Abstract
A single-step synthesis of 3-O-ethyl-l-ascorbic acid was performed without the induction of protecting groups. Sodium l-ascorbate reacted with ethyl bromide in DMSO to give 3-O-ethylascorbic acid in a yield of 51.0%. 3-O-Ethylascorbic acid enhanced dibutyryl cyclic AMP-induced neurite outgrowth in PC12 cells.
2-O-α-d-Glucopyranosyl-l-ascorbic acid (AA-2G), a stable ascorbic acid derivative developed by Yamamoto et al.,Citation1–4) has been approved by the Japanese Government as a quasi-drug principal ingredient for skin care and as a food additive, and it is now widely used as a medical additive in commercial cosmetics. AA-2G exhibits vitamin C activity in vitro and in vivo after enzymatic hydrolysis to ascorbic acid by α-glucosidase.Citation5–7) We have recently chemically synthesized the monoacylated derivative of AA-2G, 6-O-dodecanoyl-2-O-α-d-glucopyranosyl-l-ascorbic acid (6-sDode-AA-2G), in order to improve the bioavailability of AA-2G.Citation8,9) 6-sDode-AA-2G had outstanding permeability in a human living skin equivalent model. The ascorbic acid derivative was susceptible to enzymatic hydrolysis by tissue esterase and α-glucosidase to produce ascorbic acid.
More recently, 3-O-ethyl-l-ascorbic acid, an ascorbic acid derivative with a simple structure and efficient transdermal activity, has been developed as an additive in commercial cosmetics. The derivative is synthesized by a three-step procedure as shown in Scheme (A).Citation10) A series of 3-O-alkylascorbic acids has been synthesized in the three-step reaction to act as radical scavengers for active oxygen species and free radicals.Citation10–12) These derivatives inhibited lipid peroxidation, and some of them also reduced coronary reperfusion-induced arrhythmias in anesthetized rats. In addition, 3-O-ethylascorbic acid has been reported to effectively inhibit the induction of nephroblastomas, an uncommon tumor mostly found in childhood in humans,Citation13) and to reduce rat mammary tumor induction.Citation14) In this study, we succeeded in a single-step synthesis of 3-O-ethylascorbic acid without the induction of protecting groups, and we examined its ability as an enhancer of neurite outgrowth-promoting activity of dibutyryl cyclic AMP (Bt2cAMP) in PC12 cells.
Some reports have appeared in the literature recently regarding the direct 3-O-alkylation of l-ascorbic acid without protecting groups. Ascorbic acid directly reacts with alkyl bromides or with alkyl mesylates using sodium hydrogen carbonate as a base in DMSO.Citation15,16) Tahir and Hindsgaul have developed a novel methodology for the preparation of 3-O-alkyl derivatives of ascorbic acid under Mitsunobu conditions.Citation17) We considered a simple reaction system consisting of sodium l-ascorbate as a starting material and an alkylation reagent in DMSO for the synthesis of 3-O-ethylascorbic acid (Scheme (B)). This one-step procedure further simplified the direct 3-O-alkylation methodCitation15) of Beifuss and co-workers. By replacing ascorbic acid with sodium ascorbate as a starting material, there was no need to add sodium hydrogen carbonate as a base to the reaction system, thereby eliminating the neutralization step in the work-up process.
Sodium ascorbate (39.6 mg, 0.2 mmol) and 1.0 mL of solvent were put into a 2.0-mL screw-capped microtube, and the tube was then placed in an M·BR-022 thermo-regulated shaker (Taitec, Saitama, Japan) and the mixture was stirred at 1300 r/min for 10 min at 40–60 °C. After adding an ethylation agent (0.8–1.6 equiv.), the mixture was further stirred at 1300 r/min at 40–60 °C. Aliquots of 50 μL were withdrawn at the indicated times and were diluted 400-fold with 15% MeOH-H2O containing 0.5% formic acid. The methanolic solution was subjected to HPLC analysis. 3-O-Ethylascorbic acid was separated by isocratic elution in an Inertsil ODS-3 column (ϕ 4.6 × 100 mm, 3 μm; GL Sciences, Tokyo, Japan) kept at 40 °C with 15% MeOH-H2O containing 0.5% formic acid at a flow rate of 0.7 mL/min. The absorbance at 245 nm was monitored.
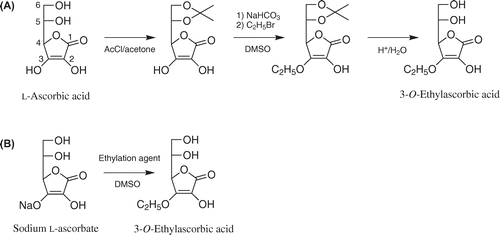
Effects of the ethylation agent and reaction temperature on 3-O-ethylascorbic acid formation are shown in Fig. (A). Generally, it is not easy to mix carbohydrates and alkyl donors in a reaction medium because of their different solubilities. As a result of screening for solvents that can dissolve sodium ascorbate and an ethylation agent, DMSO was chosen as a solvent to obtain a good yield of 3-O-ethylascorbic acid (data not shown). The reaction of sodium ascorbate and each ethylation agent (1.0 equiv.) was carried out in DMSO for 12 h at 40, 50, and 60 °C. At all the reaction temperatures, the reaction with ethyl bromide gave high yields. The yield was best when sodium ascorbate and ethyl bromide reacted at 50 °C. In order to improve the yield, the reaction of sodium ascorbate and ethyl bromide (0.8, 1.0, 1.2, 1.4, and 1.6 equiv.) was carried out in DMSO at 50 °C for 24 h (Fig. (B)). With 0.8 and 1.0 equivalents of ethyl bromide, the reaction yield gradually increased and reached a plateau (54% and 60%, respectively) at 6 h and then remained fairly constant until the experiment ended in 24 h. With 1.2 equivalents of ethyl bromide, the reaction yield reached a maximum (61%) at 3 h and only slightly decreased by the subsequent reaction. With 1.4 and 1.6 equivalents of ethyl bromide, the reaction yield reached a maximum (62%) at 3 h and rapidly decreased by the subsequent reaction. The maximum reaction yield was slightly better than that with 1.2 equivalents of ethyl bromide, but the periods for good yields of 3-O-ethylascorbic acid were far shorter than that with 1.2 equivalents of ethyl bromide. These results indicated that the optimal reaction conditions for simple synthesis of 3-O-ethylascorbic acid were a molar ratio of sodium ascorbate to ethyl bromide of 1:1.2, a reaction temperature of 50 °C, a reaction time of 3 h, and use of DMSO as the reaction solvent.
Fig. 1. Effect of ethylation agent and reaction temperature (A) and effect of ethyl bromide content and reaction time (B) on 3-O-ethylascorbic acid formation.
Notes: (A) The reaction mixture contained sodium ascorbate (39.6 mg, 0.2 mmol) and each ethylation agent (1.0 equiv.) in 1.0 mL of DMSO. The reaction was carried out at 40, 50, and 60 °C for 12 h. The reaction mixture was analyzed by HPLC. Each value is the mean ± SD of three independent experiments. (B) The reaction mixture contained sodium ascorbate (39.6 mg, 0.2 mmol) and ethyl bromide in 1.0 mL of DMSO: ethyl bromide contents of 0.8 equiv. (
The optimal reaction conditions were applied to scale-up the 3-O-ethylation of ascorbic acid. Sodium ascorbate (1.98 g, 10.0 mmol) was dissolved in DMSO (50 mL) and was stirred for 10 min at 50 °C. After adding ethyl bromide (896 μL, 12.0 mmol), the mixture was further stirred for 3.5 h at 50 °C. The reaction mixture was diluted twofold with water and was subjected to column chromatography to remove DMSO and unreacted starting materials. The diluted reaction mixture was chromatographed in a DIAION HP20 column (ϕ 3.0 × 33 cm; Mitsubishi Chemical Co., Tokyo) eluted with a stepwise gradient of MeOH-H2O solvent system (0, 10, 20, and 30%, v/v). The 3-O-ethylascorbic acid-containing fraction (30% MeOH eluate) was concentrated to dryness. The residue was recrystallized from EtOAc to obtain 1.04 g (51.0% yield) of 3-O-ethylascorbic acid. The results of analysis of the purified compound by 1HNMR, HRMS, and optical rotation are shown as follows. 1HNMR (600 MHz, CD3OD) δΗ: 1.41 (3H, t, J = 7.2 Hz), 3.69 (2H, d, J = 6.6 Hz), 3.88 (1H, dt, J = 1.8, 6.6 Hz), 4.59 (2H, q, J = 7.2 Hz), 4.81 (1H, d, J = 1.8 Hz). ESI-HRMS m/z [M−H]−: calcd for C8H11O6: 203.0561, found: 203.0562. [α]25D + 48.1° (c 1.0, MeOH). The product was determined to be 3-O-ethylascorbic acid by its 1HNMR spectrum and mass spectrum, and by comparing the 1HNMR spectrum with that in a previous report.Citation10) The results indicate that the described method here enables preparation of 3-O-ethylascorbic acid very easily and quickly compared with the conventional method,Citation10) and suggest that the simple single-step synthesis of 3-O-ethylascorbic acid can be applied to the procedure of a large scale.
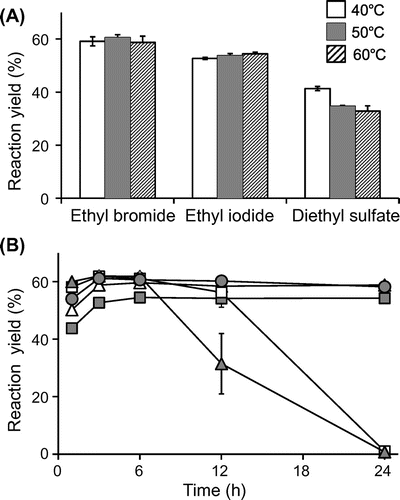
The ability of 3-O-ethylascorbic acid as an enhancer of neurite outgrowth-promoting activity of Bt2cAMP was investigated in PC12 cells. Evaluation of neurite outgrowth-promoting activity was carried out basically according to our previous procedure.Citation18) Briefly, PC12 cells (RIKEN Cell Bank, Tsukuba, Japan) were grown in RPMI 1640 medium supplemented with 5% heat-inactivated fetal bovine serum, 10% heat-inactivated horse serum, 100 units/mL penicillin G sodium salt, and 100 μg/mL streptomycin sulfate, and then incubated in a humidified atmosphere containing 95% air and 5% CO2 at 37 °C. The suspended PC12 cells in the medium were seeded in 96-well plates coated with porcine tendon collagen at a density of 4.0 × 103 cells/90 μL/well. After 24 h, 10 μL of a medium containing each sample and Bt2cAMP was added to the cultured cells. Neurite formation was examined 24 h after the treatment. The number of cells bearing neurites longer than one cell body diameter after treatment was divided by the total number of cells, which amounted to 300–400 cells per well. 3-O-Ethylascorbic acid showed neurite outgrowth-promoting activity at 3–30 μM (Fig. ). Ascorbic acid had neurite outgrowth-promoting activity at 0.75–7.5 μM. The percentage of cells with neurites (25.9%) at 10 μM of 3-O-ethylascorbic acid was higher than the maximum level (23.9% at 2.5 μM) of neurite outgrowth induced by ascorbic acid, although a higher concentration of 3-O-ethylascorbic acid than that of ascorbic acid was required for neurite outgrowth promotion. The stability of 3-O-ethylascorbic acid (1.0 mM) in a medium at 37 °C was evaluated on the basis of the remaining ratio determined by HPLC. It was found that 97.1 ± 1.9% (n = 4) of 3-O-ethylascorbic acid remained intact after 24 h (unpublished data). These results suggested that 3-O-ethylascorbic acid per se, not ascorbic acid released from 3-O-ethylascorbic acid, might exhibit neurite outgrowth-promoting activity. Therefore, a new activity of 3-O-ethylascorbic acid for enhancement of Bt2cAMP-induced neurite outgrowth in PC12 cells was revealed.
Fig. 2. Effects of 3-O-ethylascorbic acid on Bt2cAMP-induced neurite outgrowth in PC12 cells.
Notes: PC12 cells were plated at 4.0 × 103 cells/well and cultured with indicated concentrations of 3-O-ethylascorbic acid (
In conclusion, we succeeded in synthesizing 3-O-ethylascorbic acid from sodium ascorbate and ethyl bromide in DMSO by a single-step procedure and found that 3-O-ethylascorbic acid enhanced Bt2cAMP-induced neurite outgrowth in PC12 cells.
Acknowledgments
The authors are grateful to the SC-NMR Laboratory of Okayama University and the MS Laboratory of the Faculty of Agriculture at Okayama University. We thank Prof. Yoshiaki Amakura at College of Pharmaceutical Sciences, Matsuyama University for the measurement of optical rotation.
References
- Yamamoto I, Muto N, Nagata E, Nakamura T, Suzuki Y. Formation of a stable l-ascorbic acid α-glucoside by mammalian α-glucosidase-catalyzed transglucosylation. Biochim. Biophys. Acta. 1990;1035:44–50.10.1016/0304-4165(90)90171-R
- Yamamoto I, Muto N, Murakami K, Suga S, Yamaguchi H. l-Ascorbic acid α-glucoside formed by regioselective transglucosylation with rat intestinal and rice seed α-glucosidases: its improved stability and structure determination. Chem. Pharm. Bull. 1990;38:3020–3023.10.1248/cpb.38.3020
- Aga H, Yoneyama M, Sakai S, Yamamoto I. Synthesis of 2-O-α-d-glucopyranosyl l-ascorbic acid by cyclomaltodextrin glucanotransferase from Bacillus stearothermophilus. Agric. Biol. Chem. 1991;55:1751–1756.10.1271/bbb1961.55.1751
- Mandai T, Yoneyama M, Sakai S, Muto N, Yamamoto I. The crystal structure and physicochemical properties of l-ascorbic acid 2-glucoside. Carbohydr. Res. 1992;232:197–205.10.1016/0008-6215(92)80054-5
- Yamamoto I, Suga S, Mitoh Y, Tanaka M, Muto N. Antiscorbutic activity of l-ascorbic acid 2-glucoside and its availability as a vitamin C supplement in normal rats and guinea pigs. J. Pharmacobio-Dyn. 1990;13:688–695.10.1248/bpb1978.13.688
- Yamamoto I, Muto N, Murakami K, Akiyama J. Collagen synthesis in human skin fibroblasts is stimulated by a stable form of ascorbate, 2-O-α-d-glucopyranosyl-l-ascorbic acid. J. Nutr. 1992;122:871–877.
- Kumano Y, Sakamoto T, Egawa M, Tanaka M, Yamamoto I. Enhancing effect of 2-O-α-d-glucopyranosyl-l-ascorbic acid, a stable ascorbic acid derivative, on collagen synthesis. Biol. Pharm. Bull. 1998;21:662–666.10.1248/bpb.21.662
- Yamamoto I, Tai A, Fujinami Y, Sasaki K, Okazaki S. Synthesis and characterization of a series of novel monoacylated ascorbic acid derivatives, 6-O-acyl-2-O-α-d-glucopyranosyl-l-ascorbic acids, as skin antioxidants. J. Med. Chem. 2002;45:462–468.10.1021/jm010379f
- Tai A, Goto S, Ishiguro Y, Suzuki K, Nitoda T, Yamamoto I. Permeation and metabolism of a series of novel lipophilic ascorbic acid derivatives, 6-O-acyl-2-O-α-d-glucopyranosyl-l-ascorbic acids with a branched-acyl chain, in a human living skin equivalent model. Bioorg. Med. Chem. Lett. 2004;14:623–627.10.1016/j.bmcl.2003.11.059
- Nihro Y, Sogawa S, Izumi A, Sasamori A, Sudo T, Miki T, Matsumoto H, Satoh T. 3-O-Alkylascorbic acids as free radical quenchers. 3. Protective effect on coronary occlusion-reperfusion induced arrhythmias in anesthetized rats. J. Med. Chem. 1992;35:1618–1623.10.1021/jm00087a017
- Nihro Y, Miyataka H, Sudo T, Matsumoto H, Satoh T. 3-O-Alkylascorbic acids as free-radical quenchers: synthesis and inhibitory effect on lipid peroxidation. J. Med. Chem. 1991;34:2152–2157.10.1021/jm00111a034
- Nihro Y, Sogawa S, Sudo T, Miki T, Matsumoto H, Satoh T. 3-O-Alkylascorbic acids as free radical quenchers. II. Inhibitory effects on some lipid peroxidation models. Chem. Pharm. Bull. 1991;39:1731–1735.10.1248/cpb.39.1731
- Ogawa K, Futakuchi M, Hirose M, Boonyaphiphat P, Mizoguchi Y, Miki T, Shirai T. Stage and organ dependent effects of 1-O-hexyl-2,3,5-trimethylhydroquinone, ascorbic acid derivatives, n-heptadecane-8-10-dione and phenylethyl isothiocyanate in a rat multiorgan carcinogenesis model. Int. J. Cancer. 1998;76:851–856.10.1002/(ISSN)1097-0215
- Futakuchi M, Hirose M, Miki T, Tanaka H, Ozaki M, Shirai T. Inhibition of DMBA-initiated rat mammary tumour development by 1-O-hexyl-2,3,5-trimethylhydroquinone, phenylethyl isothiocyanate, and novel synthetic ascorbic acid derivatives. Eur. J. Cancer Prev. 1998;7:153–159.
- Beifuss U, Kunz O, Aguado GP. Regioselective O-alkylation of ascorbic acid for the efficient synthesis of lipophilic antioxidants. Synlett. 1999;1999:147–149.10.1055/s-1999-2522
- Beifuss U, Kunz O, Voss G. Regioselective synthesis of 3-O-alkyl ethers of ascorbic acid without protecting groups in a single step. Tetrahedron. 2000;56:357–361.10.1016/S0040-4020(99)01013-3
- Tahir H, Hindsgaul O. Regio- and chemoselective alkylation of l-ascorbic acid under Mitsunobu conditions. J. Org. Chem. 2000;65:911–913.10.1021/jo990606+
- Zhou X, Tai A, Yamamoto I. Enhancement of neurite outgrowth in PC12 cells stimulated with cyclic AMP and NGF by 6-acylated ascorbic acid 2-O-α-glucosides (6-Acyl-AA-2G), novel lipophilic ascorbate derivatives. Biol. Pharm. Bull. 2003;26:341–346.10.1248/bpb.26.341