Abstract
Increased abnormal oocytes due to meiotic chromosome misalignment and spindle defects lead to elevated rates of infertility, miscarriage, and trisomic conceptions. Here, we investigated the effect of biotin deficiency on oocyte quality. Three-week-old female ICR mice were fed a biotin-deficient or control diet (0, 0.004 g biotin/kg diet) for 21 days. On day 22, these mouse oocytes were analyzed by immunofluorescence. Due to biotin, undernutrition increased the frequency of abnormal oocytes (the biotin deficient vs. control: 40 vs. 16%). Next, the remaining mice in the biotin-deficient group were fed a control or biotin-deficient diet from day 22 to 42. Although biotin nutritional status in the recovery group was restored, the frequency of abnormal oocytes in the recovery group was still higher than that in the control group (48 vs. 18%). Our results indicate that steady, sufficient biotin intake is required for the production of high-quality oocytes in mice.
Graphical Abstract
Biotin-deficient diet induces chromosome misalignment and spindle defects in mouse oocytes that persist even after biotin is re-administered.

Key words:
No good-quality oocytes (chromosomal and spindle abnormalities oocytes) are the major factors in infertility, fetal loss (miscarriage), and conceptions, resulting in birth defects such as trisomy 21 (Down syndrome).Citation1) Recent studies have shown that macronutrients affect the oocyte maturationCitation2,3) and that high-fat diets in mice induce the formation of abnormal oocytes that has chromosome misalignment and spindle defects.Citation2) Caloric restriction without malnutrition, depressed the aging-related increase in the oocyte aneuploidy and chromosomal misalignment.Citation4)
Biotin deficiency affects fetal development and growth.Citation5) Biotin is a water-soluble vitamin that acts as a prosthetic group of carboxylases,Citation6) regulates gene expression,Citation7,8) and has a wide range of effects on the systemic processes such as development of embryo,Citation9,10)immunity,Citation11) growth,Citation12) and metabolism.Citation13) Biotin deficiency in experimental animals causes growth retardation, alopecia, dermatitis, and neurological impairment.Citation14) Several studies in rodents have reported that biotin deficiency also affects fetal development,Citation5) with biotin deficiency during pregnancy being found to increase the rates of abnormal fetal development and growth, as well as affect the rates of absorption and embryonic death in rodents.Citation5,15,16)
Báez-Saldaña et al.Citation17) also reported that biotin deficiency in young female mice induced ovary atrophy, estrus arrest, reduction in the oocyte of primordial and graafian follicles, and increase in serum estradiol, a hormone that was involved in oocyte growth. This study suggests that biotin deficiency decreased oocyte growth in the ovaries, but the effect of biotin deficiency on oocyte quality remained unclear. To our knowledge, however, no study has directly examinedthe effects of biotin deficiency on oocyte quality.
Here, we investigated the direct effect of biotin deficiency and its subsequent restoration on oocyte quality.
Materials and methods
Mice
Animals were allowed free access to food and water, and body weight was measured for every two days. Food intake of mice in metabolic cages was measured daily. Temperature was maintained at approximately 20 °C with 60% humidity and a 12-h light/dark cycle (lights on at 6:00 and off at 18:00). The care and treatment of the experimental animals conformed to the guidelines of the ethical treatment of laboratory animals set by the University of Shiga Prefecture (Shiga, Japan).
Deficiency experiment
Female ICR mice (3 weeks old) were obtained from Charles River Laboratories (Tokyo, Japan) and immediately divided into two groups (control group, n = 21; biotin-deficient group, n = 30). For each group, approximately half (control group, n = 11; biotin-deficient group, n = 15) were individually housed in metabolic cages (LC-0335; CLEA Japan, Tokyo, Japan) to collect 24 h urine samples and half (control group, n = 10; biotin-deficient group, n = 15, five mice/plastic cage) in plastic cages. On day 22, mice in metabolic cages (n = 5 per group) were sacrificed to analyze the oocyte quality, while those in plastic cages (n = 5 per group) were sacrificed to analyze the biotin concentrations in the liver, ovaries, and uterus.
Recovery experiment
On day 22, the remaining mice of the biotin-deficient group in metabolic cages (n = 10) and plastic cages (n = 10) were randomly divided into two groups. Group 1 consisted of mice in individual metabolic cages (n = 5) and one plastic cage (n = 5) that maintained a biotin-deficient diet. Group 2 (the recovery group) consisted of mice in individual metabolic cages (n = 5) and one plastic cage (n = 5) that were fed a control diet for 20 days. The remaining mice in individual metabolic cages (n = 6) and one plastic cage (n = 5) were continuously fed the control diet. On Day 42, the mice housed in metabolic cages were sacrificed to analyze the oocyte quality, and five mice housed in plastic cages were sacrificed to analyze the biotin concentrations in the liver, uterus, and ovaries.
Diet
The control group was fed 30% egg white solids diet supplemented with 0.004 g biotin/kg diet, while the biotin-deficient group was fed 30% egg white solids diet (Table ), as previously described.Citation17) Egg white solids were obtained from CLEA Japan, Inc. (Tokyo, Japan), which contains avidin that forms a non-absorbable complex with biotin in the alimentary tract and results in biotin deficiency. So a sufficient quantity of biotin was therefore added to the control diet.
Table 1. Diet compositions.
Oocyte retrieval and classification
To get oocytes, mice which were randomly selected on day 20 or day 40 were superovulated with an intraperitoneal injection of 5 IU pregnant mare serum gonadotrophin (PMSG; product No. E164A; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) followed by 5 IU human chorionic gonadotrophin (hCG; product No. E801A; ASKA Pharmaceutical) after 46 to 48 h. Oocytes were collected 18 h after hCG injection in EmbryoMax® FHM HEPES buffered medium (FHM; Millipore Corp., Billerica, MA, USA). Retrieved oocytes were denuded of cumulus cells by 5% hyaluronidase, (Sigma-Aldrich Inc., Tokyo, Japan) for 5 min at room temperature. Oocytes were counted and classified using a Hoffman light microscope as mature metaphase II (MII) or dead (condensed and fragmented cytoplasm). Oocytes from the experimental groups were analyzed in the blind study.
Immunofluorescence
Oocytes were collected from the control group (oocyte number; n = 101) and the biotin-deficient group (oocyte number; n = 123) on day 22, and from the control group (oocyte number; n = 240), the recovery group (oocyte number; n = 134), and the biotin-deficient group (oocyte number; n = 158) on day 42. Oocytes were collected by inducing superovulation and then puncturing the oviducts with forceps at which point they were washed in PBS and then moved in 5% protease (Sigma–Aldrich Inc., Tokyo, Japan) to soften and remove the zona pellucida. Oocytes were then extensively washed with PBS and fixed in PBS containing 4% paraformaldehyde for 15 min at room temperature. Oocytes were permeabilized with 0.2% Triton X-100 in PBS for 20 min at room temperature and blocked for 1 h in 5% goat serum (Sigma-Aldrich Inc., Tokyo, Japan) in PBST (0.05% Tween20, PBS) at room temperature. Oocytes were then washed with PBST and incubated for 1 h in a 1:4000 dilution of mouse anti-α-tubulin antibody (Cell Signaling Technology, Inc., Danvers, MA, USA) in PBST containing 5% goat serum at room temperature, after which they were washed again and incubated for 1 h in a 1:500 dilution of goat anti-mouse IgG with Alexa Flour-488 and 4′6-diamidino-2-phenylindole dihydrochloride (DAPI) (Dojindo Laboratories, Kumamoto, Japan). After washing, oocytes were mounted using PermaFluor aqueous mounting medium (Thermo Fisher Scientific Inc. Kanagawa, Japan) and analyzed by confocal fluorescence microscopy (FV10i; Olympus, Inc., Tokyo, Japan). Oocytes were classified as abnormal if: (1) the chromosomes failed to align on an otherwise normal meiotic spindle; (2) the spindle exhibited serious malformations; or (3) both spindle and chromosome alignment were normal, but one more pair of chromosomes was far removed from the spindle equator, most commonly behind one spindle pole. Oocytes with barrel-shaped bipolar spindles and distinct and well-organized microtubule fibers, along with tightly aligned chromosomes on the metaphase plate, were classified as normal.
Biotin assay in urine, liver, plasma, uterus, and ovaries
Twenty-four hour urine samples on day 19 and day 39 were collected from mice in metabolic cages with amber bottles containing 1 mL of 1 mol/L HCl and were stored at −30 °C until use. To avoid the effect of superovulation on metabolism in mice, we collected a 24 h urine sample prior to PMSG injection. Biotin concentrations in urine samples on day 19 and day 39, and in the liver, uterus, and ovaries at day 22 and day 42 were assayed via a microbiological method with Lactobacillus plantarum ACTT 8014.Citation18)
Measurement of 3-hydoroxyisovaleric acid in urine
In rodents and humans, urinary excretion of 3-hydroxyisovaleric acid (3-HIA) is an early and sensitive indicator of biotin deficiency.Citation19–23) Concentrations of 3-HIA, a metabolite of L-leucine in 24 h urine samples on day 19 and day 39 were measured via high-performance liquid chromatography (HPLC) in accordance with the method described by Watanabe et al.Citation19) The 3-HIA (Tokyo chemical industry Co., Ltd,. Tokyo, Japan) derivatized with 2-nitiophenylhydrazine hydrochloride (Tokyo chemical industry Co., Ltd. Tokyo, Japan) was detected using the HITACHI HPLC system (Hitachi, Ltd. Tokyo, Japan) and the HPLC column was a YMC-Pack FA (particle size 5 μm, 250 × 6.0 mm) (YMC Co., Ltd., Kyoto, Japan).
Serum estradiol analyses
On day 22 and day 42, blood samples were collected into EDTA-2Na tubes (Terumo Co., Ltd., Tokyo, Japan) from the carotid artery. The collected samples were centrifuged at 1700 × g for 30 min at 4 °C to obtain plasma samples which were stored at −80 °C until use.
Estradiol concentration was determined in mice plasma on day 22 and day 42 using an enzyme-linked immunosorbent assay (ELISA) kit (catalog No. 582251; Cayman Chemical Company, Ann Arbor, MI, USA) in accordance with the manufacturer’s instructions.
Estrus cycle
The estrus cycle of mice in the metabolic cages was evaluated by daily vaginal smears at 9:00 from Day 22 to Day 40. The bulb was gently depressed to expel a ~25–50 μL water at the opening of vaginal canal. This step was repeated for four to five times. Placed the fluid on glass slide, and allowed the smear to completely dry at room temperature. The dry glass slide was stained with 1.6% Giemsa stain solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 10 min, and dried at room temperature. The vaginal smear was detected at the estrus stage with a microscope (Carl Zeiss Japan). Estrus cycle is composed of “proestrus → estrus → metestrus → diestrus.” Estrus stages were defined as proestrus (60–100% nucleated epithelial cells), estrus (70–100% cornified squamous epithelial cells), metestrus (~50% cornified epithelial cells and 50% leukocytes), and diestrus (60–100% leukocytes).Citation24) Mice that exhibited at least three consecutive four- or five-day cycles were considered to have a regular cycle, while those that displayed a prolonged cycle (≥6 days) had an irregular cycle.
Statistical analyses
Data on day 22 were analyzed by student t-test, and those on day 42 by one-way ANOVA, followed by Tukey’s multiple comparison tests. p <0.05 was considered to be significant. All statistical analyses were conducted using GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Effects of biotin deficiency on body weight, food intake, uterus, and ovary weight
Animal body weights did not differ markedly between the control and biotin-deficient groups on day 22 (Table ). Total food intake over 21 days (09:00 on day 1 to 09:00 on day 22) was lower in the biotin-deficient group than in the control group (Table ). Liver weight vs. body weight was higher in the biotin-deficient group than in the control group on day 22 (Table ). Uterus and ovary weight vs. body weight did not differ markedly between the control and biotin-deficient groups on day 22 (Table ).
Table 2. Effects of biotin deficiency.
Effects of biotin restoration on body weight, food intake, uterus, and ovary weight
Animal body weights did not differ markedly between the three groups on day 22. Final body weight, body weight gain for the next 20 days, and total food intake for the next 20 days (09:00 on day 22 to 09:00 on day 41) did not differ markedly between the recovery and control groups but were lower than either in the biotin-deficient group (Table ). Liver weight vs. body weight was higher in the recovery and biotin-deficient groups than in the control group. Uterus weight vs. body weight decreased in the order of the control group, followed by recovery group, and then biotin-deficient group (Table ). The characteristic physiological uterus weight vs. body weight was 50% lower in the biotin-deficient group than in the control group (Table ). Ovary weight vs. body weight did not differ markedly between the three groups (Table ).
Table 3. Effects of biotin restoration.
The biotin-deficient group exhibited clinical symptoms associated with biotin deficiency such as alopecia, approximately three weeks into receiving the biotin-deficient diet. While alopecia persisted on the head of the recovery group mice approximately 1 week after starting the control diet, symptoms had disappeared by 2 weeks after starting. Alopecia caused by biotin deficiency was therefore resolved by feeding a biotin-containing control diet for 14 days.
Effect of biotin deficiency on oocyte numbers
The total number of live and dead oocytes did not differ markedly between the control and biotin-deficient groups on day 22 (Table ) or among control, recovery, and biotin-deficient groups on day 42 (Table ).
Frequency of chromosome misalignment and spindle defects in oocytes
The frequency of abnormal oocytes due to chromosome misalignment and spindle defects in oocytes was 2.4 times higher in the biotin-deficient group than in the control group on day 22 (control vs. biotin-deficient: 16.2 vs. 39.5%) (Fig. and Fig. (A)). The spindles in normal oocytes were converged to one centrosome (Fig. (A)). However, the spindles in almost all abnormal oocytes of the biotin-deficient group were converged separately to some centrosome (Fig. (B)). A few abnormal oocytes in the biotin-deficient group were chromosome misalignment (Fig. (B)). Frequency of abnormal oocytes was higher in the recovery group than in the control group, and its percentage was roughly the same as in the biotin-deficient group on day 42 (Control vs. biotin-deficient vs. recovery: 18.1 vs. 58.5 vs. 48.1%) (Figs. (B) and ). The oocytes that had separating spindles and/or chromosome misalignment were observed in the biotin-deficient and recovery groups (Fig. (B) and (C)), but not in the control group (Fig. (A)).
Fig. 1. Spindle abnormalities in oocytes induced by biotin deficiency.
Notes: Representative examples of meiotic spindles in oocytes from indicated mice (n = 37–38 oocytes analyzed per group) after labeling α-tubulin antibody (green) and counter-staining DNA with DAPI (aqua blue). (A) control group, (B) biotin-deficient group.
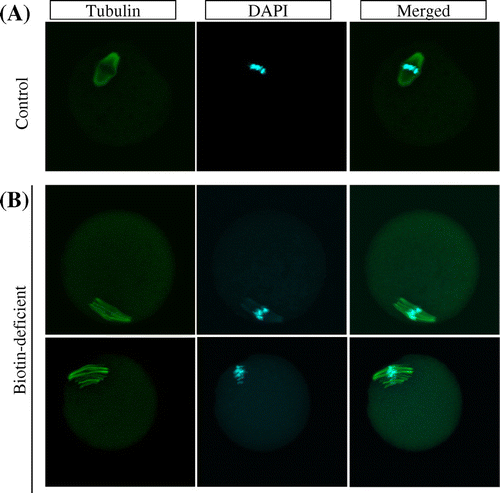
Fig. 2. Higher frequency of abnormal oocytes induced by biotin deficiency not restored by re-feeding of biotin-containing diet.
Notes: Abnormal oocyte number is the summation of chromosome alignment and spindle defect in oocytes. (A) Day 22, (B) Day 42. (C) control; BD; biotin deficient group; R, recovery group. n = 37–54 oocytes analyzed per group.
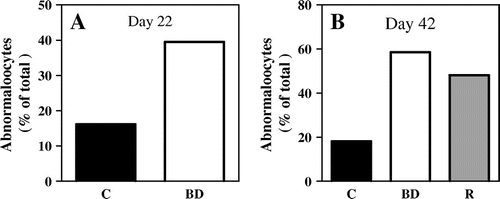
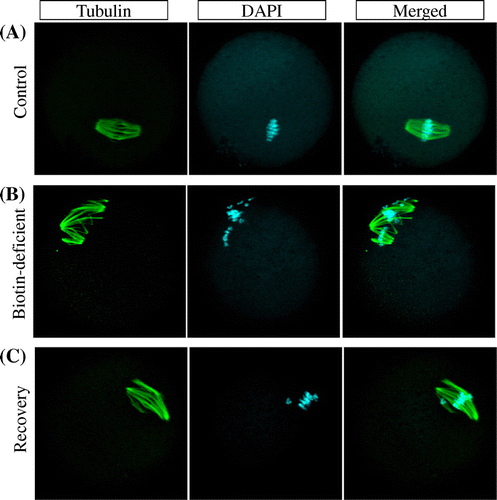
Fig. 3. Meiosis aneuploidy induced by biotin deficiency, not restored by biotin re-feeding.
Notes: Representative examples of meiotic spindles in oocytes from indicated mice (n = 41–66 oocytes analyzed per group) after labeling α-tubulin antibody (green) and counter-staining DNA with DAPI (aqua blue). (A) control group, (B) biotin-deficient group, and, (C) recovery group.
Effect of biotin-deficiency on biotin urinary excretion and concentrations of the liver, uterus, and ovaries
Urinary excretion of biotin was markedly lower in mice fed on biotin-deficient than in those on the control diet on day 19 (Table ). Biotin concentrations not only in the liver but also in the uterus and ovaries were lower in the biotin-deficient group than in the control group on day 22 (Table ).
Effect of biotin restoration on biotin urinary excretion and concentration in the liver, uterus, and ovaries
The urinary excretion of biotin in the recovery group did not differ markedly from that in the control group on day 39 (Table ). Biotin concentrations in the liver, uterus, and ovaries of the recovery group were restored to the same levels as in the control group (Table ). As expected, the biotin concentrations in the liver, uterus, and ovaries were lower in the biotin-deficient group than either in the control or recovery groups.
Urinary excretion of 3-HIA
For the surrogate indicator of biotin deficiency, 3-HIA, a metabolite of L-leucine was measured. 3-HIA was not detected in urine samples of the control group on day 19 (Table ) but was detected in the biotin-deficient group on the same day (Table ). The result suggests that biotin deficiency affect the metabolism in the systemic level at the time of day 19. Re-feeding the control diet reduced urine 3-HIA levels to almost zero on day 39 (Table ). 3-HIA urinary excretion in the biotin-deficient group was higher than the control and recovery groups on day 39. The results mean that abnormal L-leucine metabolism caused by biotin deficiency was repaired by re-feeding the control diet.
Estradiol level in plasma
Estradiol concentrations in plasma did not differ markedly between the control and biotin-deficient groups on day 22 (Table ) or among the control, recovery, and biotin-deficient groups on day 42 (Table ).
Estrus cycle
Estrus cycles in the biotin-deficient group were longer than in the control group but showed no marked difference when compared with the recovery group (Table ). Specifically, two of the five mice in the recovery group had a normal estrus cycle, while three had abnormal cycles.
Discussion
While biotin deficiency in mice is known to affect not only the outcome of pregnancyCitation9,10) but also the growth of oocytes,Citation17) whether or not the compound influences oocyte quality is unknown. Here, we clarified that biotin deficiency in mice does, indeed, induce the production of abnormal oocytes, such as those with chromosome misalignment and spindle defects. Further, we confirmed that biotin concentrations in liver, uterus, and ovaries were found to be lower in the biotin-deficient group than in the control group. Alopecia has been used as an index of physical sign of biotin deficiency in humanCitation25) and animal experiments.Citation17,20)
We also demonstrated that a number of abnormal oocytes were increased due to biotin deficiency before animals actually exhibited any clinical symptoms of deficiency, such as decreased body weight or alopecia.
Báez-Saldaña et al.Citation17) has reported that serum estradiol concentration was 1.5 times higher in the mice which were fed the biotin-deficient diet for 8 weeks than in the control mice which were fed the control diet. Additionally, ovary weight vs. body weight was decreased 0.34 times in the biotin-deficient group.Citation17) In the current study, estradiol concentration and ovary weight vs. body weight in biotin-deficient group were not significantly different from the control group on day 22 and day 42. So, the potential of ovary dysfunction might be low in biotin-deficient group. We considered that long term biotin-deficiency might cause ovary dysfunction; however, short-term biotin deficiency did not cause. It has been reported that biotin deficiency during pregnancy (for 18 days) caused teratology fetus.Citation9) However, we showed that uterus weight vs. body weight did not differ between the control and the biotin-deficient groups on day 22. So, we considered that there was no relationship between uterus weight loss and decrease in the pregnancy outcome.
In biotin-deficient mice, activity of 3-methylcrotonyl-CoA carboxylase (MCC) is reduced in the liver,Citation20,26–28) resulting in high excretion of 3-HIA, which is a metabolite of L-leucine in the urine.Citation20,25,26)MCC catalyzes the metabolism of 3-methylcrotonyl-CoA to 3-methylglutaconyl-CoA, which involves with L-leucine catabolismCitation6,19) Reduction in this enzyme reaction like biotin deficiency leads to the accumulation of the substrate 3-methylcrotonyl-CoA. Accordingly, accumulated 3-methylcrotonyl-CoA is hydrolyzed to form 3-hydroxyisovaleroyl-CoA by enoyl-CoA hydrolase and then converted to 3-HIA, which is quickly eliminated through urine. MCC is expressed not only in the liver but also in the extrahepatic tissue (kidney, heart, and skeletal muscle).Citation29) Because it has been reported that urine 3-HIA is a useful biomarker as biotin deficiency,Citation20) we measured 3-HIA in urine as a surrogate indicator of biotin deficiency. In the present study, 3-HIA was not detected in the urine of control mice but was detected in the urine of biotin-deficient mice, strongly suggesting that biotin deficiency affects systemic metabolism.
A number of biotin-dependent enzymes have been identified: acetyl-CoA carboxylases (ACC), propionyl-CoA carboxylase (PCC), and pyruvate carboxylase (PC). ACC participates in the regulation of fattyacid biosynthesis,Citation30) and PCC catalyzes essential steps in the metabolism of cholesterol and odd-chain fatty acids,Citation5) while PC catalyzes an essential step in gluconeogenesis and plays important roles in lipogenesisCitation31,32) and glucose-induced insulin release.Citation33) Biotin deficiency results in increased LDL cholesterol and decreased VLDL levels in serum, as well as decreased activity of all carboxylases in the liver.Citation28) We did not, however, measure metabolism involving other biotin-dependent enzymes.
In addition, we performed a recovery experiment to determine whether or not chromosome misalignment, spindle defects, and abnormal L-leucine metabolism caused by biotin deficiency could be restored upon re-feeding a biotin-sufficient diet. Results showed, however, that the misalignment and spindle defects were unable to be restored. We believe that a number of factors may be involved in this failure to restore the misalignment and spindle defects, such as biotin content of the diet, and the duration or timing of biotin re-feeding. However, in contrast, improved uterus weight vs. body weight and improved estrus cycle normality were observed in the recovery group, suggesting that the restoration of reproductive function in recovery mice may indeed be possible. Although we cannot exclude the possibility that biotin deficiency induced irreversible alteration in oocytes, abnormalities in oocytes caused by biotin deficiency may be able to be restored in recovery mice which were fed a diet that includes biotin for longer than 20 days or a diet including additional biotin via egg white solids or re-fed white egg solids before Day 22.
Urine 3-HIA was not detected in the recovery group, indicating that biotin-deficient mice fed the control diet for 20 days recovered from abnormal L-leucine metabolism due to biotin deficiency. Recovery from metabolic abnormalities might therefore be faster than from oocyte abnormalities.
The present data show that keeping good nutritional conditions are important for suppressing oocyte abnormalities. During meiosis in oocyte, microtubule is built and scrapped, and chromosome is aligned with chromatin within very short time. Thus, meiosis oocyte needs a lot of energy and also nutrients, and the related compounds for re-building, for example γ-tubulinCitation34) and mediator,Citation35,36) are re-built within very short time. It is known that glycolysis and β-oxidation of fatty acid expressions are very weak in mouse oocyte.Citation37) Therefore, oocyte consumes pyruvate as the main energy source Citation38–40): Pyruvate is supplied from extra oocyte, for example, from cumulus cell where pyruvate is made from glucose via glycolysis, and the resulting pyruvate is transported to oocyte from cumulus cell. The pyruvate is metabolized to acetyl-CoA and oxaloacetate in oocytes. Biotin needs the formation of oxaloacetate from pyruvate which is catalyzed by the biotin-enzyme PC. PC gene expression is known to increase at meiosis in oocytes.Citation41) This suggests that PC plays a pivotal role in making energy for meiosis maturation.Citation38,41)
Recently, biotin is known to be related to gene expression via biotinylation of histones H2A, H3, and H4,Citation42–44) which is dependent on the biotin supply in Drosophila,Citation45) further supporting the potential role of biotin in maintaining oocyte quality. To our knowledge, this is the first report to focus on the relationship between biotin deficiency and oocyte quality in mice. Further elucidation of this relationship between oocytes and biotin will require an investigation of metabolic disorders, involving biotin enzymes and transcription disorders, and biotin itself.
Author contributions
AT and KS designed the study, AT and KS drafted the manuscript, TN reviewed the manuscript, and AT performed the experiment. All authors reviewed and approved the final manuscript.
Competing interests
The authors disclose no potential conflicts of interest.
Additional information
Funding
Notes
Abbreviations: 3-HIA, 3-hydoroxyisovaleric acid; DAPI, 4′6-diamidino-2-phenylindole dihydrochloride.
References
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Human Genet. 1985;70:11–17.10.1007/BF00389450
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217.10.1371/journal.pone.0049217
- Muhlhauser A, Susiarjo M, Rubio C, Griswold J, Gorence G, Hassold T, Hunt PA. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol. Reprod. 2009;80:1066–1071.10.1095/biolreprod.108.074815
- Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Nat. Acad. Sci. USA. 2011; 108:12319–12324.10.1073/pnas.1018793108
- Watanabe T. Dietary biotin deficiency affects reproductive function and prenatal development in hamsters. J. Nutr. 1993;123:2101–2108.
- Tong L. Structure and function of biotin-dependent carboxylases. Cell. Mol. Life Sci. 2013;70:863–891.10.1007/s00018-012-1096-0
- Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin. J. Nutr. Biochem. 2003;14:680–690.10.1016/j.jnutbio.2003.07.001
- Zempleni J. Uptake, localization, and noncarboxylase roles of biotin. Annu. Rev. Nutr. 2005;25:175–196.10.1146/annurev.nutr.25.121304.131724
- Watanabe T, Endo A. Teratogenic effects of maternal biotin deficiency on mouse embryos examined at midgestation. Teratology. 1990;42:295–300.10.1002/(ISSN)1096-9926
- Watanabe T. Morphological and biochemical effects of excessive amounts of biotin on embryonic development in mice. Experientia. 1996;52:149–154.10.1007/BF01923361
- Báez-Saldaña A, Ortega E. Biotin deficiency blocks thymocyte maturation, accelerates thymus involution, and decreases nose-rump length in mice. J. Nutr. 2004;134:1970–1977.
- Báez-Saldaña A, Gutiérrez-Ospina G, Chimal-Monroy J, Fernandez-Mejia C, Saavedra R. Biotin deficiency in mice is associated with decreased serum availability of insulin-like growth factor-I. Eur. J. Nutr. 2009;48:137–144.10.1007/s00394-009-0773-8
- Dakshinamurti K. Biotin–a regulator of gene expression. J. Nutr. Biochem. 2005;16:419–423.10.1016/j.jnutbio.2005.03.015
- Dakshinamurti K, Chauhan J. Biotin. Vitam. Horm. 1989;45:337–384.10.1016/S0083-6729(08)60398-2
- Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J. Nutr. 2003;133:2519–2525.
- Mock DM. Marginal biotin deficiency is teratogenic in mice and perhaps humans: a review of biotin deficiency during human pregnancy and effects of biotin deficiency on gene expression and enzyme activities in mouse dam and fetus. J. Nutr. Biochem. 2005;16:435–437.10.1016/j.jnutbio.2005.03.022
- Báez-Saldaña A, Camacho-Arroyo I, Espinosa-Aguirre JJ, Neri-Gómez T, Rojas-Ochoa A, Guerra-Araiza C, Larrieta E, Vital P, Díaz G, Chavira R, Fernandez-Mejia C. Biotin deficiency and biotin excess: effects on the female reproductive system. Steroids. 2009;74:863–869.10.1016/j.steroids.2009.06.004
- Fukui T, Iinuma K, Oizumi J, Izumi Y. Agar plate method using Lactobacillus plantarum for biotin determination in serum and urine. J. Nutr. Sci. Vitaminol. 1994;40:491–498.10.3177/jnsv.40.491
- Watanabe T, Oguchi K, Ebara S, Fukui T. Measurement of 3-hydroxyisovaleric acid in urine of biotin-deficient infants and mice by HPLC. J. Nutr. 2005;135:615–618.
- Mock DM, Mock NI. Lymphocyte propionyl-CoA carboxylase is an early and sensitive indicator of biotin deficiency in rats, but urinary excretion of 3-hydroxypropionic acid is not. J. Nutr. 2002;132:1945–1950.
- Horvath TD, Matthews NI, Stratton SL, Mock DM, Boysen G. Measurement of 3-hydroxyisovaleric acid in urine from marginally biotin-deficient humans by UPLC-MS/MS. Anal. Bioanal. Chem. 2011;401:2805–2810.10.1007/s00216-011-5356-x
- Eng WK, Giraud D, Schlegel VL, Wang D, Lee BH, Zempleni J. Identification and assessment of markers of biotin status in healthy adults. Br. J. Nutr. 2013;110:321–329.10.1017/S0007114512005065
- Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased biotin status in experimental biotin deficiency. Am. J. Clin. Nutr. 1997;65:951–958.
- McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. 2012;15:e4389.
- Wastell HJ, Bartlett K, Dale G, Shein A. Biotinidase deficiency: a survey of 10 cases. Arch. Dis. Child. 1988;63:1244–1249.10.1136/adc.63.10.1244
- Rodríguez-Meléndez R, Pérez-Andrade ME, Díaz A, Deolarte A, Camacho-Arroyo I, Cicerón I, Ibarra I, Velázquez A. Differential effects of biotin deficiency and replenishment on rat liver pyruvate and propionyl-CoA carboxylases and on their mRNAs. Mol. Genet. Metab. 1999;66:16–23.10.1006/mgme.1998.2777
- Rodríguez-Fuentes N, López-Rosas I, Román-Cisneros G, Velázquez-Arellano A. Biotin deficiency affects both synthesis and degradation of pyruvate carboxylase in rat primary hepatocyte cultures. Mol. Genet. Metab. 2007;92:222–228.10.1016/j.ymgme.2007.06.021
- Suchy SF, Wolf B. Effect of biotin deficiency and supplementation on lipid metabolism in rats: cholesterol and lipoproteins. Am. J. Clin. Nutr. 1986;43:831–838.
- Gallardo ME, Desviat LR, Rodríguez JM, Esparza-Gordillo J, Pérez-Cerdá C, Pérez B, Rodríguez-Pombo P, Criado O, Sanz R, Morton DH, Gibson KM, Le TP, Ribes A, de Córdoba SR, Ugarte M, Peñalva MA. The molecular basis of 3-methylcrotonylglycinuria, a disorder of leucine catabolism. Am. J. Human Genet. 2001;68:334–346.10.1086/318202
- Kim KH. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu. Rev. Nutr. 1997;17:77–99.10.1146/annurev.nutr.17.1.77
- Freytag SO, Utter MF. Induction of pyruvate carboxylase apoenzyme and holoenzyme in 3T3-L1 cells during differentiation. Proc. Nat. Acad. Sci. USA. 1980; 77:1321–13255.10.1073/pnas.77.3.1321
- Jitrapakdee S, Slawik M, Medina-Gomez G, Campbell M, Wallace JC, Sethi JK, O’rahilly S, Vidal-Puig AJ. The peroxisome proliferator-activated receptor-gamma regulates murine pyruvate carboxylase gene expression in vivo and in vitro. J. Biol. Chem. 2005; 280:27466–27476.10.1074/jbc.M503836200
- MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J. Biol. Chem. 1995;270:20051–20058.
- Combelles CM, Albertini DF. Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of gamma-tubulin. Dev. Biol. 2001;239:281–294.10.1006/dbio.2001.0444
- Lu Q, Dunn RL, Angeles R, Smith GD. Regulation of spindle formation by active mitogen-activated protein kinase and protein phosphatase 2A during mouse oocyte meiosis. Biol. Reprod. 2002;66:29–37.10.1095/biolreprod66.1.29
- Wang X. Liu XT, Dunn, Ohl DA, Smith GD. Glycogen synthase kinase-3 regulates mouse oocyte homologue segregation. Mol. Reprod. Dev. 2003;64:96–105.10.1002/(ISSN)1098-2795
- Yamada M, Takanashi K, Hamatani T, Hirayama A, Akutsu H, Fukunaga T, Ogawa S, Sugawara K, Shinoda K, Soga T, Umezawa A, Kuji N, Yoshimura Y, Tomita M. A medium-chain fatty acid as an alternative energy source in mouse preimplantation development. Sci. Rep. 2012;2:1–9.
- Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oöcyte and zygote. Proc. Nat. Acad. Sci. USA. 1967;58:560–567.10.1073/pnas.58.2.560
- Downs SM, Humpherson PG, Leese HJ. Pyruvate utilization by mouse oocytes is influenced by meiotic status and the cumulus oophorus. Mol. Reprod. Dev. 2002;62:113–123.10.1002/(ISSN)1098-2795
- Harris SE, Leese HJ, Gosden RG, Picton HM. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol. Reprod. Dev. 2009;76:231–238.10.1002/mrd.v76:3
- Ma JY, Li M, Luo YB, Song S, Tian D, Yang J, Zhang B, Hou Y, Schatten H, Liu Z, Sun QY. Maternal factors required for oocyte developmental competence in mice: transcriptome analysis of non-surrounded nucleolus (NSN) and surrounded nucleolus (SN) oocytes. Cell Cycle. 2013;12:1928–1938.10.4161/cc
- Camporeale G, Shubert EE, Sarath G, Cerny R, Zempleni J. K8 and K12 are biotinylated in human histone H4. Eur. J. Biochem. 2004;271:2257–2263.10.1111/j.1432-1033.2004.04167.x
- Chew YC, Camporeale G, Kothapalli N, Sarath G, Zempleni J. Lysine residues in N-terminal and C-terminal regions of human histone H2A are targets for biotinylation by biotinidase. J. Nutr. Biochem. 2006;17:225–233.10.1016/j.jnutbio.2005.05.003
- Kobza K, Camporeale G, Rueckert B, Kueh A, Griffin JB, Sarath G, Zempleni J. K4, K9 and K18 in human histone H3 are targets for biotinylation by biotinidase. FEBS J. 2005;272:4249–4259.10.1111/j.1742-4658.2005.04839.x
- Smith EM, Hoi JT, Eissenberg JC, Shoemaker JD, Neckameyer WS, Ilvarsonn AM, Harshman LG, Schlegel VL, Zempleni J. Feeding Drosophila a biotin-deficient diet for multiple generations increases stress resistance and lifespan and alters gene expression and histone biotinylation patterns. J. Nutr. 2007;137:2006–2012.
- Reeves RG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997;127:838S–841S.
