Abstract
Chitinase hydrolyzes the β-1,4-glycosidic bond in chitin. In higher plants, this enzyme has been regarded as a pathogenesis-related protein. Recently, we identified a class III chitinase, which functions as a calcium storage protein in pomegranate (Punica granatum) seed (PSC, pomegranate seed chitinase). Here, we solved a crystal structure of PSC at 1.6 Å resolution. Although its overall structure, including the structure of catalytic site and non-proline cis-peptides, was closely similar to those of other class III chitinases, PSC had some unique structural characteristics. First, there were some metal-binding sites with coordinated water molecules on the surface of PSC. Second, many unconserved aspartate residues were present in the PSC sequence which rendered the surface of PSC negatively charged. This acidic electrostatic property is in contrast to that of hevamine, well-characterized plant class III chitinase, which has rather a positively charged surface. Thus, the crystal structure provides a clue for metal association property of PSC.
Graphical Abstract
Crystal structure of a class III chitinase with calcium accumulating property was solved. The negatively charged surface may enable the metal binding on it.
Chitinase (EC 3.2.1.14) catalyzes the hydrolysis of a β-1,4 glycosidic bond in chitin, which is a β-1,4-linked linear polymer of N-acetyl-D-glucosamine (NAG)n. This class of enzyme is ubiquitously distributed in a wide range of organisms from bacteria to higher plants and animals, and plays various roles, such as protecting from bacterial diseases and chitinous polysaccharide degradation.Citation1,2) According to the CAZy (carbohydrate-active enzymes) data base,Citation3) chitinases can be divided into two families, i.e. glycoside hydrolase (GH) family 18 (GH-18) and 19 (GH-19) based on their reaction mechanisms (retaining or inverting) and primary structure. Many higher plants also produce various chitinases. Although no endogenous substrate for plant chitinase has been found in plants, chitin is commonly found as an abundant component of fungal cell wall and the exoskeleton of arthropods. Since some of these chitin-containing organisms are defined as major pathogens against plants, plant chitinase is usually regarded as a pathogenesis-related protein.Citation4) Plant chitinases have been classified into three members, class I, II, and III by Shinshi et al., according to their primary structures.Citation5) More recently, they are further divided into seven classes, I–VII.Citation6) In general, class I and II chitinases possess GH-19 catalytic domain and α + β structure, whereas class III chitinase has GH-18 catalytic domain with (β/α)8 barrel folding. Judging from the primary structure, the plant class III chitinase shares some common characteristics, i.e. it possesses catalytic “DXDXE motif” in its sequence, conserved three disulfide bonds, and the folding rather similar to that of bacterial and fungal chitinases, in spite of poor sequence similarity. In addition, there is a protein family homologous to class III chitinase that does not possess the GH enzymatic activity, but functions as a carbohydrate-binding protein (lectin) or seed storage protein, such as concanavarin B,Citation7) nanovarin,Citation8) and chitinase like lectin (chi-lectin).Citation9) These members have similar primary structures and three-dimensional structure (TIM-barrel (β/α)8-fold) to that of the plant type III chitinase,Citation10) although the active site acidic residues are substituted.
Recently, we identified a new class III chitinase, which functions as calcium storage protein in pomegranate (Punica granatum) seeds. This pomegranate seed chitinase (PSC) is catalytically active and is capable of binding calcium ions with high capacity and low affinity. In the present study, to address the mechanism for the association between the PSC and metal ions, we have crystallized a recombinant PSC. The high-resolution X-ray crystallography analysis has revealed the metal co-ordination environment in PSC. The structural comparison of putative chitinase active site and substrate binding cleft is also discussed.
Materials and methods
Expression and purification of the recombinant PSC:
The procedure for cDNA cloning of PSC was described elsewhere.Citation11) The cDNA fragment encoding mature region of the protein without the N-terminal signal peptide was inserted into the NcoI/BamHI restriction site of pET21d vector (Novagen, San Diego, CA) using a primer set, (5′-GATCACCATGGGCGACATTGCCATTTAC-3′) and (5′-GACAGGATCCTCAAACTTGATCCTTTATGG-3′). The expression plasmids were transformed into the Escherichia coli strain OrigamiB (DE3) (Novagen, San Diego, CA). Protein expressions were performed at 20 °C after isopropyl-β-D-thiogalactopyranoside induction. In order to stabilize the expressed protein, calcium chloride was added to the medium to a final concentration of 2.5 mM. The cells were harvested and disrupted by sonication, followed by protein extraction with an extraction buffer (20 mM Tris–HCl, pH 7.8, 0.15 M NaCl) containing a protease inhibitor cocktail (Nacalai tesque, Kyoto, Japan). The recombinant PSC was expressed as soluble proteins, and was further purified by ammonium sulfate precipitation, anion exchange chromatography by Q-Sepharose (GE Healthcare, Piscataway, NJ), and size exclusion chromatography using a Superdex 200 pg 16/60 column (GE Healthcare). Concentrations of purified proteins were adjusted by the absorbance at 280 nm.Citation13)
Crystallization, data collection, and structure determination of PSC:
Initial crystals were obtained in few months using a crystallization screening kit (Crystal screen I; Hampton Research, Aliso Viejo, CA). The crystallization condition was optimized using the hanging-drop vapor-diffusion method. The optimized crystallization drops were prepared by mixing equal volumes of mother liquid (35% PEG3350, 0.1 M Tris–HCl pH 8.5, 0.2 M MgCl2) and protein solution containing 15 mg ml−1 of the recombinant PSC. Thin board-like monoclinic crystals (space group P21; a = 58.5, b = 80.0, c = 97.5 Å, β = 103°; VM = 1.92 Å3/Da for four subunits per an asymmetric unit) appeared in two weeks at 20 °C. Diffraction data on a native crystal were collected to 1.6 Å resolution at 100 K at the SPring-8 beamline BL38B1 after flush cooling. No cryoprotectant was needed for the crystals obtained in this condition. Diffraction data were processed, merged, and scaled with HKL-2000 (HKL Research, Charlottesville, CA).Citation14) Data processing statistics are shown in Table . The structure of PSC was determined by molecular replacement using the MolrepCitation15) program in CCP4i suite 1.4.4Citation16), and using the structure of fungal chitinase (PDB code 2XVP)Citation17) as a search model. Refinement was performed using the REFMAC5Citation18) and PHENIX programsCitation19,20). During the refinement with PHENIX, the metal–ligand distances were loosely restrained using the ReadySet program in the PHENIX package. The structure was visualized and rebuilt using COOT 0.6.2Citation21) and further modified on σ-weighted (2|Fo| − |Fc|) and (|Fo| − |Fc|) electron density maps. Figs. were produced by PyMOL (DeLano Scientific, San Carlos, CA). The secondary structure was assigned using DSSP program.Citation22) The images of the electrostatic potential of the protein surface were generated using the APBS application (Fig. ).
Table 1. Data collection and refinement statistics for the crystals of PSC.
Structural factors and coordinates have been deposited in the RCSB Protein Data Bank with accession codes, 4TOQ.
Results and discussion
Overall structure of PSC
The recombinant PSC was crystallized in the monoclinic (P21) crystal with four monomers in an asymmetric unit. The overall coordinate error of the final model was 0.15 Å. As shown in Table , root mean square deviations of bond lengths and angles from ideality were calculated to be 0.010 Å and 1.146°. These values are well within the accepted limits, indicating that the structure has tight stereochemical constraints. A Ramachandran plot of the final structure showed that 96.7% and 3.3% of all residues were in the most favored and allowed regions, respectively. Refinement statistics are shown in Table .
Since the PSC contains the signal peptide, the N-terminal 26 residues are absent from the mature protein.Citation11) Therefore, the 27th glycine from the initiated methionine is designated as the N-terminus as shown in Fig. . The final model is composed of chains A, B, C, and D. The chains A, C, and D contain complete amino acid residues from the N-terminus (Gly1) to the C-terminus (Val273), whereas the residues from 146 to 148 in chain B were missing because of the disorder. We have described the structure of PSC using the illustration of chain A in this article, unless it is specially denoted. The N-terminal Gly1 and the C-terminal Val273 positions adjacently that enables a weak interaction via hydrogen bond between the C-terminal oxygen and the N-terminal nitrogen at the distance of 3.3 Å (Fig. S1). The secondary structural assignment is shown in Fig. . The overall structure of PSC consists of ten β-strands and eight α-helices, of which β/α motifs form a TIM-barrel structure (Fig. (a)). Additionally, five short 310-helices composed of three residues are seen in the structure (Figs. , and (a)). Among the strands, eight parallel β-strands (β1–β8) form the core of the enzyme, while the eight α-helices form the outer surface of the enzyme. The overall structure of PSC is highly conserved among related plant proteins, such as hevamine (PDB ID, 2HVM), concanavalin B (1CNV), and chi-lectin from tamarind (4B16).The main chain rmsds between the PSC and hevamine, concanavalin B, and chi-lectin from tamarind were calculated as 0.688, 1.29, and 1.39 Å with 67.4, 26.4, and 28.9% sequence identities, respectively. The deduced active site (Asp125 and Glu127) and non-proline cis-peptide, which have a crucial role in substrate binding, are conserved in the PSC structure (Fig. (a)), suggesting that the PSC is active class III chitinase. In fact, we demonstrated the chitin hydrolytic activity of the PSC in our previous report,Citation11) the kcat/Km value of PSC is much lower than that of another active class III chitinase hevamine.Citation24,25)
Fig. 1. Primary structure of PSC and multiple sequence alignment of PSC with various plant chitinase families.
Note: The PDB accession IDs of hevamine, concanavalin B (conB), and tamarind chitin-binding lectin (chi-lectin) are 2HVM, 1CNV, and 4B15. Conserved residues are shown in black. Secondary structural elements are given with indicators, arrowheads, springs, and gray bars, which represent the α-helices, β-strands, and 310 helices, respectively. The residues forming the conserved cis-peptides are shown as #, while the catalytic center residues are shown as *.
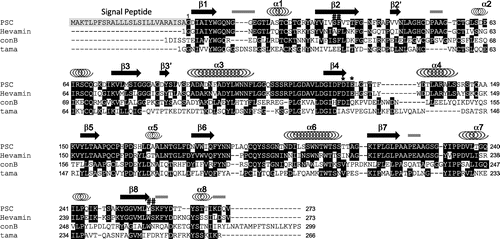
Fig. 2. Crystal structure of PSC.
Note: Overall structure of PSC is shown in different orientations, with the secondary structure assignment as follows: α-helices, β-strands, and 310 helices are shown as red, yellow, and blue, respectively. The secondary assignment is corresponding to that of Fig. .
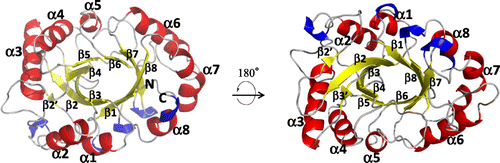
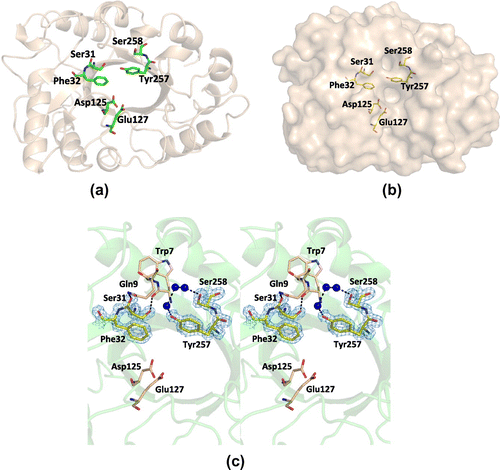
Fig. 3. The three-dimensional structure around the conserved cis-peptides.
Note: (a) The orientation of active site (Asp125 and Glu127) and cis-peptides (Ser31 and Phe32, and Tyr257 and Ser258). These amino acid residues are shown as sticks. (b) Surface representation of PSC. The active site and cis-peptides are shown as sticks near the active site cleft. (c) The detailed structure around the cis-peptides (stereo view). This region is stabilized by the interaction with adjacent residues, such as Trp7 and Gln9, and water molecules. The electron density of the 2|Fo| − |Fc| map for this site is contoured at 1.2σ and shown in blue mesh.
Activity center and conserved non-proline cis-peptide
The active site (Asp125 and Glu127) of PSC is well superimposable with that of hevamine, whereas the glutamate is substituted by glutamine in concanavalin B and both of the two residues are absent in the sequence of chi-lectin. On the other hand, the non-proline cis-peptides are seen in all the four members, although the amino acid sequences are not strictly conserved. The cis-peptides are formed between Ser31 and Phe32, and Tyr257 and Ser258 in PSC. The cis-peptides related Phe32 and Tyr257 of PSC are corresponding to Phe32 and Trp255 of hevamine, which is reported to form the substrate-binding cleft.Citation26) Similarly, these residues also form the cleft in PSC (Fig. (b)). These cis-conformations are stabilized by the hydrogen bond network around (Fig. (c)). As shown in Fig. (c), the cis-conformation is unambiguously determined in our structural model.
Metal association to PSC
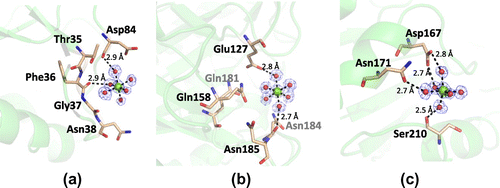
Originally, PSC has been identified as a high-capacity calcium binding protein. In accordance, the present crystal structure contained some metal binding sites in their molecular surfaces. We assigned magnesium to the major electron densities having proper coordination environment on the surface of PSC, because high concentration (0.2 M) of magnesium salts was contained in the crystallization condition. Since the PSC associates calcium ions with low affinity,Citation11,12) it is possible that magnesium ions can associate with PSC in such extreme conditions. There are at least 13 magnesium ions clearly identified in our structural model. Typical coordination environments of the associated magnesium ions are shown in Fig. (a)–(c). As shown in the figure, magnesium ions are coordinated by six ligands such as waters, resulting in octahedral bipyramidal shape. In these cases, coordinated water molecules are hydrogen-bonded to the oxygen atoms in the side chain or the main chain of amino acids. The distances from oxygen atoms to coordinated water molecules are in the range of 2.5–2.9 Å, while those from magnesium to coordinated water molecules are approximately 2.1 Å (Fig. (a)–(c)). Some other electron densities with the shape of water-coordinated metal were observed during the refinement process; however, we omitted them from the final coordinate file because of their low occupancies.
Fig. 4. Metal association property of PSC.
Note: (a)–(c) The structures of three magnesium-binding sites and their coordination environments. Magnesium ions are shown as green spheres, while the water molecules are shown as red spheres. The electron density of the 2|Fo| − |Fc| map for this site is contoured at 1.2σ and shown in blue mesh.
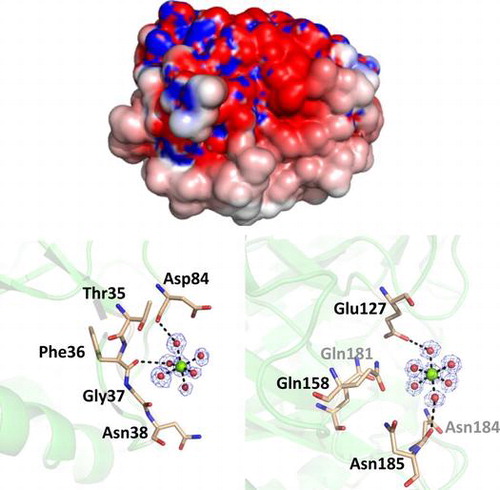
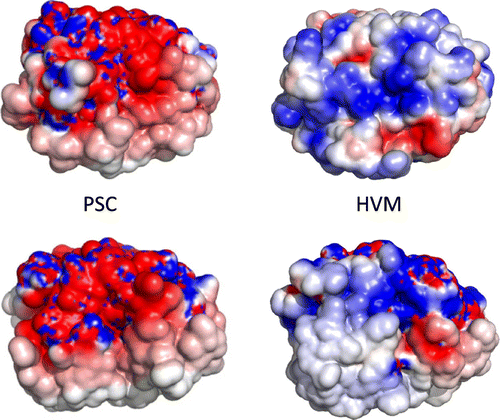
In our previous report, we discussed that the high amounts of aspartate residues contribute to the high capacity for calcium ions.Citation11) In fact, there are nine unconserved aspartate and two glutamate residues in the mature region of PSC (Fig. ). These PSC-specific acidic residues are Asp2, Asp21, Asp51, Asp62, Glu63, Asp71, Asp84, Glu90, and Asp94. All of these acidic residues are exposed to the surface of PSC and render the surface more acidic, in contrast to hevamine (Fig. ). Similar to the case of the PSC, a calcium storage protein calsequestrin also possesses the negatively charged surface, which contributes to its function of calcium sequestration. The difference in surface electrostatic potential seems not to affect the optimal pH in chitin hydrolytic activity, i.e. the optimal pH of PSC and hevamine are around 4.5, commonly. However, kinetic parameters are largely different from each other, e.g. the Km values are 48 μM for PSC (unpublished data) and 14.3 μM for hevamine,Citation25) and kcat/Km values are 0.12 M−1 s−1 for PSCCitation11) and 2.5–5.4 × 104 M−1 s−1 for hevamine, respectively. Although the molecular mechanism of the differences in the kinetic parameters and enzymatic property is not clear, it is possible that the difference in surface electrostatic potential affects the substrate affinity or association.
Fig. 5. The surface electrostatic potential of PSC (left) and hevamine (right) from two different orientations were rendered from the −2 (red; electronegative) to + 2 kT (blue, electropositive) range, where k is the Boltzmann constant and T is the absolute temperature (K).
Note: The orientations of upper and lower panels are corresponding to that of left and right panel in Fig. .
Conclusion
In this study, we determined the crystal structure of PSC, a class III chitinase with high calcium-binding capacity from pomegranate seed, at 1.6 Å resolution. The overall structure and structure of catalytic sites and cis-peptides essential for substrate association were well conserved with those of active class III chitinases. Further, some metal-binding sites and negatively charged surfaces were proven. However, the structural basis of high-capacity and low-affinity calcium-binding property is not fully unveiled, because this crystallization condition did not contain calcium ion. Currently, in order to clarify the molecular mechanism of calcium storage in PSC, we attempted to crystallize PSC in this condition with calcium.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.962475
Supplemental Materials
Download PDF (161.9 KB)Additional information
Funding
References
- Fukamizo T. Chitinolytic enzymes: catalysis, substrate binding, and their application. Curr. Protein Pept. Sci. 2000;1:105–124.10.2174/1389203003381450
- Adrangi S, Faramarzi MA. From bacteria to human: a journey into the world of chitinases. Biotechnol. Adv. 2013;31:1786–1795.10.1016/j.biotechadv.2013.09.012
- Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 2009;37:D233–D238.10.1093/nar/gkn663
- Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K. Plant chitinases. Plant J. 1993;3:31–40.10.1046/j.1365-313X.1993.t01-1-00999.x
- Shinshi H, Neuhaus JM, Ryals J, Meins F. Structure of a tobacco endochitinase gene: evidence that different chitinase genes can arise by transposition of sequences encoding a cysteine-rich domain. Plant Mol. Biol. 1990;14:357–368.10.1007/BF00028772
- Beintema JJ. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994;350:159–163.10.1016/0014-5793(94)00753-5
- Hennig M, Jansonius JN, Terwisscha van Scheltinga AC, Dijkstra BW, Schlesier B. Crystal structure of concanavalin B at 1.65 A resolution. An “inactivated” chitinase from seeds of Canavalia ensiformis. J. Mol. Biol. 1995;254:237–246.10.1006/jmbi.1995.0614
- Hennig M, Pfeffer-Hennig S, Dauter Z, Wilson KS, Schlesier B, Nong VH. Crystal structure of narbonin at 1.8 Å resolution. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1995;51:177–189.10.1107/S0907444994009807
- Patil DN, Datta M, Dev A, Dhindwal S, Singh N, Dasauni P, Kundu S, Sharma AK, Tomar S, Kumar P. Structural investigation of a novel N-acetyl glucosamine binding chi-lectin which reveals evolutionary relationship with class III chitinases. PLoS ONE. 2013;8:e63779.10.1371/journal.pone.0063779
- Terwisscha van Scheltinga AC, Hennig M, Dijkstra BW. The 1.8 Å resolution structure of hevamine, a plant chitinase/lysozyme, and analysis of the conserved sequence and structure motifs of glycosyl hydrolase family 18. J. Mol. Biol. 1996;262:243–257.10.1006/jmbi.1996.0510
- Yang H, Zhang T, Masuda T, Lv C, Sun L, Qu G, Zhao G. Chitinase III in pomegranate seeds (Punica granatum Linn.): a high-capacity calcium-binding protein in amyloplasts. Plant J. 2011;68:765–776.10.1111/j.1365-313X.2011.04727.x
- Lv C, Masuda T, Yang H, Sun L, Zhao G. High-capacity calcium-binding chitinase III from pomegranate seeds (Punica granatum Linn.) is located in amyloplasts. Plant Signal. Behav. 2011;6:1963–1965.
- Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423.10.1002/pro.v4:11
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromol. Crystallogr, Part A. 1997;276:307–326.
- Vagin A, Teplyakov A. MOLREP : an automated program for molecular replacement. J. Appl. Crystallogr. 1997;30:1022–1025.10.1107/S0021889897006766
- Potterton E, Briggs P, Turkenburg M, Dodson E. A graphical user interface to the CCP 4 program suite. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2003;59:1131–1137.10.1107/S0907444903008126
- Rush CL, Schüttelkopf AW, Hurtado-Guerrero R, Blair DE, Ibrahim AF, Desvergnes S, Eggleston IM, van Aalten DM. Natural product-guided discovery of a fungal chitinase inhibitor. Chem. Biol. 2010;17:1275–1281.10.1016/j.chembiol.2010.07.018
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1997;53:240–255.10.1107/S0907444996012255
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2002;58:1948–1954.10.1107/S0907444902016657
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Echols N, Headd JJ, Hung L-W, Jain S, Kapral GJ, Kunstleve RWG, McCoy AJ, Moriarty NW, Oeffner RD, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106.10.1016/j.ymeth.2011.07.005
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004;60:2126–2132.10.1107/S0907444904019158
- Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637.10.1002/(ISSN)1097-0282
- Holst MJ, Saied F. Numerical solution of the nonlinear Poisson–Boltzmann equation: developing more robust and efficient methods. J. Comput. Chem. 1995;16:337–364.10.1002/(ISSN)1096-987X
- Bokma E, Barends T, Terwisscha van Scheltinga AC, Dijkstra BW, Beintema JJ. Enzyme kinetics of hevamine, a chitinase from the rubber tree Hevea brasiliensis. FEBS Lett. 2000;478:119–122.10.1016/S0014-5793(00)01833-0
- Bokma E, Rozeboom HJ, Sibbald M, Dijkstra BW, Beintema JJ. Expression and characterization of active site mutants of hevamine, a chitinase from the rubber tree Hevea brasiliensis. Eur. J. Biochem. 2002;269:893–901.10.1046/j.0014-2956.2001.02721.x
- Terwisscha van Scheltinga AC, Kalk KH, Beintema JJ, Dijkstra BW. Crystal structures of hevamine, a plant defence protein with chitinase and lysozyme activity, and its complex with an inhibitor. Structure. 1994;2:1181–1189.10.1016/S0969-2126(94)00120-0
- Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang CH. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 1998;5:476–483.10.1038/nsb0698-476
- Sanchez EJ, Lewis KM, Danna BR, Kang C. High-capacity Ca2+ binding of human skeletal calsequestrin. J. Biol. Chem. 2012;287:11592–11601.10.1074/jbc.M111.335075
