Abstract
The effects of modern dressings on inflammation, which represent the earliest phase of wound healing, are poorly understood. We investigated the effects of modern hydrocellular foam dressings (HCFs) on wound healing and on the gene expression levels of the inflammatory markers—interleukin (IL)-1β, IL-6, and IL-10—in rat periwound skin and granulation tissue by quantitative reverse transcription-polymerase chain reaction. HCF absorbed significantly higher volume of water than hydrocolloid dressing (HCD) and increased the contraction of wounds. Polymorphonuclear neutrophils were massively infiltrated to the wound edge and boarded between granulation and dermis in the HCD group. IL-1β, IL-6, and IL-10 mRNA levels were significantly decreased in the periwound skin around the wounds and granulation tissue covered with HCF. These findings suggest that HCF may promote wound healing along with decrease in inflammation by reducing gene expression levels of IL-1β, IL-6, and IL-10.
Graphic·Abstract
HCF promotes wound healing along with decreases in inflammation by reducing gene expression levels of IL-1β, IL-6 and IL-10 in rat periwound skin and granulation tissue.
Modern wound dressings provide a moist environment for wound healing. This quite new concept, which was widely recognized in the 1960s,Citation1) has been made possible in clinical practice by the development of commercially available wound dressings. Many different types of dressings are recommended for wound management. Some researchers introduced the concept of interactive dressings that can alter the local wound environment, e.g. alginates, collagen films, foams, hyaluronic acid products, hydrocolloids, and hydrogels.Citation2) These dressings are either occlusive or semi-occlusive, and result in the accumulation of water vapor at the wound surface, which helps maintain a moist wound environment.
Hydrocellular foam dressings (HCFs) are recently being used as the first choice of dressing for the treatment of moderate to heavily exuding wounds, which require excessive wound fluid management to provide optimum conditions at the wound site, while maintaining a moist wound environment.Citation3) The contact layer is non-adherent, so the foam dressing will not stick to the sores. The waterproof outer layer aids in the prevention of bacterial contamination. Although these two major types of dressings are widely used in clinical settings, there is currently no consensus regarding which dressing is superior for managing wounds.
Wound healing involves three major sequential phases: inflammation; proliferation, and remodeling. Inflammation, the earliest phase, is considered as a critical period for wound healing. Inflammatory cytokines such as interleukin (IL)-1β, IL-6, and IL-10 mediate the cellular responses. It is generally accepted that excessive inflammation can retard the wound healing process, and optimization of this process is therefore important.Citation4,5) The second phase of wound healing, which is migration and proliferation, starts with a reduction in the inflammatory activities of immune cells and accompanying the migration of several types of cells to the wound, including endothelial cells, fibroblasts, and keratinocytes. The subsequent proliferation of these cells repair the wound with the induction of angiogenesis and granulation. The final remodeling stage occurs with the re-differentiation of keratinocytes to form epidermal barrier.Citation6) Furthermore, Kunugiza et al.Citation7) suggested that a polyurethane foam dressing limited the scab formation and accelerated the new vessel formation in granulation tissue, leading to the promotion of wound healing. However, the effects of these modern dressings on the inflammation phase of wound healing are poorly understood. A better understanding of the effects of wound dressings on the inflammatory response will facilitate the scientific choice of the optimal dressings to promote the wound healing. We therefore focused on the HCF-induced wound healing and its effects on gene expression levels of the inflammatory markers IL-1β, IL-6, and IL-10.
In the present study, we used an animal model to examine the effects of HCFs on wound healing at the molecular level by measuring gene expression levels of IL-1β, IL-6, and IL-10 in periwound skin and granulation tissue by using quantitative reverse transcription-polymerase chain reaction (RT-PCR).
Materials and methods
Materials
The HCF dressing ALLEVYN Non-Adhesive, a HCF dressing that consists of hydrophilic polyurethane and polyethylene glycol, was purchased from Smith & Nephew Medical Ltd. (Hull, UK), and the hydrocolloid dressing (HCD) Duoactive CGF was purchased from Conva Tec Japan Co. Ltd. (Tokyo, Japan). The SV Total RNA Isolation System was purchased from Promega Corp. (Madison, WI, USA), and the High-Capacity cDNA Reverse Transcription Kit and the SYBR Green PCR Master Mix were obtained from Applied Biosystems (Foster City, IN, USA).
Water absorptivity
Water absorptivities of HCD and HCF were determined by gravimetric method. Initially, these dressings were cut into pieces (a diameter of 8 mm) and their dry weights were accurately measured. Each dressing was immersed in a plate containing 1 ml of pure water and incubated for 30 min. The wet weight of these dressings was measured after the removal of excess surface water in the plate. We calculated the water absorption by subtracting their dry weights from their wet weights. HCD was used as the control which is to be widely used for managing open wounds in clinical settings.Citation8)
Animals
Male specific-pathogen-free nine-week-old Wistar rats were purchased from Japan SLC Inc. (Shizuoka, Japan). Animals were given food and ultrafiltered water ad libitum, and were maintained on a 12-h/12-h light/dark cycle.
Full-thickness cutaneous wound model
Two full-thickness wounds, with a diameter of 1.5 cm, were created in the dorsolateral skin using sterile scissors, under sedation by an intraperitoneal injection of Somnopentyl (pentobarbital sodium; Kyoritsu Seiyaku Corporation, Tokyo, Japan) (30 mg/kg body weight). The subcutaneous fat layer was completely dissected to expose the fascia. Each wound was covered with either HCF or HCD. HCD was used as the control to maintain moist environment and suppress scab formation. The dressings were randomly assigned to each side in individual rats. A film dressing was also used as a secondary dressing to keep the HCF and HCD attached to the wound. After wounding the wound area was measured every day until 6 days using an image analysis software (IMAGEJ version 1.42; NIH, Bethesda, MD), and the results were expressed relative to the initial wound area. Periwound skin (5 mm thick, taken from the edge of the wound) and granulation tissue samples were collected on day 3 under anesthesia and were used to prepare RNA. The experimental protocol was approved by the Animal Research Committee of the University of Tokyo. All animals were treated according to the Guide for the Care and Use of Laboratory Animals of the NIH.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from the rat skin and granulation tissue using the SV Total RNA Isolation System. Reverse transcription was performed using a High-capacity cDNA Reverse Transcription Kit. The amplification products obtained were detected using a SYBR Green PCR Master Mix and real-time RT-PCR (Stratagene MX 3000P, Agilent Technologies, Japan). Amplifications were performed under the following conditions: 40 cycles at 95 °C for 30 s and 60 °C for 1 min after preheating at 95 °C for 10 min. The relative expression levels of each target gene were calculated using the Ct method using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as an internal control. The sequences of the IL-1β, IL-6, IL-10, and GAPDH primers were as follows: IL-1β forward, 5′-GCAGCTTTCGACAGTGAGGA-3′ and IL-1β reverse, 5′-ACAGCTTCTCCACAGCCACA-3′ (GenBank accession no.: NM_031512.2); IL-6 forward, 5′- AGTCGGAGGCTTAATTACAT-3′ and IL-6 reverse, 5′-TTGGAAGCATCCATCATTTC-3′ (GenBank accession no.: NM_012589.2); IL-10 forward, 5′-GCCAAGCCTTGTCAGAAATG-3′ and IL-10 reverse, 5′-TTCTTCACCTGCTCCACTGC-3′ (GenBank accession no.: NM_012854.2); GAPDH forward, 5′-TGGTGAAGGTCGGTGTGAAC-3′ and GAPDH reverse, 5′-GACTGTGCCGTTGAACTTGC-3′ (GenBank accession no.: NM_017008.4).
Histologic analysis
The wound specimens, including the wound edge and the surrounding skin, were excised during post-wounding on day 3 and fixed in 4% paraformaldehyde diluted with 0.1 M phosphate buffer (pH 7.4) for light microscopic observations. After fixation, light microscopic samples were dehydrated in a graded series of ethanol and embedded in paraffin. Serial sections (5-μm thick) were cut and stained with hematoxylin and eosin.
Statistical analysis
Results are expressed as mean ± SE. Repeated measures of analysis of covariance with the Student-Newman-Keuls test was performed at each time to compare the differences in wound area between the sides covered with HCD and HCF. Differences in gene expression between the two groups were determined using Student’s t-test. The significance level was set at p < 0.05.
Results
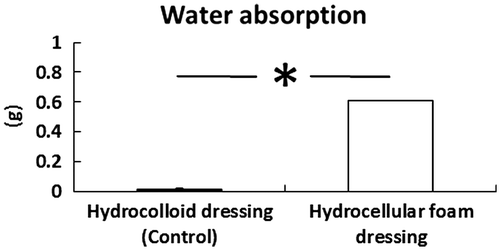
Effects of HCF on water absorption
The effects of HCF on water absorption were investigated. HCF absorbed significantly higher volume of water than HCD (Fig. ).
Effects of HCF on wound healing in rat skin
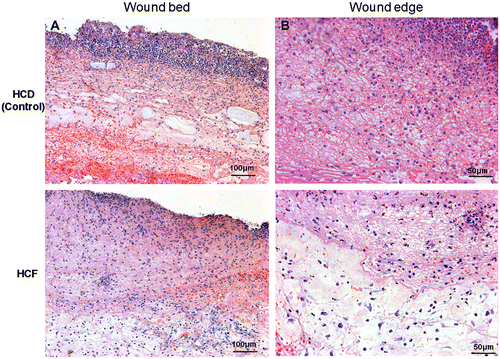
The time-course effects of HCF on wound healing in rat skin are shown in Fig. (A) and (B). When compared with those treated with HCD, gross observations revealed increased contraction of wounds in those treated with HCF and we did not observe redness in the periwound skin and adverse skin events caused due to the removal of these dressings (Fig. (A)). We detected significant differences in the wound size on days 4 and 6 post-wounding (Fig. (B)). Massive infiltration of polymorphonuclear neutrophils at the wound edge and the border between the granulation tissue and the dermis, and inflammatory edematous changes were seen in the wound bed in the HCD group (Fig. ).
Fig. 2. Effects of HCF on wound healing in rat skin.
Note: Gross observations (A) and relative wound areas and (B) revealed increased wound contraction induced by HCF, compared with HCD. Results are expressed as mean ± SE (n = 3). *p < 0.05 indicates the significant difference between wounds covered by HCD and HCF.
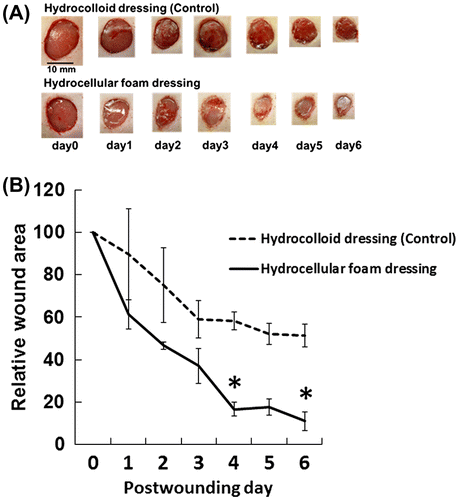
Fig. 3. Histologic analysis of the wound edge and the wound bed.
Note: Periwound skin and granulation tissue samples were collected on day 3 and used to assess histologic analysis. Hematoxylin and eosin staining showed severe inflammation and edema in the wound edge (A) and the wound bed (B) of the HCD group, compared with the HCF group. Magnification in (A) × 10, (B) × 20.
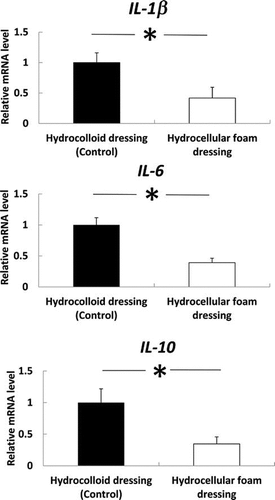
Effects of HCF on gene expression levels of IL-1β, IL-6, and IL-10 in periwound skin
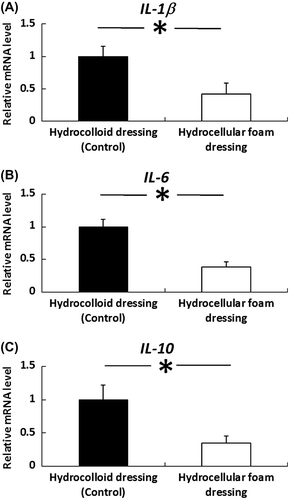
The effects of HCF on the gene expression levels of IL-1β, IL-6, and IL-10 in periwound skin were investigated. IL-1β, IL-6, and IL-10 mRNA levels were significantly decreased in periwound skin around the wounds covered with HCF, compared with those covered with HCD (Fig. ).
Fig. 4. Effects of HCF on the gene expression levels of IL-1β, IL-6 and IL-10 in the skin around the wound.
Note: Periwound skin samples were collected on day 3 and used to assess the gene-expression levels of IL-1β, IL-6, and IL-10. IL-1β, IL-6, and IL-10 mRNA levels in the periwound skin were measured by quantitative RT-PCR and expressed as values relative to GAPDH. Results are expressed as mean ± SE (n = 4). *p < 0.05 the indicates significant difference between HCD and HCF.
Effects of HCF on gene expression levels of IL-1β, IL-6, and IL-10 in granulation tissue
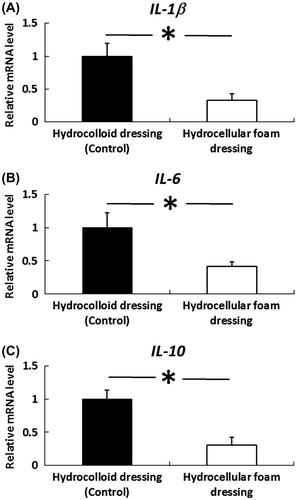
The effects of HCF on the gene expression levels of IL-1β, IL-6, and IL-10 in granulation tissue were investigated. IL-1β, IL-6, and IL-10 mRNA levels were also significantly decreased in granulation tissue covered with HCF, compared with those covered with HCD (Fig. ).
Fig. 5. Effects of HCF on the gene expression levels of IL-1β, IL-6, and IL-10 in the granulation tissue.
Note: Granulation tissue samples were collected on day 3 and used to assess the gene-expression levels of IL-1β, IL-6, and IL-10. IL-1β, IL-6, and IL-10 mRNA levels in granulation tissue were measured by quantitative RT-PCR and expressed as values relative to GAPDH. Results are expressed as mean ± SE (n = 4). *p < 0.05 indicates significant difference in rat granulation tissue between HCD and HCF.
Discussion
HCF wound dressings induce a moist environment that has been reported to promote wound healing.Citation9) However, the effects of these modern dressings on the inflammation phase of wound healing are poorly understood. The present study therefore examined the effects of HCF on wound healing, and on the gene expression levels of inflammatory cytokines such as IL-1β, IL-6, and IL-10. These results provide the first evidence to indicate that HCF promotes wound healing by reducing the gene-expression levels of IL-1β, IL-6, and IL-10 in rat periwound skin and granulation tissue, compared with HCD.
HCF consists of sponge-like polyurethane with a high water absorption rate. In the present study, HCF absorbed significantly higher volume of water than HCD. Our data also demonstrated increased wound contractions in the wounds treated with HCF, compared with those treated with HCD, suggesting that the different retention forms of the exudate may cause different changes in cytokine and/or growth factor levels on the wound surface.
Our results clearly demonstrated the acceleration of wound healing by the use of HCF along with reduced inflammatory markers. We investigated the gene-expression levels of inflammatory cytokines such as IL-1β, IL-6, and IL-10 in rat periwound skin and granulation tissue, which are responsible for wound inflammation. In the initial stages of wound healing, immune cells clear foreign pathogens and produce proinflammatory cytokines.Citation10,11) Sato and OhshimaCitation12,13) suggested that IL-1β, IL-6, and IL-10 were expressed in macrophages during skin wound healing. While cytokines and growth factors seem to be necessary for wound healing, excessive levels of inflammatory cytokines have been detected in non-healing wounds.Citation5,6) In the present study, HCF decreased IL-1β, IL-6, and IL-10 mRNA levels in the skin around the wound. We observed that the mRNA levels of IL-1β, IL-6, and IL-10 were substantially lower around the wounds covered with HCFs, compared to those covered with film dressings (data not shown). Overall, these results suggest that HCFs have beneficial effects on inflammation, which represents the earliest phase of wound healing, in both periwound skin and granulation tissue of full-thickness wounds. Further studies are needed to determine the role of exudate retention manner by investigating the direct effects of the exudate retained on the wound surface of the cells related to wound healing. Surprisingly, epithelialization was already observed in the inflammatory phase in the HCF group, which may be partly responsible for improved wound healing. Further investigation of the molecular mechanisms responsible for this phenomenon would promote the understanding of new modes of wound healing offered by HCF dressings.
In summary, we demonstrated that HCF may promote wound healing that is associated with the decrease in IL-1β, IL-6, and IL-10 mRNA levels in rat periwound skin and granulation tissue, compared with HCD. HCF-induced changes, in cytokine and/or growth factor levels at the wound surface, may contribute to the decrease in IL-1β, IL-6, and IL-10 gene expression levels because our previous studies showed that HCF changed the composition of cytokine and/or growth factor in the wound fluid at the wound surface by absorbing the fluid (data not shown). The results of the present study will help to identify novel pharmacological targets, which can protect against inflammatory tissue damage and promote tissue recovery, and contribute to the elucidation of the mechanisms underlying the effects of HCF-induced moist environments on wound healing and further the practice of evidence-based wound care.
References
- Hinman CD, Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature. 1963;200:377–378.10.1038/200377a0
- Kondo S, Kuroyanagi Y. Development of a wound dressing composed of hyaluronic acid and collagen sponge with epidermal growth factor. J. Biomater. Sci. Polym. Ed. 2012;23:629–643.10.1163/092050611X555687
- Banks V, Bale S, Harding K, Harding EF. Evaluation of a new hydrocellular foam dressing. J. Wound Care. 1997;6:266–269.
- Garner WL, Karmiol S, Rodriguez JL, Smith DJ Jr, Phan SH. Phenotypic differences in cytokine responsiveness of hypertrophic scar versus normal dermal fibroblasts. J. Invest. Dermatol. 1993;101:875–879.10.1111/jid.1993.101.issue-6
- Cooney R, Iocono J, Maish G, Smith JS, Ehrlich P. Tumor necrosis factor mediates impaired wound healing in chronic abdominal sepsis. J. Trauma. 1997;42:415–420.10.1097/00005373-199703000-00008
- Bello YM, Phillips TJ. Recent advances in wound healing. JAMA. 2000;283:716–718.10.1001/jama.283.6.716
- Kunugiza Y, Tomita T, Moritomo H, Yoshikawa H. A hydrocellular foam dressing versus gauze: effects on the healing of rat excisional wounds. J. Wound Care. 2010;19:10–14.10.12968/jowc.2010.19.1.46093
- Heyneman A, Beele H, Vanderwee K, Defloor T. A systematic review of the use of hydrocolloids in the treatment of pressure ulcers. J. Clin. Nurs. 2008;17:1164–1173.10.1111/j.1365-2702.2007.02218.x
- Salisbury RE, Bevin AG, Dingeldein GP, Grisham J. A clinical and laboratory evaluation of a polyurethane foam. Arch. Surg. 1979;114:1188–1192.10.1001/archsurg.1979.01370340094016
- Simpson DM, Ross R. The neutrophilic leukocyte in wound repair. J. Clin. Invest. 1972;51:2009–2023.10.1172/JCI107007
- Ross R, Odland G. Human wound repair. II. Inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. J. Cell Biol. 1968;39:152–168.10.1083/jcb.39.1.152
- Sato Y, Ohshima T. The expression of mRNA of proinflammatory cytokines during skin wound healing in mice: a preliminary study for forensic wound age estimation (II). Int. J. Legal Med. 2000;113:140–145.10.1007/s004140050285
- Sato Y, Ohshima T, Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem. Biophys. Res. Commun. 1999;265:194–199.10.1006/bbrc.1999.1455