized in Table . The amounts of avenanthramides with 4,5-dihydroxyanthranilic acid were 26.9–16.5% of corresponding avenanthramides with 5-hydroxyanthranilic acid. In this experiment we extracted 10 groats together, and 9 samples were analyzed. The results of analysis still showed a large variation in the avenanthramide contents, indicating that the amounts largely differ among groats.
Fig. 4. Analysis of avenanthramides in oat groats by MRM of LC-MS/MS analysis.
Notes: Compound 1 (A), avenanthramide C (B), compounds 2 (C) and 3 (D), and avenanthramides A (E), B (F), L (G), and D (H) were extracted from oat groats and analyzed by LC-MS/MS.
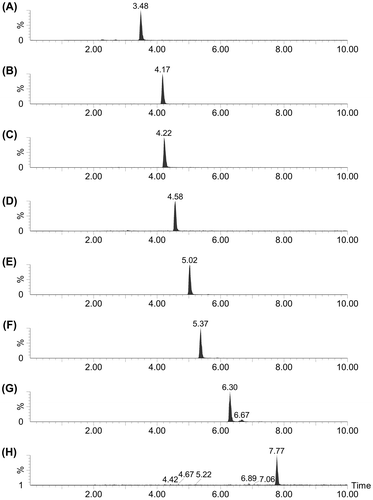
Table 1. Amounts of avenanthramides in oat groats. Ten groats were extracted and analyzed. The values are the average of nine replicates with SD.
DPPH radical-scavenging activity of avenanthramides
We evaluated anti-oxidant activity by the DPPH method according to Oki et al.Citation23) Trolox equivalent of the DPPH radical-scavenging activity of avenanthramides is summarized in Table . Avenanthramide composed of 4,5-dihydroxyanthranilic acid scavenged DPPH radical more effective than the corresponding avenanthramides composed of 5-hydroxyanthranilic acid. In the comparison of the activity of avenanthramides with 5-hydroxyanthranilic acid, the radical-scavenging activity of avenanthramides was dependent on the hydroxycinnamoyl moiety; the order of activity was caffeoyl > feruloyl > p-coumaroyl. This tendency was also observed for avenanthramides with 4,5-dihydroxyanthranilic acid, although the difference in the activities of avenanthramides with p-coumaroyl and feruloyl moiety was small.
Table 2. Trolox equivalent of avenanthramides determined by DPPH assay. Values indicate Trolox amounts (moles) that show the same DPPH radical-scavenging activity with 1 mol of each avenanthramide. Abbreviated names and compound numbers were shown in parentheses. Values are averages of 3 replicates with SD.
Radical-scavenging activity of avenanthramides was evaluated by DPPH assay by Peterson et al., Fagerlund et al., and Bratt et al.Citation18,19,30) The order of activity in our assay for avenanthramide A, B, and C was the same as their results. As demonstrated for hydroxycinnamic acids, ortho substitution with an electron-donating methoxy group in the ferulic acid moiety is considered to increase its activity by stabilizing the phenoxy radical.Citation31−33) On the other hand, the presence of a second hydroxyl group in the ortho position of the 4-hydroxy group in p-coumaric acid is known to increase radical-scavenging activity by additional resonance stabilization and o-quinone formation.Citation33−35) The small Trolox equivalent of avenanthramide D indicates that the contribution of the hydroxyl group of p-coumaroyl moiety to DPPH radical scavenging is marginal.
The 5-hydroxyl group on anthranilic acid was shown to also contribute to the radical-scavenging activity of avenanthramides, because the activity of avenanthramide A is substantially higher than that of avenanthramide D. The introduction of another hydroxyl group at 4-position of anthranilate further increased activity. This occurred for the same reason as the increase in radical-scavenging activity in the introduction of the ortho hydroxyl group in the caffeoyl moiety. Moreover, the relatively large Trolox equivalent value of 2 indicates that the activity of 2 is mainly attributable to 4,5-dihydroxyanthranilic acid in the molecule. Similarly, the enhancement of DPPH radical-scavenging activity by introducing a methoxy group at 4-position on the anthranilic moiety in avenanthramides was reported by Fagerlund et al.Citation19) Considering the high radical-scavenging activity of 1–3 and their relatively larger amounts, it is likely that they significantly contribute to the anti-oxidant activity of oat seed extract.
Avenanthramides have been shown to have bioactivities mainly in relation to the anti-inflammatory process.Citation14,36,37) It would be of interest to assay the activity of newly identified avenanthramides in various experimental systems.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.946390.
Supplemental Materials
Download MS Word (36.9 KB)Acknowledgments
This study was supported by the Foundation for Dietary Scientific Research and by the Adaptable and Seamless Technology Transfer Program (A-step) through Target-driven R&D, Japan Science and Technology Agency.
References
- Collins FW. Oat phenolics: avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agric. Food Chem. 1989;37:60–66.10.1021/jf00085a015
- Collins FW. Oat phenolics: structure, occurrence, and function. In: Webster FH, editor. Oats: chemistry and technology. St. Paul, MN: American Association of Cereal Chemists; 1986. p. 227–295.
- Mayama S, Tani T, Matsuura Y, Ueno T, Fukami H. The production of phytoalexins by oat in response to crown rust, Puccinia coronata f. sp. avenae. Physiol. Plant Pathol. 1981;19:217–226.10.1016/S0048-4059(81)80024-0
- Mayama S, Matsuura Y, Iida H, Tani T. The role of avenalumin in the resistance of oat to crown rust, Puccinia coronata f. sp. avenae. Physiol. Plant Pathol. 1982;20:189–199.10.1016/0048-4059(82)90084-4
- Miyagawa H, Ishihara A, Nishimoto T, Ueno T, Mayama S. Induction of avenanthramides in oat leaves inoculated with crown rust fungus, Puccinia coronata f. sp. avenae. Biosci. Biotechnol. Biochem. 1995;59:2305–2306.10.1271/bbb.59.2305
- Bordin APA, Mayama S, Tani T. Potential elicitors for avenalumin accumulation in oat leaves. Jpn. Ann. Phytopath. Soc. Japan 1991;57: 688–695.10.3186/jjphytopath.57.688
- Okazaki Y, Isobe T, Iwata Y, Matsukawa T, Matsuda F, Miyagawa H, Ishihara A, Nishioka T, Iwamura H. Metabolism of avenanthramide phytoalexins in oats. Plant J. 2004;39:560–572.10.1111/tpj.2004.39.issue-4
- Ishihara A, Matsukawa T, Miyagawa H, Ueno T, Mayama S, Iwamura H. Induction of hydroxycinnamoyl-CoA: hydroxyanthranilate N-hydroxycinnamoyltransferase (HHT) activity in oat leaves by victorin C. Z. Naturforsch. 1997;52c:756–760.
- Ishihara A, Miyagawa H, Matsukawa T, Ueno T, Mayama S, Iwamura H. Induction of hydroxyanthranilate hydroxycinnamoyl transferase activity by oligo-N-acetylchitooligosaccharides in oats. Phytochemistry. 1998;47:969–974.10.1016/S0031-9422(98)80055-1
- Matsukawa T, Isobe T, Ishihara A, Iwamura H. Occurrence of avenanthramides and hydroxycinnamoyl-CoA: hydroxyanthranilate N-hydroxycinnamoyltransferase activity in oat seeds. Z. Naturforsch. 2000;55c:30–36.
- Yang Q, Xuan Trinh H, Imai S, Ishihara A, Zhang L, Nakayashiki H, Tosa Y, Mayama S. Analysis of the involvement of hydroxyanthranilate hydroxycinnamoyltransferase and caffeoyl-CoA 3-O-methyltransferase in phytoalexin biosynthesis in oat. Mol. Plant-Microbe Interact. 2004;17:81–89.10.1094/MPMI.2004.17.1.81
- Schrickel DJ. Oat production, value and use. In: Webster FH, editor. Oats: chemistry and technology. St. Paul, MN: American Association of Cereal Chemists; 1986. p. 1–11.
- Kurtz ES, Wallo W. Colloidal oatmeal: history, chemistry and clinical properties. J. Drugs Dermatol. 2007;6:167–170.
- Liu L, Zubik L, Collins FW, Marko M, Meydani M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis. 2004;175:39–49.10.1016/j.atherosclerosis.2004.01.044
- Sur R, Nigam A, Grote D, Liebel F, Southall MD. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Dermatol. Res. 2008;300:569–574.10.1007/s00403-008-0858-x
- Azuma H, Banno K, Yoshimura T. Pharmacological properties of N-(3′,4′-dimethoxycinnamoyl)anthranilic acid (N-5′), a new anti-atopic agent. Br. J. Pharmacol. 1976;58:483–488.10.1111/bph.1976.58.issue-4
- Isaji M, Miyata H, Ajisawa Y. Tranilast: a new application in the cardiovascular field as an antiproliferative drug. Cardiovasc. Drug Rev. 1998;16:288–299.10.1111/cdr.1998.16.issue-3
- Peterson DM, Hahn M, Emmons CL. Oat avenanthramides exhibit antioxidant activities in vitro. Food Chem. 2002;79:473–478.10.1016/S0308-8146(02)00219-4
- Fagerlund A, Sunnerheim K, Dimberg LH. Radical-scavenging and antioxidant activity of avenanthramides. Food Chem. 2009;113:550–556.10.1016/j.foodchem.2008.07.101
- Stöckigt J, Zenk MH. Chemical syntheses and properties of hydroxycinnamoyl-coenzyme A derivatives. Z. Naturforsch. 1975;30c:352–358.
- Miyagawa H, Ishihara A, Nishimoto T, Ueno T, Mayama S. Induction of avenanthramides in oat leaves inoculated with crown rust fungus, Puccinia coronata f. sp. avenae. Biotechnol. Biochem. 1995;59:2305–2306.10.1271/bbb.59.2305
- Breuer H, Drossard JM, Ermann PH, Straub H, Treuner UD. 2-Oxo-1-[[(substituted sulfonyl)amino]carbonyl]azetidines, procedure for their preparation, and their use as antibacterials. Eur. Pat. Appl EP 251143 A1 19880107; 1988.
- Oki T, Kobayashi M, Nakamura T, Okuyama A, Masuda M, Shiratsuchi H, Suda I. Changes in radical-scavenging activity and components of mulberry fruit during maturation. J. Food Sci. 2006;71:18–22.
- Blaakmeer A, van der Wal D, Stork A, van Beek TA, de Groot A, van Loon JJA. Structure-activity relationship of isolated avenanthramide alkaloids and synthesized related compounds as oviposition deterrents for Pieris brassicae. J. Nat. Prod. 1994;57:1145–1151.10.1021/np50110a003
- Wise ML. Effect of chemical systemic acquired resistance elicitors on avenanthramide biosynthesis in oat (Avena sativa). J. Agric. Food Chem. 2011;59:7028–7038.10.1021/jf2008869
- Wise ML. Avenanthramides: chemistry and biosynthesis. In: Chu Y, editor. Oats: nutrition and technology. Oxford: John Wiley & Sons; 2014. p. 195–226.
- Collins FW. Oat biochemistry and biological functionality. In: Webster FH, editor. Oats: chemistry and technology. 2nd ed. Online book. St. Paul, MN AACC International Books; 2012. p. 157–217.
- Dimberg LH, Theander O, Lingnert H. Avenanthramides – a group of phenolic antioxidants in oats. Cereal Chem. 1993;70:637–641.
- Reinhard K, Matern U. Different types of microsomal enzymes catalyze ortho- or para-hydroxylation in the biosynthesis of carnation phytoalexins. FEBS Lett. 1991;294:67–72.10.1016/0014-5793(91)81345-9
- Bratt K, Sunnerheim K, Bryngelsson S, Fagerlund A, Engman L, Andersson RE, Dimberg LH. Avenanthramides in oats (Avena sativa L.) and structure−antioxidant activity relationships. J. Agric. Food Chem. 2003;51:594–600.10.1021/jf020544f
- Cuvelier ME, Richard H, Berset C. Comparison of the antioxidative activity of some acid-phenols: structure–activity relationship. Biosci. Biotechnol. Biochem. 1992;56:324–325.10.1271/bbb.56.324
- Terao J, Karasawa H, Arai H, Nagao A, Suzuki T, Takama K. Peroxyl radical scavenging activity of caffeic acid and its related phenolic compounds in solution. Biosci. Biotechnol. Biochem. 1993;57:1204–1205.10.1271/bbb.57.1204
- Chen JH, Ho C. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997;45:2374–2378.10.1021/jf970055t
- Graf E. Antioxidant potential of ferulic acid. Free Radical Biol. Med. 1992;13:435–448.10.1016/0891-5849(92)90184-I
- Brand-Willams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995;28:25–30.
- Guo W, Wise ML, Collins FW, Meydani M. Avenanthramides, polyphenols from oats, inhibit IL-1β -induced NF-κB activation in endothelial cells. Free Radical Biol. Med. 2008;44:415–429.10.1016/j.freeradbiomed.2007.10.036
- Nie L, Wise ML, Peterson DM, Meydani M. Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis. 2006;186:260–266.10.1016/j.atherosclerosis.2005.07.027