Abstract
We investigated the effect of theaflavins (TFs) on membrane barrier of Caco-2 cells. For fluorescein-transport experiments, the apparent permeability (Papp) of fluorescein in Caco-2 cells pretreated with 20 μM TFs were significantly decreased compared with that in untreated cells. Although the respective monomeric catechins did not show any Papp reduction, purpurogallin pretreatment resulted in a significant Papp reduction similar to that of TF-3′-O-gallate (TF3′G) pretreatment. This indicates that the benzotropolone moiety may play a crucial role in the Papp reduction or tight junction (TJ)-closing effect induced by TFs. In TF-3′-O-gallate-pretreated Caco-2 cells, fluorescein transport was completely restored by compound C (AMPK inhibitor). In addition, TF3′G significantly increased both the mRNA and protein expression of TJ-related proteins (occludin, claudin-1, and ZO-1) as well as the phosphorylation of AMPK. It was, thus, concluded that TFs could enhance intestinal barrier function by increasing the expression of TJ-related proteins through the activation of AMPK in Caco-2 cells.
Graphical Abstract
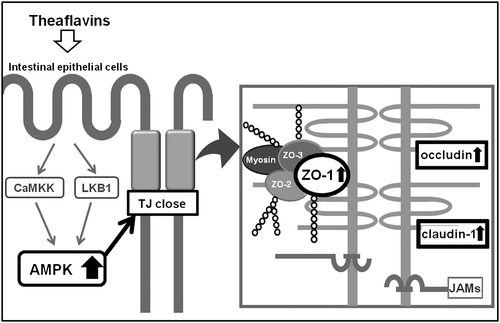
Theaflavins can close the tight junction or improve the intestinal membrane barrier via AMPK activation in Caco-2 cells.
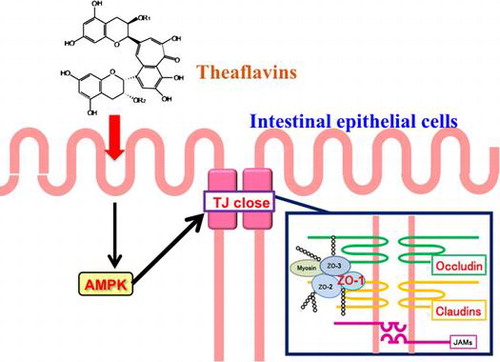
Theaflavins (TFs), oxidative coupling dimers of catechins, are major polyphenol pigments in black tea, contributing to its characteristic orange–red color.Citation1) Recently, TFs have attracted great interest due to their physiological functions, such as anti-hyperglycemic, antioxidant, and anti-inflammation effects.Citation2,3) In our studies on TF-induced functions, we have also demonstrated an alternative physiological role of TFs on the intestinal transport system as well as on reduced absorption across the membrane.Citation4) TFs suppressed intestinal transport of small peptides via the downregulation of AMP-activated protein kinase (AMPK)-mediated peptide transporter (PEPT1) in Caco-2 cell monolayers.Citation4,5) We previously found that TFs induced AMPK activation in Caco-2 cells. Thus, TFs may influence other AMPK-related intestinal transport systems, since Zheng and Cantley have found that epithelial AMPK activation was closely associated with enhanced tight junction (TJ) integrity in MDCK cells.Citation6) In addition, Scharl and co-workers supported the relation of epithelial barrier function with AMPK activation.Citation7) These reports provide additional rationale for our investigation on the effect of TFs on the paracellular transport system.
TJs localized at the junction of the apical–lateral membrane are a major determinant of intestinal barrier function and paracellular permeability.Citation8,9) TJs are composed of a complex combination of integral transmembrane proteins, including occludin, claudins, and junctional adhesion molecules (JAMs) as well as cytosolic proteins, such as the zonula occludens (ZO) protein family.Citation8) Although information is still limited, recent studies found intestinal TJ regulation by food-derived factors, including polyphenols.Citation8,10) Suzuki and co-workers reported that kaempferol, a natural flavonoid, showed a protective effect on TJ barrier integrity via ZO-2 and claudin-4 expression and a redistribution of occludin and claudin-1/-3.Citation11) Thus, it is important to know how TJs are regulated and whether or not natural compounds may regulate the opening or closing of TJs. In this study, we investigated the effect of TFs on the paracellular transport system by using fluorescein as a TJ transport model markerCitation12) in Caco-2 cells. Further experiments were also performed to identify the mechanism underlying TF-induced intestinal barrier function with regard to TJ-related protein expression.
Materials and methods
Materials
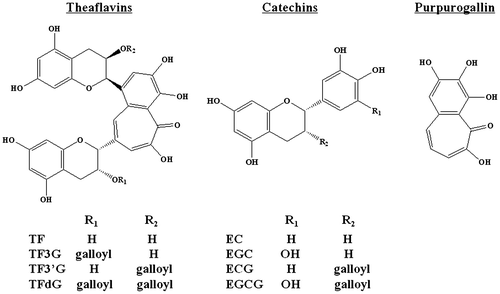
Theaflavin (TF), TF-3-O-gallate (TF3G), TF-3′-O-gallate (TF3′G), TF-3,3′-O-digallate (TFdG), (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECG), and (-)-epigallocatechin gallate (EGCG) were purchased from Nacalai Tesque Co. (Kyoto, Japan). Purpurogallin was purchased from Pfaltz & Bauer Inc. (Waterbury, CT). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from GIBCO Life Technologies (Grand Island, NY). Penicillin was purchased from Meiji Seika Co. (Tokyo, Japan). Insulin, fluorescein sodium salt, and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). Seeding basal medium, enterocyte differentiation medium, and MITO+™ serum extender for Caco-2 cell culture were purchased from BD Biosciences (Franklin Lakes, NJ). Compound C, an AMPK inhibitor, and GF109203X, a non-specific protein kinase C (PKC) inhibitor, were obtained from Merck Millipore (Darmstadt, Germany). Other reagents were of analytical grade and were used without further purification.
Cell culture
Caco-2 cells between passages 50 and 60 were propagated and maintained under standard cell culture conditions as described previously.Citation4,5) For Caco-2 transport experiments, cells were seeded into BD Falcon™ cell culture insert (PET membrane, 0.9 cm2, 1.0 μm pore size; BD Biosciences) coated with type I collagen (collagen gel culturing kit, Cellmatrix type I-A, Nitta Gelatin, Osaka, Japan) at a density of 4.0 × 105 cell/mL. Seeded cells were cultured in a seeding basal medium containing MITO+™ serum extender for 48 h. The medium was replaced with an enterocyte differentiation medium containing MITO+™ serum extender every day for three days to allow the cells to form monolayers. The integrity of the monolayers was evaluated by measuring transepithelial electrical resistance (TEER) using a Multi-channel Voltage/Current EVC-4000 system (World Precision Instruments, Sarasota, FL). Monolayers with a TEER value of >100 Ω cm2 were used for Caco-2 transport experiments.
Cell treatment
Pretreatment of Caco-2 cell monolayers in the BD Falcon™ cell culture inserts with test samples (TFs, catechins, or purpurogallin) was performed by replacing 10% FBS in DMEM with medium containing sample (5, 10, or 20 μM) in the presence or absence of inhibitor (10 μM compound C or 10 μM GF109203X) and incubated for 1, 3, 6, or 24 h at 37 °C, in an humidified atmosphere of 5% CO2 in air. Cells incubated in the medium without test samples were used as a control. The Caco-2 cell monolayers were used for the fluorescein transport studies. The medium containing test samples was prepared by diluting a stock solution of each sample dissolved in DMSO with 10% FBS in DMEM. The final concentration of DMSO was 0.5% throughout the experiments to avoid any cell damage by DMSO.Citation13)
Fluorescein transport study
Fluorescein transport experiments were performed with an Ussing Chamber system (Model U-2500; Warner Instrument Co., Hamden, CT) using Caco-2 cell monolayers pretreated as described above. Prior to the transport experiments, Caco-2 cell monolayers in the transwell inserts were gently rinsed with Hanks’s balanced salt solution (HBSS) buffer (pH 7.4, adjusted with 10 mM HEPES [4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid]) to wash out any free sample from the insert. The insert was then carefully mounted in the Ussing Chamber using blunt forceps. An aliquot (7.5 mL) of HBSS buffer (pH 7.4) was added to both the apical and basolateral sides, since fluorescein restrictively transports through the paracellular (TJ) route in Caco-2 cells when both sides had no proton gradient (in this study, pH 7.4).Citation12) After equilibration for 15 min at 37 °C, transport experiments were started by replacing the apical buffer with fresh HBSS buffer containing 100 μM fluorescein. During 30 min-transport experiments, solutions on both sides were bubbled continuously with a mixture of O2:CO2 (95:5), and an aliquot of the solution was taken from the basolateral side every 5 min. Next, fresh HBSS buffer (100 μL) was added to the basolateral side at each time point. The fluorescence intensity of the collected solutions was measured with a fluorescence spectrophotometer (Wallac ARVO SX 1420 multilabel counter; Perkin-Elmer Life Sciences, Tokyo, Japan) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
Apparent permeability coefficient
The apparent permeability coefficient (Papp) was calculated from the following equation:
where dC/dt is the change in concentration at the basolateral side over time (mmol/s), V is the volume of solution in the basolateral compartment (7.5 mL), A is the surface area of the membrane (0.2826 cm2), and C0 is the initial concentration at the apical side (mmol). The Papp of fluorescein in Caco-2 cells pretreated with test samples was expressed as a percentage of Papp of control (%).
Real-time reverse transcription PCR
Caco-2 cells were cultured on a six-well plate under the same cell culture conditions as described above. Total RNA was isolated from Caco-2 cells pretreated with or without 10 μM TF3′G for 1 and 3 h using an SV Total RNA Isolation System (Promega Corporation, Madison, WI). The complementary DNA was produced from 1 μg of total cellular RNA by reverse transcription (Promega Corporation) according to the manufacturer’s instructions. The Mx3000P QPCR system (Stratagene Inc., Tokyo, Japan) and SYBR Premix Ex Taq II were used for real-time PCR amplification of TJ-related proteins (occludin, claudin-1/-3/-4, ZO-1/-2, and JAM-1) and β-actin with the following cycle profile: 1 cycle at 95 °C for 10 s, 47 cycles at 95 °C for 30 s (denaturation step), at 60 °C for 30 s (annealing step), and at 72 °C for 1 min (extension phase). At the end of each extension phase, observed fluorescence was used for quantitative analyses. A melting point analysis was carried out by heating the amplicon from 55 to 95 °C, and a characteristic melting point curve was obtained for each product. Primer sequences were as follows: occludin, forward primer, 5′-TCCTATAAATCCACGCCGGTTC-3′ and reverse primer, 5′-CTCAAAGTTACCACCGCTGCTG-3′; claudin-1 forward primer, 5′-GCACATACCTTCATGTGGCTCAG-3′ and reverse primer, 5′-TGGAACAGAGCACAAACATGTCA-3′; claudin-3 forward primer, 5′-ACATCATCACGTCGCAGAACATC-3′ and reverse primer, 5′-AGTGCCAGCAGCGAGTCGTA-3′; claudin-4 forward primer, 5′-CATCGGCAGCAACATTGTCAC-3′ and reverse primer, 5′-GCACCACGCAGTTCATCCATAG-3′; ZO-1 forward primer, 5′-CGGGACTGTTGGTATTGGCTAGA-3′ and reverse primer, 5′-GGCCAGGGCCATAGTAAAGTTTG-3′; ZO-2 forward primer, 5′-CTAGCAGCGATCAACTTAGGGACAA-3′ and reverse primer, 5′-CCCAGGAGTTTCATTACCAGCAA-3′; JAM-1 forward primer, 5′-CAGAGGTGATTCATGGCTCTGTG-3′ and reverse primer, 5′-TTCCAGGCTGGCAATAACTGAC-3′; β-actin forward primer, 5′-TGGCACCCAGCACAATGAA-3′ and reverse primer, 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. As an internal control, β-actin primers were used for RNA template normalization. Quantification was reported by proprietary software, as cycle threshold (Ct). The expression of target genes relative to the housekeeping genes was calculated as the difference between Ct values for the two groups. All PCR reactions were done in triplicates.
Western blot analysis
The relative amounts of TJ-related proteins (occludin, claudin-1, and ZO-1) in Caco-2 cells treated with or without 10 μM TF3′G for 1 and 3 h were detected by Western blot analysis. Proteins extracted from Caco-2 cells with RIPA buffer (radio immunoprecipitation assay) were mixed with an equal volume of sample buffer (20% glycerol, 4% sodium dodecylsulfate, 3% dithiothreitol, 0.002% bromophenol blue, and 0.125 M Tris–HCl, pH 6.8). The supernate of the lysate was collected by centrifugation at 20,800 × g for 10 min at 4 °C, and the amount of protein was quantified with a DC protein assay kit (Bio-Rad Laboratories, Inc., Berkeley, CA). The supernate was mixed with an equal volume of SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer and boiled in water for 5 min. An aliquot (15 μg/lane) was applied to 10% SDS-PAGE for 1 h at 20 mA and transferred onto a polyvinylidene difluoride (PVDF) membrane (Hybond-P, GE Healthcare, MJ) for 1.5 h at 40 mA. The membrane was blocked with 5% (w/v) enhanced chemiluminescence (ECL) membrane-blocking reagent in Tris-buffered saline containing 0.05% Tween 20 (TBST) for 1 h at room temperature, and then probed with a primary antibody to either occludin (1:1000 dilution, Santa Cruz Biotechnology, Inc., Dallas, TX), claudin-1 (1:50 dilution, Thermo Fisher Scientific Inc., Runcorn, UK), ZO-1 (1:200 dilution, Santa Cruz Biotechnology), or β-actin (1:1000 dilution, Applied Biological Materials Inc., Richmond, BC, Canada) overnight at 4 °C. Finally, the membrane was incubated with the secondary antibody (HRP-conjugated donkey anti-rabbit IgG antibody at a 1:1000 dilution, GE Healthcare) for 1 h at room temperature. The immunoreactive proteins on the membrane were detected with ECL plus detection reagent using an ImageQuant LAS 4000 (GE Healthcare). Quantitation of the occludin, claudin-1, and ZO-1 amounts was determined by ImageQuant TL 7.0 software (GE Healthcare). The levels of occludin, claudin-1, and ZO-1 in Caco-2 cells were calculated by the ratio of their levels to β-actin, and the data were expressed as quantity relative to the control level.
Assay for phosphorylation of AMPK
Caco-2 cell monolayers in a six-well microplate were treated with or without 10 μM TF3′G in the presence or absence of 10 μM compound C for 3 h at 37 °C. Proteins were extracted from Caco-2 cells using RIPA buffer containing PhosSTOP (Roche, Indianapolis, IN), and the supernate was obtained as mentioned above. The amounts of p-AMPK and AMPK proteins were evaluated by Western blot analysis. An aliquot (15 μg) of the prepared sample was applied to 10% SDS-PAGE for 50 min at 20 mA and transferred onto PVDF membrane for 1.5 h at 40 mA. The membrane was incubated with 5% ECL membrane-blocking reagent for 1 h at room temperature. The membrane was probed with a primary antibody for phospho (Thr172)-AMPKα1 (1:1000 dilution, Merck Millipore, Bedford, MA, USA) overnight at 4 °C, and then incubated with the secondary antibody (HRP-conjugated donkey anti-rabbit IgG antibody) for 1 h at room temperature. The immunoreactive proteins on the membrane were analyzed with ECL detection reagents and Image Quant LAS 4000. For removing the p-AMPK, the membrane was incubated in stripping buffer (2% SDS, 0.1 M mercaptoethanol, and 62.5 mM, Tris–HCl, pH 6.8) for 30 min, and then washed with TBS-T for 1 h. The membrane was blocked with 5% ECL-blocking reagent for 1 h at room temperature and reprobed with primary antibody for anti-AMPKα1 (1:1000 dilution, Merck Millipore) overnight at 4 °C, and then incubated with the secondary antibody (HRP-conjugated donkey anti-rabbit IgG antibody) for 1 h at room temperature, as described above. Quantification of p-AMPK and AMPK was performed by an Image Quant TL 7.0 software (GE Healthcare). Relative band intensities of p-AMPK and AMPK were expressed as a percentage of control (or of TF3′G-untreated cells).
Statistical analysis
Results are expressed as the mean ± SEM. The statistical significance between groups was analyzed using one-way ANOVA followed by the Tukey-Kramer’s t-test for post hoc analysis. Other statistical evaluations were performed by the Student’s t-test. All analyses were conducted with the Star View J 5.0 (SAS Institute Inc., Cary, NC).
Results
Effect of TFs on fluorescein transport across Caco-2 cell monolayers
To investigate the effect of TFs (TF, TF3G, TF3′G, and TFdG) on intestinal barrier function in Caco-2 cell monolayers, TEER and fluxes of fluorescein were measured. Caco-2 cell monolayers were pretreated individually with 20 μM TFs for 24 h before the transport experiments. As shown in Fig. , the Papp of fluorescein in control Caco-2 cells (control: 3.7 ± 0.1 × 10−6 cm/s) was significantly reduced by TF pretreatment in the descending order of TF3′G (1.2 ± 0.1 × 10−6 cm/s) > TF3G, TFdG (1.4 ± 0.2 × 10−6 cm/s) > TF (1.7 ± 0.1 × 10−6 cm/s). This result revealed that the TFs used in this study significantly inhibited paracellular (TJ) permeability regardless of the TF structures. TF3′G which had a potent Papp reduction effect was used throughout the remainder of our studies. As shown in Fig. (A), the TEER value of Caco-2 cell monolayers (control: 110 ± 1.5 Ω cm2) was significantly increased by TF3′G-pretreatment (e.g. 50 μM: 145 ± 16.1 Ω cm2) for 24 h, suggesting that the Papp reduction by TF may be caused by TF-induced enhancement of intestinal barrier function. The effect of 20 μM TF3′G pretreatment time on the Papp of fluorescein was examined (Fig. (B)). As a result, Papp decreased with time (1–24 h) and a significant reduction was obtained for >3 h-pretreatment. As shown in Fig. (C), at 3-h pretreatment, as low as 5 μM, TF3′G could reduce fluorescein transport across Caco-2 cell monolayers. Further experiments, however, were performed in Caco-2 cells pretreated with 10 μM TF3′G for 3 h by considering experimental errors at 5 μM (Fig. (C)).
Fig. 1. Effect of TF on fluorescein Papp values in Caco-2 cells pretreated with 20 μM TF for 24 h.
Note: The TF used for this experiment were TF, TF3G, TF3′G, and TFdG. Apparent permeability coefficient (Papp) values (in cm/s × 10−6) are expressed as the mean ± SEM (n = 3–5). Different letters represent the statistical differences at p < 0.05 among the groups by the Tukey-Kramer’s t-test.
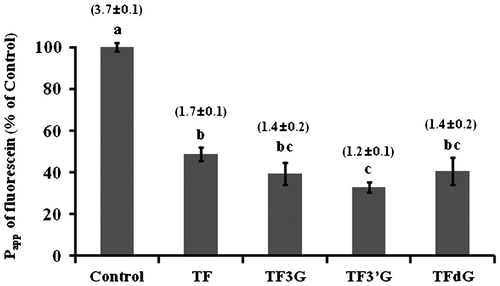
Fig. 2. Effect of TF3′G on intestinal barrier function in Caco-2 cells.
Note: (A) Effect of TF3′G concentration on the TEER value. Caco-2 cells were pretreated with either 10, 20, or 50 μM TF3′G for 24 h. (B) Time-dependent effect of TF3′G on fluorescein transport. Caco-2 cells were pretreated with or without 20 μM TF3′G. (C) Concentration-dependent effect of TF3′G on fluorescein transport. Caco-2 cells were incubated for 3 h. Values are expressed as the mean ± SEM (n = 3–5). Different letters represent the statistical differences at p < 0.05 among the groups by the Tukey-Kramer’s t-test.
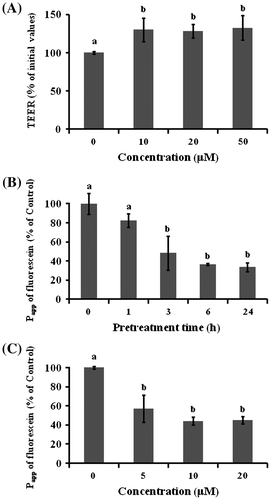
Effect of TF3′G and its related compounds on fluorescein transport
Catechins and purpurogallin with a benzotropolone moiety similar to that of TF3′G (Fig. ) were examined for fluorescein transport across Caco-2 monolayers to obtain insight on the structural requirements of TFs for paracellular permeability inhibition. As shown in Fig. , four catechins did not show any significant Papp reduction, while the Papp of fluorescein was significantly reduced in Caco-2 cells pretreated with 10 μM purpurogallin (51% of control in Papp), which is similar to level observed with TF3′G pretreated cells (46% of control in Papp). This result indicated that the benzotropolone moiety having a characteristic seven-ring structure in TFs was a crucial structural factor responsible for TF-induced intestinal barrier function in Caco-2 cell monolayers.
Fig. 4. Effect of TF3′G and related compounds on fluorescein transport.
Note: Caco-2 cells were pretreated with or without 10 μM of either TF3′G, EC, ECG, EGC, EGCG, or purpurogallin for 3 h. Values are expressed as the mean ± SEM (n = 3–5). Different letters represent the statistical differences at p < 0.05 among the groups by the Tukey-Kramer’s t-test.
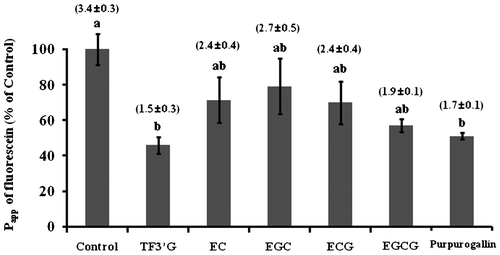
Effect of AMPK or PKC inhibitor on fluorescein transport in TF3′G-pretreated Caco-2 cells
Fluorescein transport in 10-μM TF3′G-pretreated Caco-2 cells was studied in the presence of either 10 μM GF109203X, a non-specific inhibitor of PKC, or 10 μM compound C, a specific inhibitor of AMPK. As shown in Fig. (A), the Papp value of fluorescein was restored by 10 μM compound C (3.6 ± 0.9 × 10−6 cm/s), while GF109203X did not show any change in fluorescein transport in the TF3′G-pretreated Caco-2 cells (1.2 ± 0.2 × 10−6 cm/s). As expected based on the compound C finding of inhibition of the action of TFs, we also found that the enhanced phosphorylation of AMPK by 10 μM TF3′G in Caco-2 cells was blocked by compound C (Fig. (B)), suggesting the possible involvement of TF3′G in the activation of AMPK, but not in the PKC pathway.
Fig. 5. Effect of PKC and AMPK inhibition on fluorescein transport.
Note: (A) For fluorescein transport analysis, Caco-2 cells were pretreated either with or without 10 μM TF3′G in the presence or absence of 10 μM GF109203X (PKC inhibitor) or 10 μM compound C (AMPK inhibitor) for 3 h at 37 °C. (B) For western blot analysis, Caco-2 cells were incubated either with or without 10 μM TF3′G in the presence or absence of 10 μM compound C. Specific bands for p-AMPK and AMPK were quantitated by densitometric analysis. Values are expressed as the mean ± SEM (n = 3–5). Different letters represent the statistical differences at p < 0.05 among the groups by the Tukey-Kramer’s t-test.
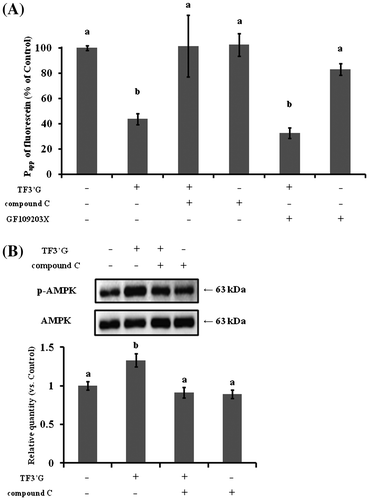
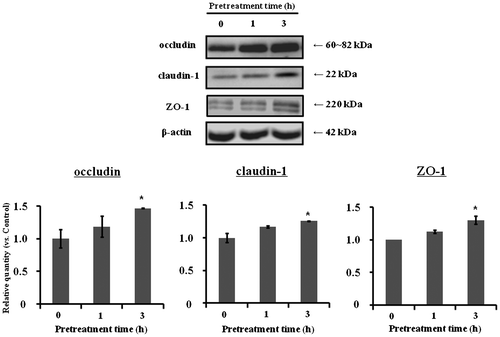
Effect of TF3′G pretreatment of Caco-2 cells on the expression of TJ-related proteins
To clarify the mechanism underlying the inhibition of paracellular transport across TF3′G-pretreated Caco-2 cell monolayers, expression of TJ-related proteins was investigated at the mRNA and protein levels. As shown in Fig. , mRNA levels for occludin, claudin-1, and ZO-1 were significantly (p < 0.05) increased at 3 hours in 10 μM TF3′G-treated Caco-2 cells, compared to control (0 h-incubation), whereas claudin-3/4, ZO-2, and JAM-1 mRNA levels were not affected. Although TJ-related protein expressions are not regulated only by mRNAsCitation14,15), further western blot experiments were focused on the TJ-related proteins (occluding, claudin-1, and ZO-1) that mRNAs were increased by TFs, since they play a crucial role to adjust permeability characteristics and tightness of epithelium.Citation15) As a result of western blot analysis, we found significant enhancement of occludin, claudin-1, and ZO-1 protein expression in 10-μM TF3′G-treated cells, as shown in Fig. .
Fig. 6. Effect of TF3′G on mRNA expression of TJ-related proteins in Caco-2 cells.
Note: Caco-2 cells were cultured either with or without 10 μM TF3′G for 1 and 3 h. Total RNA was isolated, and the expression level of mRNA was measured by real-time reverse transcription PCR using specific primers for occludin, claudin-1/3/4, ZO-1/2, and JAM-1. Values are expressed as the mean ± SEM (n = 3–5). (*) p < 0.05, significantly different from untreated cells. Significant differences between control (0 h) and TF3′G groups were evaluated by the unpaired Student’s t-test.
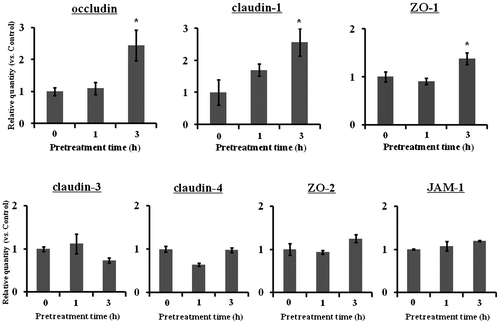
Fig. 7. Effect of TF3′G on the expression of TJ-related protein in Caco-2 cells.
Note: Caco-2 cells were treated either with or without 10 μM TF3′G for 1 and 3 h. Specific bands for occludin, claudin-1, ZO-1, and β-actin were quantitated by densitometric analysis. Values are expressed as the mean ± SEM (n = 3–5). (*) p < 0.05, significantly different from untreated cells. Significant difference between control (0 h) and TF3′G groups were evaluated by the unpaired Student’s t-test.
Discussion
The human intestine absorbs dietary nutrients and protects against noxious molecules via the intestinal barrier systems.Citation9,10) One of protective intestinal barrier systems involves the TJ-closing effect that is crucial for the maintenance of barrier integrity.Citation10,16,17) In this study, we have demonstrated for the first time that dimeric catechins, TFs, bearing a characteristic benzotropolone seven-ring structure were important for closing the TJ paracellular route in Caco-2 cells. The TF benzotropolone moiety, but not monomeric catechin moieties, was required for inhibiting fluorescein paracellular transport through the TJ route (Figs. and ). When using experimental conditions of <24 h-pretreatment with <100 μM TF3′G, we found no toxicity to the Caco-2 cells by WST-8 assay (Supplemental Fig. 1), suggesting that the transport inhibition effect of TFs may result from a change in signaling pathways towards the TJ closing route, and was not due to a toxic effect.
In previous studies, we demonstrated biochemical activity of TFs. We found that TFs inhibited intestinal peptide transport via the downregulation of the peptide transporter PEPT1 by activated AMPK in Caco-2 cells.Citation4,5) In the present study, we further showed that TFs activated AMPK resulting in enhanced TJ-related protein expression and closure of the TJ route (Figs. ). The TF-stimulated TJ proteins were occludin, claudin-1, and ZO-1, whereas the levels of claudin-3/4, ZO-2, and JAM-1 were not affected by TF (Figs. and ). It has been reported that TF enhancement of occludin, claudin-1, and ZO-1 is important in both regulating TJ structure and paracellular permeability.Citation10,11,18,19) Claudin-1 and occludin are considered to be part of the structural backbone of the TJ system, and are involved in reduced paracellular permeability by tightening the TJ.Citation10,11,18) ZO-1 acts as an adaptor that connects transmembrane proteins to the perijunctional actomyosin ring.Citation10,11)
As reported by Ulluwishewa et al. TJ-related protein expression was induced by certain signaling pathways.Citation10) Cario et al. reported that PKC stimulation induced TJ proteins, including ZO-1.Citation20) In contrast, the AMPK activation pathway is involved in alternative signaling for TJ protein expression, including occludin and ZO-1.Citation16) In this study, we successfully demonstrated that TFs were involved in the AMPK/TJ expression signaling pathway, but not in the PKC pathways. However, it still remains unclear which upstream signaling mechanisms (liver kinase B1 (LKB1), Ca2+/calmodulin-dependent protein kinase kinase (CaMKK)Citation21) or transforming growth factor-β-activated kinase (TAK-1)Citation22−Citation24)) are involved in the AMPK phosphorylation by TFs in Caco-2 cells. We are now using LKB1- and TAK-1-knocked down Caco-2 cells to determine the upstream signals.
Several groups have reported that natural compounds enhance the TJ-closing effect and increase intestinal barrier function. Suzuki and Hara reported that quercetin, a flavonoid, promoted the assembly of TJ proteins (occludin, claudin-1, and ZO-2) through the inhibition of the PKCδ isoform in Caco-2 cells, while myricetin did not alter the expression of TJ proteins.Citation8) Kaempferol also increases the expression of claudin-4 and ZO-2.Citation11) These results clearly indicate the structural importance of the hydroxyl group in the flavonoid B ring for the expression of TJ proteins involved in the PKC pathways, but detailed structural features remained unclear. In this study, we demonstrated the key role of the benzotropolone ring in condensed catechins in AMPK activation, and further show no role in PKC inhibition for the TJ-closing effect. Thus, the mechanism of polyphenols for activation of different TJ-related expression pathways must be examined in the future.
In conclusion, we demonstrated that TFs did in fact have the ability to close the TJ route in Caco-2 cells, likely through the expression of AMPK-mediated occludin, claudin-1, and ZO-1 proteins (Fig. ). Thus, the ingestion of TFs would be expected to improve degraded intestinal barrier function by e.g. inflammatory bowel disease in future animal experiments.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.951027.
Supplemental Materials
Download MS Word (66 KB)Acknowledgments
The authors thank Junko Takeda in Kyushu University for her technical supports. The authors also declare no conflict of interest.
Additional information
Funding
References
- Tanaka T, Inoue K, Betsumiya Y, Mine C, Kouno I. Two types of oxidative dimerization of the black tea polyphenol theaflavin. J. Agric. Food Chem. 2001;49:5785–5789.
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am. J. Clin Nutr. 2000;71:1698S–702S.
- Sang S, Lambert JD, Tian S, Hong J, Hou Z, Ryu JH, Stark RE, Rosen RT, Huang MT, Yang CS, Ho CT. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg. Med Chem. 2004;12:459–467.
- Takeda J, Park HY, Kunitake Y, Yoshiura K, Matsui T. Theaflavins, dimeric catechins, inhibit peptide transport across Caco-2 cell monolayers via down-regulation of AMP-activated protein kinase-mediated peptide transporter PEPT1. Food Chem. 2013;138:2140–2145.
- Park HY, Kunitake Y, Matsui T. Benzotropolone moiety in theaflavins is responsible for inhibiting peptide-transport and activating AMP-activated protein kinase in Caco-2 cells. Functional Foods in Health and Disease. 2013;3:111–121.
- Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 2007;104:819–822.
- Scharl M, Paul G, Barrett KE, McCole DF. AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J. Biol Chem. 2009;284:27952–27963.
- Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonnula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J. Nutr. 2009;139:965–974.
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol Life Sci. 2013;70:631–659.
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776.
- Suzuki T, Tanabe S, Hara H. Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. J. Nutr. 2011;141:87–94.
- Konishi Y, Hagiwara K, Shimizu M. Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci. Biotechnol. Biochem. 2002;66:2449–2457.
- Da Violante G, Zerrouk N, Richard I, Provot G, Chaumeil JC, Arnaud P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco2/TC7 colon tumor cell cultures. Biol. Pharm Bull. 2002;25:1600–1603.
- Koizumi J, Kojima T, Ogasawara N, Kamekura R, Kurose M, Go M, Harimaya A, Murata M, Osanai M, Chiba H, Himi T, Sawada N. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol. 2008;74:432–442.
- De Walle JV, Sergent T, Piront N, Toussaint O, Schneider YJ, Larondelle Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl Pharmacol. 2010;245:291–298.
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625.
- Camilleri M, Madsen K, Spiller R. Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012;24:503–512.
- Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 1998;143:391–401.
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134:1031–1049.
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238.
- Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int. J. Obes (Lond). 2008;32:S7–S12.
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol Chem. 2006;281:25336–25343.
- Chen A, Davis BH, Sitrin MD, Brasitus TA, Bissonnette M. Transforming growth factor-beta 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)(2)D(3). Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G864–G874.
- Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc. Natl. Acad. Sci. U.S.A. 2006; 103: 17378–17383.