Abstract
Asthma is a major public health concern. Its greatest risk factor is house dust mite (HDM). Dermatophagoides microceras (Der m) is a type of HDM, and in central Taiwan, there is approximately 80% prevalence of sensitization to Der m. FIP-fve is a fungal immunomodulatory protein (FIP) isolated from the fungus Flammulina velutipes, and exhibits anti-inflammatory properties. To investigate whether FIP-fve affects Der m-induced asthma and inflammation, we evaluated hyper-responsiveness (AHR), pathological changes, and cytokines in mice. We demonstrated that oral FIP-fve decreased Der m-induced airway AHR, airway inflammation, cell infiltration, and expression of cytokines in the bronchoalveolar lavage fluid of Balb/c mice. The results of this study suggest that FIP-fve suppresses asthma, inflammation, and respiratory pathogenesis stimulated by Der m. FIP-fve is able to maintain immunomodulatory activity even in simulated gastric fluid and intestinal fluid. FIP-fve could be a safe and stable agent for suppression of allergic asthma.
Graphic Abstract
Dermatophagoides Microceras (Der m) induced IL-6, TNF-α and MIP-1α secretion in bronchoalveolar lavage fluid.
Der m-induced neutrophils and lung inflammation resulted in asthma.
FIP-fve suppressed Der m-induced asthma and inflammation-related cytokines.
Asthma is a major public health concern affecting millions of people each year.Citation1) Its most important risk factor is house dust mite. Perennial HDM allergen exposure induces inflammatory diseases, which are manifested as asthma, atopic dermatitis (AD), or allergic rhinitis.Citation2) It has been suggested that approximately 20% of the population in industrialized countries develop a sensitization to HDM allergens.Citation3)
Allergens of HDMs, including Dermatophagoides pteronyssinus (Der p), Dermatophagoides farinae (Der f), Der m, and Blomia tropicalis (Blo t), are important inducers of symptoms.Citation4) Der p 1 (cysteine protease of Der p) and Der f 1 (cysteine protease of Der f) are two of the most well-known allergens. They cause the production of pro-inflammatory cytokines and allergic rhinitis.Citation5) Der p 1 increases the permeability of epithelial cells in vivo, presumably through degradation of tight junction proteins, and induces a more specific response. The interaction of epithelial cells and Der p 1 can induce cytokine production, such as interleukin 6 (IL-6) and interleukin 8 (IL-8), or induce fluid secretion from submucosal glands.Citation6) Der f induces acute AD skin lesions and causes epidermal and dermal thickening, dermal infiltration of CD4+ T cells, eosinophils, and Th2 (Type 2 T helper) cytokine expression.Citation7,8) However, a relatively higher proportion of co-sensitization of Der m compared with the other three antigens – Der p, Der f, and Blo t – has been found in Taiwanese allergic children,Citation4) while Der m has rarely been reported except in atopic children in Western countries.Citation4) In the previous study, we focused on Der m from local glycyphagid mites in central Taiwan.Citation4)
Allergen exposure induces immune responses that result in naive CD4+T-cell differentiation into Th2 cells and establish CD4+ T-cell memory.Citation9) Th2 cytokines, such as interleukin 4 (IL-4), interleukin 5 (IL-5), and interleukin 13 (IL-13), are essential in allergic disorders.Citation10) Th2 cells and their cytokines can trigger the development of allergic asthma, and the obstruction of these cytokines is an effective method for alleviating symptoms of asthma both in experimental animal models and in human subjects.Citation1)
Fungal immunomodulatory protein (FIP-fve) is an immunomodulatory protein isolated and purified from the edible golden needle mushroom, Flammulina velutipes. It has a molecular mass of ~13 kDa and comprises 114 amino acid residues.Citation11) FIP-fve is a glycoprotein and presents a 1.7 Å X-ray structure by single anomalous diffractionCitation12) in which a potential N-glycosylation site is present at position 54.Citation13) The hinge for the activity of FIPs is stabilized predominantly by hydrophobic interactions within the N-terminal helices.Citation14) Furthermore, FIP-fve has a high degree of amino acid sequence homology with LZ-8, and with 70 invariant amino acid residues.Citation11) FIP-fve has also been found to stimulate IFN-γ (interferon-gamma) production in human peripheral blood mononuclear cells (hPBMCs) via the modulation of Ca2+ release.Citation15,16) IFN-γ is secreted from such local memory Th1 (Type 1 T helper) cells to provide protection against secondary challenge.Citation17) Mice receiving oral FIP-fve treatment during sensitization to OVA demonstrated a Th1-skewing effect with the development of the allergen-specific immune response and showed protection from systemic anaphylaxis-like symptoms.Citation18) Vaccination with Th1-directing adjuvants inhibited the development of allergen-induced Th2-type responses via activated CD4+T cells.Citation19)
Der m is one of the major allergens in central Taiwan, but it was rarely reported. FIP-fve has the ability of stimulating Th1 cytokine production. We purposed that orally administrated FIP-fve may able to switch the immune response towards Th1 while Der m challenge. To evaluate the hypothesis, we fed Balb/c mice with FIP- fve orally and sensitized with Der m extract.
Materials and methods
Purification of fungal immunomodulatory protein (FIP-fve)
FIP-fve was purified as previously described.Citation20) In brief, the fruiting bodies of F. velutipes (300 g) were homogenized following soaking in ice-cold 5% acetic acid in the presence of 0.05 M 2-mercaptoethanol. The collected soluble proteins were precipitated by the addition of ammonium sulfate to 80% saturation. The precipitate was dialyzed against the dialysis solution (10 mM sodium acetate, pH 5.2), and the dialyzed proteins were centrifuged, then the supernatant was collected for crude extract. The dialysate equilibrated with 10 mM sodium acetate (pH 5.2) was applied to a CM-52 column (2 × 5 cm). The column was washed with equilibration buffer and then eluted with 200 ml 0–0.5 M NaCl in 10 mM sodium acetate. Finally, approximately 80 mg of purified FIP-fve was collected. To confirm the purification, crude extract and purified FIP-fve were analyzed by 12% SDS-PAGE and stained with Coomassie blue (0.26%) for 30 min. The stained gel was destained by destaining buffer (50% methanol and 3% glycerol) overnight.
Animals
Female Balb/c mice at 6–8 weeks of age and with body weights of 20–25 g were purchased from the National Laboratory Animal Center in Taiwan. All animal housing requirements and procedures were performed in accordance with the Animal Use Committee of Chung Shan Medical University. Mice were individually housed in rack-mounted stainless steel cages with access to food and water and grouped as follows: (1) the control group had intranasal application of normal saline; (2) Der m group mice fed normal saline and were intranasal with 6.25 mg/kg/day of Der m extract for 10 days; (3) FIP-fve/Der m group mice were intranasal with 6.25 mg/kg/day of Der m extract for 10 days. Each mouse was administered 10 mg/kg FIP-fve, 2 days before and up to 10 days, after intranasal application of Der m.
Airway hyper-responsiveness and bronchoalveolar lavage fluid collection
The effect of FIP-fve on RSV-induced AHR was measured using a whole-body barometric plethysmography (Model PLY 3211; Buxco Electronic Inc., Sharon, CT). During inspiration and expiration with increasing doses of inhaled methacholine (Sigma-Aldrich, St. Louis, MO), the change in the chamber pressure was calculated and recorded as Penh, which is a dimensionless parameter used to evaluate pulmonary resistance. Each mouse rested in a chamber and received an initial baseline challenge with saline, followed by increasing doses of inhaled methacholine (0, 5, 10, 20, and 40 mg/ml). This was followed by a 3-min spray of nebulized methacholine, after which the breathing frequency was read and recorded for 3 min. The respiratory cycle resulted in box pressure waveforms that constitute the peak expiratory pressure (PEP), the peak inspiratory pressure, and the time of expiration. Penh was calculated by the following formula: Penh = Pause × peak expiratory pressure (PEP)/peak inspiratory pressure (PIP). Finally, the Penh values were averaged and reported as baseline saline values in percentages.Citation21,22). After measurement of AHR, lungs were lavaged via the trachea with 1 ml of normal saline for collecting bronchoalveolar lavage fluid (BALF). The cellularity of the BALF was investigated using a hemocytometer. The cells were centrifuged onto slides, fixed and then stained with Liu’s staining. The remaining BALF was stored at −70 °C until used in the assay.
Mouse cytokine array
A mouse cytokine array (Proteome ProfilerTM Array, R&D system Catalog Number ARY006) was conducted according to the manufacturer’s instructions to analyze the cytokine profiles of the BALF of mice. Briefly, the sample was first mixed with the detection antibody at room temperature for 1 h and was then added to the array membrane. The membrane was incubated at 2–8 °C on a shaker overnight. After incubation, the membrane was washed. Horseradish peroxidase-conjugated streptavidin was then added to the membrane and incubated at room temperature on a shaker for 30 min. Following washing, array signals were detected by a chemiluminescence imaging system (MultiGel-21). Finally, the mean pixel densities of spots were analyzed using ImageJ analysis software.
Cytokine measurement
BALF was collected via the trachea with 1 ml of normal saline lavation. Airway inflammation was evaluated by IL-6 in the BALF and assessed using an ELISA kit (88-7066-88; eBioscience) according to the manufacturer’s protocol. The ELISA plate was read at 450 nm using a Bio-Rad ELISA reader. Samples were compared using a standard curve containing seven dilution points, which were the relevant recombinant cytokines on each assay plate.
Histological analysis
To assess pathological changes, the lungs of the mice were soaked in 10% formaldehyde for fixation and embedded in paraffin immediately after sacrificed by CO2. Lung tissue sections were stained with hematoxylin and eosin (H&E) after being cut from the paraffin blocks.
Statistical analysis
Kruskal–Wallis H test was used for non-parametric multiple comparisons. Then, Mann–Whitney was used to test the differences between control and Der m subgroups, and then to test the differences between Der m and FIP-fve/Der m subgroups. A p value <0.025 was considered statistically significant after adjustment, for multiple comparisons. All statistical analyses were performed using the SPSS statistical software program (version 17.0; SPSS, Inc,Chicago, IL).
Results
Examination of the purity and activity of FIP-fve
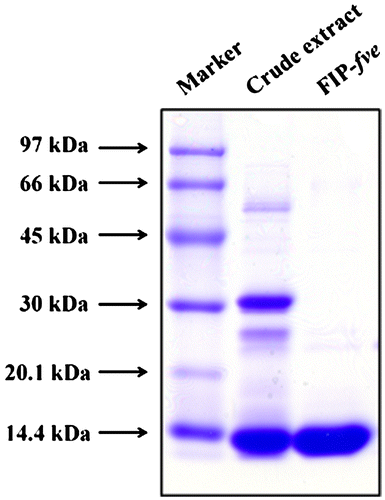
Approximately 80 mg of purified FIP-fve was obtained from fruiting bodies of F. velutipes (300 g). To examine purity, the protein solution was analyzed by SDS-PAGE performed in 12% slab gels and then stained with Coomassie brilliant blue. FIP-fve was present as a single band on SDS-PAGE at 13 kDa (Fig. ). The activity of FIP-fve was checked with an ELISA assay of IFN-γ on human peripheral blood monocytes. After 0 and 50 μg/ml of FIP-fve treatment for 48 h, <16 and 1198 ± 68 ρg/ml, respectively, of IFN-γ were detected from the supernatant of human peripheral blood monocytes (data not show).
Fig. 1. SDS-PAGE analysis of FIP-fve.
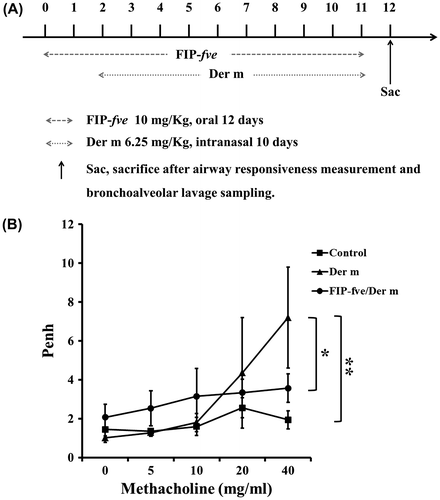
The effect of FIP-fve on Der m-induced AHR
To determine whether administered FIP-fve affected Der m-induced airway-associated syndrome, FIP-fve (10 mg/kg) was orally administered daily from 2 days before Der m challenge to day 10 after challenge (Fig. (A)). Mice inhaled methacholine at doses of 0, 5, 10, 20, and 40 mg/ml, and then a whole-body barometric plethysmography was used to detect AHR (Fig. (B)). The mice with intranasal application of Der m were significantly more sensitive to methacholine exposure compared with the control group (p = 0.017). AHR of the mice administered with FIP-fve (FIP-fve/Der m group) was lower than that of the Der m-challenged mice (Der m group) (p = 0.003).
Fig. 2. Experiment for intranasal application of Der M and oral application of FIP-fve.
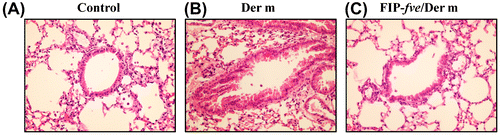
Effect of FIP-fve treatment on Der m-induced lung inflammation in mice
The effect of FIP-fve treatment on overall lung inflammation in the Balb/c mice was investigated via histological staining. Tissue sections were fixed in formaldehyde and stained with H&E. The control group (Fig. (A)) showed no inflammation. Mice with intranasal application of Der m (Fig. (B)) revealed the extensive mucosal folds, epithelial cell shedding, bronchiole smooth muscle mild hypertrophy, bronchial wall and basement membrane thickening, and shape irregularity, as well as numerous inflammatory cells infiltration of peribronchial and perivascular airway tissue compared to the control group. In the FIP-fve/Der m group (Fig. (C)), goblet cell metaplasia was significantly reduced when compared with the Der m group.
Fig. 3. Effect of FIP-fve treatment on airway responsiveness in Der m-challenged mice.
The effect of FIP-fve on infiltrating cells in mice lungs
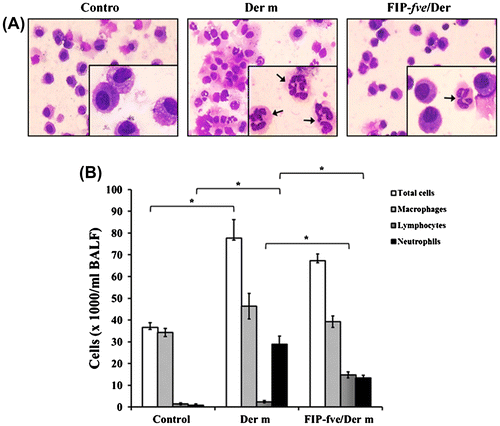
Cells infiltration of the lungs is the characteristic of allergic asthma.Citation22) To investigate the effect of FIP-fve on infiltrating cells, we evaluated the changes in the proportion of macrophages, lymphocytes, and neutrophils in the BALF among the three mice groups. After sacrifice, lungs were lavaged using the trachea with 1 ml of normal saline to collect the BALF. The cells were centrifuged onto slides, fixed, and then stained with Liu’s staining (Fig. (A)). We found that macrophages, neutrophils, and total cells increased in the mice challenged with Der m compared to the control group. In the FIP-fve/Der m group, neutrophils significantly decreased but lymphocytes increased (p = 0.021) (Fig. (B)).
Fig. 4. The effects of FIP-fve treatment on inflammatory cell infiltration in the BALF of Der m-challenged mice.
The effect of FIP-fve on Der m-induced cytokine and total IgEexpression
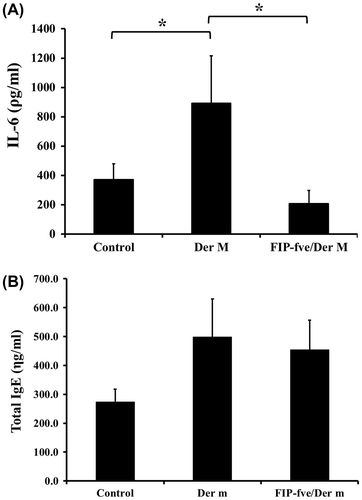
We evaluated the cytokine profiles of the BALF of mice using a mouse cytokine array kit. Supplemental material showed the names of cytokines. The data showed that cytokines, including IL-1α, IL-4, IL-5, IL-6, IL-7, IL-10, IL-13, IL-12 p70, IL-23, IL-27, the chemokine (C-X-C motif) ligand 1 (CXCL1), macrophage inflammatory protein 1 alpha (MIP-1α), and TNF-α, were up-regulated by Der m and suppressed with FIP-fve treatment (Fig. (A) and (B)). Furthermore, we examined the effects of FIP-fve on IL-6 in the BALF (Fig. (A)). The results showed that IL-6 in the mice challenged with Der m increased (893.3 ± 321.7 ρg/mL) compared with the control group (371.4 ± 108.4 ρg/mL; p = 0.016). FIP-fve administered during the Der m challenge phase significantly decreased IL-6 levels to 207.4 ± 89.4 ρg/mL (p = 0.016). The serum total IgE tended to increase after the Der m challenge (499.4 ± 130.1 ηg/mL; p = 0.057) compared with the control group (273.5 ± 44.2 ηg/mL), but no difference was found between Der m and FIP-fve/Der m group (454.7 ± 101.7 ηg/mL; p = 0.686) (Fig. (B)).
Fig. 5. Protein analysis by cytokine array.
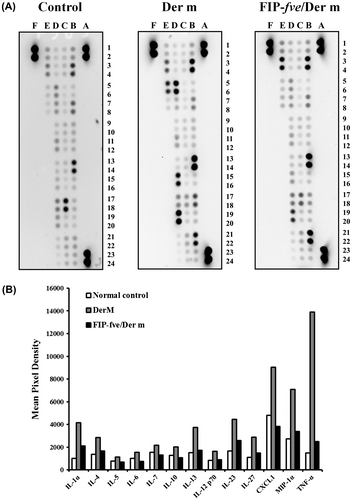
Fig. 6. Effect of FIP-fve on IL-6 and total IgE expression in mice.
Discussion
In this study, we demonstrated that intranasal application with Der m extract significantly increased AHR, lung inflammation, neutrophil infiltration, and IL-6 secretion in the BALF of Balb/c mice, and that FIP-fve showed negative effects on these Der m-induced inflammations. This indicated that FIP-fve administration can eliminate Der-induced inflammatory responses.
An increased number of eosinophils are a classic feature of allergic diseases, such as allergic asthma, allergic rhinitis, and AD. In recent studies, neutrophils have been observed as a novel characteristic in allergic asthma. Kämpe et al. reported that neutrophilia was found in induced sputum in children with non-atopic asthma.Citation23) Patients with asthma and with neutrophilic airway inflammation have increased systemic inflammation and are associated with severe clinical diseases. Systemic inflammation may result from the pathophysiology of neutrophilic asthma; neutrophilic asthma had higher IL-6 levels compared with non-neutrophilic asthma.Citation24) Wood et al. reported that sputum neutrophils increased in subjects with neutrophilic asthma, but there was no difference in non-neutrophilic asthma, including eosinophilic asthma and paucigranulocytic asthma, vs. healthy control subjects.Citation24) In our data, we did not observe the presence of eosinophil in BALF. It may indicate that Der m-induced asthma is classified as neutrophilic asthma. Eosinophils were increased in the BALF of the OVA-treated mice.Citation22) In Der m-treated mice, eosinophils were not observed in BALF. It may indicate that OVA-induced asthma is classified as non-neutrophilic asthma. In addition, Huang et al. showed OVA-stimulated eotaxin increasing in the BALF of mice.Citation25) In our study, there was no difference in eotaxin expression between Der m-treated mice and control mice. However, similar patterns of degranulation after allergen exposure were found in allergic rhinitis and allergic asthma between systemically activated eosinophils and neutrophils. In both allergic groups, the released quantity of eosinophil cationic protein, eosinophil peroxidase, and myeloperoxidase was about the same in all allergen-challenged models.Citation23)
Patients with chronic severe or acute fatal asthma exhibit a feature of neutrophilia in airways.Citation26) In a recent study, mice were challenged intranasally with HDM extract for 10 days and 5 times per week for 5 weeks as an acute and a chronic HDM models, respectively. Granulocyte colony-stimulating factor (G-CSF), TNF-α, IP-10, and CXCL1 increased in the lung of the acute HDM model. In the chronic HDM model, soluble collagen and tissue inhibitors of metalloproteinase-1 in the lung were significantly up-regulated similar to mucus overproduction.Citation27 Our data also showed that CXCL1 and TNF-α increased after the Der m challenge. Inhibition of HIF-1α activity abrogated the production of vascular endothelial growth factor A (V EGF-A) and CXCL1 through an up-regulation of the HIF-1α negative regulators Prolyl Hydroxylases 1 (PHD1) and Prolyl Hydroxylases 3 (PHD3). Reducing HIF-1α expression in lung macrophages may be a strategy to suppress allergen-induced airway inflammation and angiogenesis.Citation28) In our study, Der m stimulated an increase in IL-6in the BALF of mice. The previous study demonstrated that HDM extract induced the release of IL-6 and increased the proliferation of bronchial smooth muscle cells, which is a remarkable characteristic of airway remodeling by reducing CEBPA (CCAAT/enhancer-binding protein (C/EBP)α) mRNA translation in asthma patients.Citation29) In addition, the Fms-like tyrosine kinase 3 ligand was found to have a therapeutic effect in allergic asthma via a reduction in the Th2 cytokines, such as IL-6 and transforming growth factor beta (TGF-β).Citation30)
In this study, we demonstrated that the oral application of FIP-fve inhibits Der m-induced airway hyper-responsiveness; this is in agreement with findings of oral FIP-fve inhibiting OVA-induced airway inflammation.Citation22) Our previous investigation reported that oral FIP-fve had an anti-inflammatory effect on OVA-induced airway inflammation and it was more effective in pre-treated FIP-fve groups compared with post-treated FIP-fve groups.Citation22) According to this study, we performed our experiments with pre-treated FIP-fve groups. Our previous study suggested that Th2 cytokines directly promote the features of asthma and IFN-γ could suppress some characteristics of allergic airway inflammation. The anti-allergic effects of FIP-fve in Th2-mediated allergic response are considered to be partly associated with the function of FIP-fve to stimulate activation of IFN-γ-releasing Th1 cells.Citation22) In HDM-challenged mice, epithelial NF-κB played a role in promoting inflammation, AHR, and fibrotic remodeling.Citation31) GMI, an immunomodulatory protein cloned from G. microsporum, has an ability to inhibit nuclear translocation of NF-κB p65.Citation32) In this study, the effect of FIP-fve in decreasing Der m-induced asthma and inflammation may inhibit nuclear translocation of NF-κB p65. In a recent study, a Der p 2-FIP-fve fusion protein (OsDp2Fve) was injected in mice and showed reduction of Der p 2-specific IgE and Th2 effector cytokines as well as Der p 2-specific IgG antibody production.Citation33) Local nasal immunotherapy (LNIT) with allergen-derived peptides and FIP-fve showed an immunotherapeutic effect on Der p 2-induced airway inflammation. After LNIT with Der p2 peptides (Der p2 28–40 and Der p2 28–40A) and FIP-fve, mixtures of both were able to inhibit Der p-induced airway hyper-responsiveness and inflammation.Citation34) Moreover, LNIT with recombinant Der p 2 (rDer p 2) in conjunction with FIP-fve was able to suppress rDer p 2-induced and even Der p-induced airway inflammation.Citation35) It was reported that non-IgE-mediated fragments of Der f 2 in conjunction with FIP-fve might have therapeutic effects on Treg cells from allergic individuals. It could up-regulate the percentage of IL-10 and TGF-β cells in Foxp3+CD4+CD25+ T cells only in D. farinae allergic individuals.Citation36) However, the DNA sequence of Der m has not been resolved; this will be determined based on the next generation sequencing technology in our future work. It was reported that mice immunized with dendritic cells (DCs) were protected from OVA aeroallergens challenge. The allergen-induced airway hyper-responsiveness, mucus production, and allergen-specific IgE and IgG1 were reduced in DC-immunized mice.Citation19) Heparin showed the ability to protect mice from Der p attack. Serum Der p-specific IgE level was lower in heparin-treated groups.Citation37) Moreover, anti-IgE antibody omalizumab (Xolair\[R\]) eliminated total IgE level in patients with allergic asthma.Citation38) In our study, total IgE level had no difference between Der m and FIP-fve/Der m group (Fig. (B)). It may indicate that Der m-specific IgE was reduced by FIP-fve with little effect in total IgE level. In addition, there may be a possibility that the suppression of allergic reaction is not associated with the decrease in antigen-specific IgE as often seen when allergic symptoms are improved. However, it is limited to detect Der m-specific IgE level so far.
Blocking toll-like receptor 4 (TLR4) with neutralizing antibodies suppressed rLZ-8-induced IL-12 p40 and IL-10 production of dendritic cells, suggesting that TLR4 plays an important role in signaling dendritic cells following incubation with rLZ-8.Citation39) FIP-fve has a high degree of amino acid sequence homology with LZ-8Citation11) and therefore may regulate anti-inflammatory factors through a similar mechanism. Moreover, FIP-fve is safer and more stable than other anti-inflammatory agents. In previous studies, FIP-fve was able to preserve immune activity even when taken orally and maintained immunomodulatory activity in an acidic environment (0.6 M hydrochloric acid, pH 2), although activity decreased following 5 M sodium hydrate (pH 13) treatment.Citation40) Furthermore, FIP-gts is an immunomodulatory protein from Ganoderma tsugae; it shows better immunomodulatory activity than FIP-fve. However, FIP-fve was more resistant to digestive enzymes in simulated gastric fluid and simulated intestinal fluid.Citation40)
In summary, our findings demonstrate that FIP-fve is a potent antagonist of Der m-induced AHR and inflammation. The data suggest that FIP-fve is a natural compound that can possibly serve as a healthy food or a pharmaceutical product for commercial development and for the suppression of HDM-mediated inflammatory and clinical syndromes.
Additional information
Funding
Notes
Abbreviations: AD, atopic dermatitis; Blo t, Blomia tropicalis; BALF, bronchoalveolar lavage fluid; CEBPA, CCAAT/enhancer-binding protein (C/EBP); Der f, Dermatophagoides farina; Der m, Dermatophagoides microceras; Der p, Dermatophagoides pteronyssinus; FIP, fungal immunomodulatory protein; G-CSF, granulocyte colony-stimulating factor; H&E, hematoxylin and eosin; HDM, house dust mite; hPBMCs, human peripheral blood mononuclear cells; AHR, hyper-responsiveness; AHR, hyper-responsiveness; IFN-γ, interferon-gamma; IL-13, interleukin 13; IL-4, interleukin 4; IL-5, interleukin 5; IL-6, interleukin 6; IL-8, interleukin 8; MIP-1α, macrophage inflammatory protein 1 alpha; OVA, ovalbumin; PEP, peak expiratory pressure; PIP, peak inspiratory pressure; PHD 1, prolyl hydroxylases 1; PHD 3, prolyl hydroxylases 3; SD, standard deviation; CXCL1, the chemokine (C-X-C motif) ligand 1; TGF-β, transforming growth factor beta; TNF-α, tumor necrosis factor alpha; Th1, type 1 t helper; Th2, type 2 t helper; VEGF-A, vascular endothelial growth factor A.
References
- Kannan AK, Sahu N, Mohanan S, Mohinta S, August A. IL-2-inducible T-cell kinase modulates TH2-mediated allergic airway inflammation by suppressing IFN-gamma in naive CD4+ T cells. J Allergy Clin Immunol. 2013;132:e811–e815.10.1016/j.jaci.2013.04.033
- Pulsawat P, Pitakpolrat P, Prompetchara E, Kaewamatawong T, Techakriengkrai N, Sirivichayakul S, Buranapraditkun S, Hannaman D, Ruxrungtham K, Jacquet A. Optimization of a Der p 2-based prophylactic DNA vaccine against house dust mite allergy. Immunol Lett. 2013;151:23–30.10.1016/j.imlet.2013.01.013
- Zock JP, Heinrich J, Jarvis D, Verlato G, Norback D, Plana E, Sunyer J, Chinn S, Olivieri M, Soon A, Villani S, Ponzio M, Dahlman-Hoglund A, Svanes C, Luczynska C, Indoor Working Group of the European Community Respiratory Health, S., II. Distribution and determinants of house dust mite allergens in Europe: the European Community Respiratory Health Survey II. J Allergy Clin Immunol. 2006;118:682–690.10.1016/j.jaci.2006.04.060
- Huang HW, Lue KH, Wong RH, Sun HL, Sheu JN, Lu KH. Distribution of allergens in children with different atopic disorders in central Taiwan. Acta Paediatr Taiwan. 2006;47:127–134.
- Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, Matsuda H, Matsuda A, Oboki K, Ohno T, Saito H, Nakae S, Sudo K, Suto H, Ichikawa S, Ogawa H, Okumura K, Takai T. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol. 2013;190:4489–4499.
- Warmbold C, Uliczka K, Rus F, Suck R, Petersen A, Silverman N, Ulmer AJ, Heine H, Roeder T. Dermatophagoides pteronyssinus major allergen 1 activates the innate immune response of the fruit fly Drosophila melanogaster. J Immunol. 2013;190:366–371.10.4049/jimmunol.1201347
- Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Nat Acad Sci U.S.A. 2010;107:2159–2164.10.1073/pnas.0904055107
- Choi JK, Oh HM, Lee S, Park JW, Khang D, Lee SW, Lee WS, Rho MC, Kim SH. Oleanolic acid acetate inhibits atopic dermatitis and allergic contact dermatitis in a murine model. Toxicol Appl Pharmacol. 2013;269:72–80.10.1016/j.taap.2013.03.001
- Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507.10.1146/annurev.physiol.010908.163200
- Ogino K, Zhang R, Takahashi H, Takemoto K, Kubo M, Murakami I, Wang DH, Fujikura Y. Allergic airway inflammation by nasal inoculation of particulate matter (PM2.5) in NC/Nga mice. PLoS ONE. 2014;9:e92710.10.1371/journal.pone.0092710
- Ko JL, Hsu CI, Lin RH, Kao CL, Lin JY. A new fungal immunomodulatory protein, Fip-fve isolated from the edible mushroom, Flammulina velutipes and its complete amino acid sequence. Eur J Biochem. 1995;228:244–249.10.1111/ejb.1995.228.issue-2
- Dauter Z, Dauter M. Anomalous signal of solvent bromides used for phasing of lysozyme. J Mol Biol. 1999;289:93–101.10.1006/jmbi.1999.2744
- Ko JL, Lin SJ, Hsu CI, Kao CL, Lin JY. Molecular cloning and expression of a fungal immunomodulatory protein, FIP-fve, from Flammulina velutipes. J Formos Med Assoc. 1997;96:517–524.
- Paaventhan P, Joseph JS, Seow SV, Vaday S, Robinson H, Chua KY, Kolatkar PR. A 1.7A structure of fve, a member of the new fungal immunomodulatory protein family. J Mol Biol. 2003;332:461–470.10.1016/S0022-2836(03)00923-9
- Ou CC, Hsiao YM, Wu WJ, Tasy GJ, Ko JL, Lin MY. FIP-fve stimulates interferon-gamma production via modulation of calcium release and PKC-alpha activation. J Agric Food Chem. 2009;57:11008–11013.10.1021/jf902725s
- Wang PH, Hsu CI, Tang SC, Huang YL, Lin JY, Ko JL. Fungal immunomodulatory protein from Flammulina velutipes induces interferon-gamma production through p38 mitogen-activated protein kinase signaling pathway. J Agric Food Chem. 2004;52:2721–2725.10.1021/jf034556s
- Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Nat Acad Sci U.S.A. 2011;108:284–289.10.1073/pnas.1005201108
- Hsieh KY, Hsu CI, Lin JY, Tsai CC, Lin RH. Oral administration of an edible-mushroom-derived protein inhibits the development of food–allergic reactions in mice. Clin Exp Allergy. 2003;33:1595–1602.10.1046/j.1365-2222.2003.01790.x
- Dubois A, Deruytter N, Adams B, Kanda A, Delbauve S, Fleury S, Torres D, Francois A, Petein M, Goldman M, Dombrowicz D, Flamand V. Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+ T lymphocytes in early life. J Immunol. 2010;185:884–891.10.4049/jimmunol.0903287
- Chang YC, Hsiao YM, Wu MF, Ou CC, Lin YW, Lue KH, Ko JL. Interruption of lung cancer cell migration and proliferation by fungal immunomodulatory protein FIP-fve from Flammulina velutipes. J Agric Food Chem. 2013;61:12044–12052.10.1021/jf4030272
- Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, Tournoy K, Louis R, Foidart JM, Noël A, Cataldo DD. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res. 2009;58:845–854.10.1007/s00011-009-0054-2
- Lee YT, Lee SS, Sun HL, Lu KH, Ku MS, Sheu JN, Ko JL, Lue KH. Effect of the fungal immunomodulatory protein FIP-fve on airway inflammation and cytokine production in mouse asthma model. Cytokine. 2013;61:237–244.10.1016/j.cyto.2012.09.024
- Kampe M, Stolt I, Lampinen M, Janson C, Stalenheim G, Carlson M. Patients with allergic rhinitis and allergic asthma share the same pattern of eosinophil and neutrophil degranulation after allergen challenge. Clin Mol Allergy. 2011;9:3.10.1186/1476-7961-9-3
- Wood LG, Baines kJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142:86–93.
- Huang WC, Chan CC, Wu SJ, Chen LC, Shen JJ, Kuo ML, Chen MC, Liou CJ. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J Ethnopharmacol. 2014;151:470–477.10.1016/j.jep.2013.10.065
- Yu M, Eckart MR, Morgan AA, Mukai K, Butte AJ, Tsai M, Galli SJ. Identification of an IFN-gamma/mast cell axis in a mouse model of chronic asthma. J Clin Invest. 2011;121:3133–3143.10.1172/JCI43598
- Bunting RA, Wertheimer J, Zhou Z, Cochlin K, Deutsch H, Raymond H, Yurkow E, Ogden CA, Kilgore K. In-depth assessment of acute and chronic house dust mite-induced asthma mouse models. J Inflamm (Lond). 2013;10:P2.
- Byrne AJ, Jones CP, Gowers K, Rankin SM, Lloyd CM. Lung macrophages contribute to house dust mite driven airway remodeling via HIF-1alpha. PLoS ONE. 2013;8:e69246.10.1371/journal.pone.0069246
- Miglino N, Roth M, Tamm M, Borger P. House dust mite extract downregulates C/EBPalpha in asthmatic bronchial smooth muscle cells. Eur Respir J. 2011;38:50–58.10.1183/09031936.00068010
- McGee HS, Stallworth AL, Agrawal T, Shao Z, Lorence L, Agrawal DK. Fms-like tyrosine kinase 3 ligand decreases T helper type 17 cells and suppressors of cytokine signaling proteins in the lung of house dust mite–sensitized and –challenged mice. Am J Respir Cell Mol Biol. 2010;43:520–529.10.1165/rcmb.2009-0241OC
- Tully JE, Hoffman SM, Lahue KG, Nolin JD, Anathy V, Lundblad LK, Daphtary N, Aliyeva M, Black KE, Dixon AE, Poynter ME, Irvin CG, Janssen-Heininger YM. Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. J Immunol. 2013;191:5811–5821.10.4049/jimmunol.1301329
- Lin CH, Hsiao YM, Ou CC, Lin YW, Chiu YL, Lue KH, Chang JG, Ko JL. GMI, a Ganoderma immunomodulatory protein, down-regulates tumor necrosis factor alpha-induced expression of matrix metalloproteinase 9 via NF-kappaB pathway in human alveolar epithelial A549 cells. J Agric Food Chem. 2010;58:12014–12021.10.1021/jf103068w
- Su CF, Kuo IC, Chen PW, Huang CH, Seow SV, Chua KY, Yu SM. Characterization of an immunomodulatory Der p 2-FIP-fve fusion protein produced in transformed rice suspension cell culture. Transgenic Res. 2012;21:177–192.10.1007/s11248-011-9518-6
- Liu YH, Kao MC, Lai YL, Tsai JJ. Efficacy of local nasal immunotherapy for Dp2-induced airway inflammation in mice: using Dp2 peptide and fungal immunomodulatory peptide. J Allergy Clin Immunol. 2003;112:301–310.10.1067/mai.2003.1619
- Liu YH, Tsai JJ. Production of salivary immunoglobulin A and suppression of Dermatophagoides pteronyssinus-induced airway inflammation by local nasal immunotherapy. Int Arch Allergy Immunol. 2005;138:161–168.10.1159/000088438
- Wu CC, Liao EC, Lee MF, Tsai JJ. Augmentation of regulatory T cells in allergic individuals by recombinant Der f 2 peptide with fungal immunomodulatory peptide fve. Ann Allergy Asthma Immunol. 2009;102:216–222.10.1016/S1081-1206(10)60084-1
- Fu LS, Tsai JJ, Chen YJ, Lin HK, Tsai MC, Chang MD. Heparin protects BALB/c mice from mite-induced airway allergic inflammation. Int J Immunopathol Pharmacol. 2013;26:349–359.
- Johansson SG, Nopp A, Öman H, Ankerst J, Cardell LO, Grönneberg R, Matsols H, Rudblad S, Strand V, Stålenheim G. The size of the disease relevant IgE antibody fraction in relation to ‘total-IgE’ predicts the efficacy of anti-IgE (Xolair) treatment. Allergy. 2009;64:1472–1477.10.1111/all.2009.64.issue-10
- Lin YL, Liang YC, Tseng YS, Huang HY, Chou SY, Hseu RS, Huang CT, Chiang BL. An immunomodulatory protein, Ling Zhi-8, induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and MAPK pathways. J Leukoc Biol. 2009;86:877–889.10.1189/jlb.0708441
- Ou C-C, Hsiao Y-M, Wang W-H, Ko J-L, Lin M-Y. Stability of fungal immunomodulatory protein, FIP-gts and FIP-fve, IFN-γ production. Food Agric Immunol. 2009;20:319–332.10.1080/09540100903247688
