Abstract
Measurements of the second-order rate constants and the singlet oxygen absorption capacity (SOAC) values for the reaction of singlet oxygen (1O2) with 23 kinds of food extracts were performed in ethanol/chloroform/D2O (50:50:1, v/v/v) solution at 35 °C. It has been clarified that the SOAC method is useful to evaluate the 1O2-quenching activity (i.e. the SOAC value) of food extracts having two orders of magnitude different rate constants from 3.18 × 104 L g−1 s−1 for tomato to 1.55 × 102 for green melon. Furthermore, comparison of the observed rate constants for the above food extracts with the calculated ones based on the concentrations of seven kinds of carotenoids included in the food extracts and the rate constants reported for each carotenoids was performed, in order to ascertain the validity of the SOAC assay method developed and to clarify the ratio of the contribution of principal carotenoids to the SOAC value.
Graphical Abstract
Measurements of singlet oxygen absorption capacity (SOAC) values for 23 kinds of vegetable and fruit extracts.

It is well known that lipid peroxyl radical () and singlet oxygen (1O2) are representative reactive oxygen species generated in biological systems. In recent years, the method to assess the total oxygen radical scavenging capacity of foods and plants has been developed, where oxygen radical indicates
.Citation1–5) On the other hand, singlet oxygen absorption capacity (SOAC) assay method to assess the total quenching activity of singlet oxygen by foods and plants has not been developed.
In biological systems, 1O2 is generated by the reaction of triplet sensitizers with molecular oxygen (3O2) (type II photosensitization reaction)Citation6,7) and by the biochemical reactions in cells and tissues exposed to oxidative stress.Citation8–11) 1O2 reacts with many kinds of biological targets including lipids,Citation12) proteins,Citation6,7) and DNA,Citation13,14) as well as radical does. Reactions with 1O2 occur mainly by chemical reaction, inducing the degradation of biological systems. Carotenoids and phenolic antioxidants (AOs) are widely present in vegetables, fruits, and edible oilsCitation15–20) and may function as efficient 1O2 quenchers in biological systems.Citation21–24)
In previous works,Citation25–27) kinetic studies of the quenching reaction of 1O2 with many kinds of AOs (such as carotenoids, vitamin E homologues, and polyphenols) were performed in ethanol/chloroform/D2O (50:50:1, v/v/v) (hereafter called as “mixed solvent” for the simplicity) and ethanol solutions at 35 °C. The overall rate constants, kQ (=kq + kr, physical quenching + chemical reaction), for the reaction of AOs with 1O2 were measured, using the competition reaction method, where endoperoxide (EP) was used as a singlet oxygen generator and 2,5-diphenyl-3,4-benzofuran (DPBF) as an UV–vis absorption probe (see Scheme ).(1)
The rate constants, kQ (S) and kQ (t1/2), were determined by analyzing the first-order rate constant (S) and the half-life (t1/2) of the decay curve of DPBF, respectively, showing good accordance with each other. Measurements of the kQ (S) and kQ (t1/2) values were performed not only for pure AOs, but also for three kinds of vegetable extracts (tomato, carrot, and red paprika extracts) containing high concentrations of carotenoids. From the results, a new assay method, which can quantify the SOAC of AOs, including carotenoids, phenolic AOs, and vegetable extracts, was proposed.Citation25–27) Relative SOAC value was defined in the following way.(2)
where [α-Toc] and [AO] are molar concentrations (M = mol/L) (or weight concentrations (g/L)) of α-tocopherol and AO, respectively. Equation (Equation2(2) ) indicates that the AOs with fast 1O2-quenching rate constants (kQ) show large relative SOAC values (i.e. high 1O2-quenching activity and high AO activity). α-Tocopherol was used as a standard compound of SOAC assay. The reason was described in a previous study.Citation25)
In the present work, first, measurements of the kQ (S), kQ (t1/2), and relative SOAC values were performed for total 23 kinds of vegetable and fruit extracts having different 1O2-quenching activity in mixed solvent at 35 °C, and detailed analyses were performed to ascertain the validity of the application of the SOAC method to general food extracts. Second, the concentrations of seven carotenoids ([Car-i] (i = 1–7)) (see Table ) included in the above food extracts were measured using a HPLC technique. Third, comparison of the kQ (S) values observed for food extracts with the sum of the product of the
values obtained for each carotenoid and the [Car-i] included in food extracts was performed, in order to clarify the ratio of the contribution of principal carotenoids to the SOAC value. From the results, it has been ascertained that the SOAC method developed is applicable to general food extracts to evaluate the 1O2-quenching activity.
Table 1. Contents of Seven Carotenoids in 23 Kinds of Vegetable and Fruit Extracts.
Materials and methods
Materials
D-α-tocopherol and DPBF were obtained from Tokyo Kasei Chemicals, Japan. Sea sand was obtained from Wako Chemicals, Japan. 3-(1,4-Epidioxy-4-methyl-1,4-dihydro-1-naphthyl)propionic acid (EP) was obtained from Wakenyaku Co. Ltd., Japan. The result of the measurement of the UV spectrum of EP indicates that the powder sample of EP includes 95.6% EP and 4.4% EP-precursor unreacted.Citation25)
All vegetable and fruit samples (see Table ) were purchased at a local market. Preparations of vegetable and fruit extracts are as followsCitation28): 1.00 g of freeze-dried powder sample from vegetable (or fruit) was mixed with 5-g sea sand. Sample and sand were transferred to an 11-mL extraction cell, and extraction was performed with ethanol:chloroform: D2O (50:50:1, v/v/v) three times, using an ASE-200 accelerated solvent extractor (Dionex Corporation, Sunnyvale, CA). The extracts were combined and the volume was adjusted to 25.0 mL with the same solvent in a volumetric flask. This solution was used to measure the SOAC value. Basically, edible portions of foods were used for the extraction. In the cases of onion, satsuma mandarin, orange melon, persimmon, banana, and green melon, the part of peel was took off before freeze-drying, and the remaining part was used for extraction.
Methods
Measurements of carotenoid concentrations included in food extracts
The method of the preparation of food extracts was described in a previous section.Citation28) In each food extract, two samples (food-1 and -2 extracts) were prepared independently by repeating the extraction. Concentrations of seven carotenoids (α-carotene (α-Car), β-carotene (β-Car), lutein (Lut), lycopene (Lyc), capsanthin (Cap), β-cryptoxanthin (β-Cry), and zeaxanthin (Zea)) (see Table ) included in 23 food extracts were measured using a HPLC technique, according to the method reported in a previous work.Citation17)
Measurements of rate constants (kQ)
Measurements of rate constants (kQ) were performed in mixed solvent, using a Shimadzu UV–vis spectrophotometer (UV-1800), equipped with a six-channel cell-positioner and an electron-temperature control unit (CPS-240A). All of the measurements were performed at 35.0 ± 0.5 °C. DPBF shows an UV–vis absorption maximum at λmax = 413 nm in mixed solvent. Measurements of the rate constants were performed by monitoring the change in absorption of DPBF at 413 nm due to the chemical reaction of DPBF with 1O2 in the absence and presence of AO for 2 h (see Fig. (B)). Details of measurement of UV–vis absorption spectra were described in previous works.Citation25–27) The production of 1O2 due to the thermal decomposition of EP occurs over 25 °C. Consequently, sample preparation was performed by adding 1.00 mL of EP solution to 2.00 mL of solution including DPBF and an AO (or food extract) in a quartz cuvette at ~20 °C, and measurements of the UV–vis absorption spectra were then started at 35 °C. It took about 5 min to prepare solutions of six cuvettes. About 3 min was necessary before the solution temperature in the cuvette rose from ~20 to 35 °C.
Fig. 1. Measurement of the second-order rate constant (kQ) for the reaction of pumpkin-1 extract with 1O2.
Notes: (A) Absorption spectrum of pumpkin-1 extract in ethanol/chloroform/D2O. The concentration of pumpkin-1 extract is 5.34 g/L. (B) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of sample (α-tocopherol and pumpkin-1 extracts) in ethanol/chloroform/D2O at 35 °C. [DPBF]t=0 = 6.26 × 10−5 M and [EP]t = 0 = 4.18 × 10−4 M. The values of [α-Toc]t = 0 and [pumpkin-1]t=0 are shown in panel B. (C) Change in absorbance of DPBF, where the correction of baseline due to pumpkin-1 extract was performed. (D) Plot of ln (absorbance) vs. t. (E) Plot of Sblank/Spumpkin-1 vs. [pumpkin-1]. (F) Plot of /
vs. [pumpkin-1].
![Fig. 1. Measurement of the second-order rate constant (kQ) for the reaction of pumpkin-1 extract with 1O2.Notes: (A) Absorption spectrum of pumpkin-1 extract in ethanol/chloroform/D2O. The concentration of pumpkin-1 extract is 5.34 g/L. (B) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of sample (α-tocopherol and pumpkin-1 extracts) in ethanol/chloroform/D2O at 35 °C. [DPBF]t=0 = 6.26 × 10−5 M and [EP]t = 0 = 4.18 × 10−4 M. The values of [α-Toc]t = 0 and [pumpkin-1]t=0 are shown in panel B. (C) Change in absorbance of DPBF, where the correction of baseline due to pumpkin-1 extract was performed. (D) Plot of ln (absorbance) vs. t. (E) Plot of Sblank/Spumpkin-1 vs. [pumpkin-1]. (F) Plot of / vs. [pumpkin-1].](/cms/asset/8fb1cddc-7ac6-4ba7-83ae-46318780e341/tbbb_a_972329_f0001_b.gif)
Analyses of the second-order rate constants ( (S) and
(S) and  ) and SOAC values
) and SOAC values
The rate constant (S) for the reaction of 1O2 with AO (or food extract) was determined by Equation (Equation3
(3) ).Citation25,29,30)
(3)
where Sblank and SAO are slopes of the first-order plots (i.e. ln (absorbance) vs. t plots) of disappearance of DPBF in the absence and presence of AO, respectively, and kd (=3.03 × 104 s−1) is the rate of natural deactivation of 1O2 in the solvent.Citation22,24) The results obtained for pumpkin-1 extract (AO) are shown in Fig. (D). Equation (Equation3(3) ) indicates that the
(S) value can be obtained from Sblank/SAO vs. [AO] plot (see Fig. (E)).
We can easily obtain Equation (Equation4(4) ), by substituting the relation for the first-order reaction (
= ln 2/SAO) into Equation (Equation3
(3) ).
(4)
where and
are the half-lives of DPBF in the absence and presence of AO, respectively. Equation (Equation4
(4) ) indicates that the
(t1/2) value can be obtained from
/
vs. [AO] plot.Citation25,26) In fact,
increases linearly with increasing the concentration of AO, as shown in Fig. (F).
As proposed in previous works,Citation25,26) the relative SOAC value for AO (or food extract) was defined as follows:(5)
where the unit of the concentration of AO ([AO]) and α-tocopherol ([α-Toc]) is g/L, and the unit of kQ is not M−1 s−1 but L g−1 s−1. Equation (Equation5(5) ) indicates that the SOAC value corresponds to the ratio (
/
) of the quenching rate of singlet oxygen (
) by AO to that (
) by α-tocopherol. As described above,
(S) value may be obtained by Sblank/SAO vs. [AO] plot (see Fig. (E)). On the other hand, Equation (Equation5
(5) ) indicates that the relative SOAC value may be easily determined by the measurement of the half-life of DPBF for only one concentration of AO (or food extract). It is very convenient to evaluate a 1O2-quenching activity of AO. However, the SOAC value calculated from the ratio (
/
) of the quenching rates of singlet oxygen is more reliable, as discussed in Results section.
Results
Concentrations of carotenoids included in 23 kinds of food extracts
Measurements of the concentrations of seven carotenoids (α-Car, β-Car, Lut, Lyc, Cap, β-Cry, and Zea) (abbreviated as [Car-i], i = 1–7) included in 23 kinds of food extracts were performed to compare the (S) (obsd.) values observed for food extracts with the
(S) (calcd.) ones calculated based on the
(S) values reported for each carotenoid and the concentrations of seven carotenoids included, as described in Discussion section. The concentrations of seven carotenoids and total concentrations of carotenoids included in 23 extracts are summarized in Table , together with those reported for tomato, carrot, and red paprika extracts in a previous work.Citation26)
β-Carotene is included in 22 food extracts except for onion, as listed in Table . α-Carotene and lutein are included in 18 and 19 extracts, respectively. Zeaxanthin and β-cryptoxanthin are included in 6 and 5 extracts, respectively. Lycopene is included only in tomato. Capsanthin is included only in red paprika. However, its concentration is very high (162.14 and 135.15 mg/100 g) in red paprika-1 and -2, respectively.
In the case of carrot extracts, the concentrations of α-carotene, β-carotene, and lutein included in carrot-1 and -2 independently prepared were similar to one another, respectively. Similar results were obtained for 18 kinds of extracts. The differences in the total carotenoid concentrations in food-1 and -2 extracts were less than by 10% for 13 kinds (and by 15% for 18 kinds) of vegetable and fruit extracts, as listed in Table . Only in the cases of broccoli and persimmon extracts, the total carotenoid concentrations included in broccoli-1 and persimmon-1 were by 31 and 37% smaller than the corresponding those in broccoli-2 and persimmon-2, respectively. The differences will be due to the inhomogeneous distribution of carotenoids included in freeze-dried powder sample and/or the experimental errors which occur in the process of solvent extraction.
Measurements of the 1O2-quenching rates (kQ (S) and kQ (t1/2)) for 23 kinds of food extracts
Measurements of the 1O2-quenching rates (kQ (S) and kQ (t1/2)) were performed in the following way: for example, the pumpkin-1 extract prepared from 1.00 g of freeze-dried powder was dissolved in 25 mL of mixed solvent. From this solution, four concentrations of pumpkin-1 extract (see samples 1–4 in Table (A)) were prepared, where the concentration of pumpkin-1 was defined as gram per liter (g/L). Similarly, the concentration of α-tocopherol as a standard sample was expressed as g/L. The concentration of α-tocopherol used for the measurement was 5.01 × 10−4 M, that is, 2.16 × 10−1 g/L (see Table (A)).
Table 2. Employed Concentrations, First-Order Decay Rates (S) and Half-Lives (t1/2) of Blank (DPBF Only), α-Tocopherol, and Samples 1 – 4 ((A) Pumpkin-1, (B) Eggplant-2, and (C) Strawberry-1) and Relative SOAC Values in Ethanol/Chloroform/D2O Solution.
The pumpkin-1 extract shows an UV–vis absorption at 370–520 nm, suggesting that high concentrations of carotenoids are included in pumpkin-1 (see Fig. (A)).Citation25,26) Decay curves of the absorbance of DPBF due to the reaction with 1O2 for pumpkin-1 are shown in Fig. (B). Baseline corrections were performed using an absorbance at 413 nm of UV–vis absorption spectrum in Fig. (A), and the decay curves corrected are shown in Fig. (C). ln (absorbance) vs. t plots are shown in Fig. (D), indicating that the decay of DPBF for pumpkin-1 follows first-order kinetics at ~10 < t < ~50 min.Citation25) The values of Spumpkin-1, Sα-Toc, and Sblank, ,
, and
obtained are listed in Table (A).
Sblank/Spumpkin-1 and /
vs. [pumpkin-1] plots are shown in Fig. , panels (E) and (F), respectively. Both the Sblank/Spumpkin-1 and
/
values increase linearly with increasing [pumpkin-1], and the plots show similar slopes, that is, similar rate constants (
(S) and
(t1/2)). The
(S) and
(t1/2) values obtained are 1.39 × 104 and 1.36 × 104 L g−1 s−1 (see Table (A)), respectively, where the values were calculated by linear least-squares method (see Table (A)).
Table 3.  (S) and
(S) and  (t1/2) Values Observed for (A) 16 Vegetable and (B) 7 Fruit Extracts in Ethanol/Chloroform/D2O Solution at 35.0oC, Relative Rate Constants (
(t1/2) Values Observed for (A) 16 Vegetable and (B) 7 Fruit Extracts in Ethanol/Chloroform/D2O Solution at 35.0oC, Relative Rate Constants ( (S)/
(S)/ (S)), Relative SOAC Values,
(S)), Relative SOAC Values,  (S) Values Calculated, and Ratio ((B)/(A)).
(S) Values Calculated, and Ratio ((B)/(A)).
Measurements were similarly performed for pumpkin-2 extract. The observed rate constants ( (S) and
(t1/2)) and the ratio of the rate constants (
(S)/
(S)) (see Table (A)) are ~20% smaller than the corresponding those of pumpkin-1, because pumpkin-2 extract includes lower concentrations of carotenoids than pumpkin-1 (see Table (A)).
Similar measurements were performed for 23 food extracts. For example, as green-colored Chinese leek-1 extract contains chlorophyll a (λmax = 430 nm, εmax = 4,5000 M−1 cm−1 in mixed solvent), absorption of chlorophyll a overlaps to those of carotenoids (λ = 370–520 nm), as shown in Fig. (A). Consequently, measurements were performed using lower concentrations of extract than those of pumpkin-1 (see Fig. (B)). The (S) and
(t1/2) values obtained for Chinese leek-1 and -2 are similar to each other, as listed in Table (A). In fact, total carotenoid concentration in Chinese leek-1 was similar to that of Chinese leek-2 (see Table ). The
(S) and
(t1/2) values and the relative rate constants (
(S)/
(S)) obtained for 16 vegetable and 7 fruit extracts are summarized in Table (A) and 3B, respectively. Experimental errors in the
(S) and
(t1/2) values were estimated to be <10%.Citation26) The
(S) values of food extracts varied notably depending on the kinds of food ones, as expected. The
(S) value (3.18 × 104 L g−1 s−1) of tomato-1 is 205 times larger than the
(S) value (1.55 × 102 L g−1 s−1) of green melon-1.
Fig. 2. Measurement of the second-order rate constant (kQ) for the reaction of Chinese leek-1 extract with 1O2.
Notes: (A) Absorption spectrum of Chinese leek-1 extract in ethanol/chloroform/D2O. The concentration of Chinese leek-1 extract is 1.34 g/L. (B) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of sample (α-tocopherol and Chinese leek-1 extracts) in ethanol/chloroform/D2O at 35 °C. [DPBF]t=0 = 6.42 × 10−5 M and [EP]t=0 = 4.33 × 10−4 M. The values of [α-Toc]t=0 and [Chinese leek-1]t=0 are shown in panel B. (C) Change in absorbance of DPBF, where the correction of baseline due to Chinese leek-1 extract was performed. (D) Plot of ln (absorbance) vs. t. (E) Plot of Sblank/SChinese leek-1 vs. [Chinese leek-1]. (F) Plot of /
vs. [Chinese leek-1].
![Fig. 2. Measurement of the second-order rate constant (kQ) for the reaction of Chinese leek-1 extract with 1O2.Notes: (A) Absorption spectrum of Chinese leek-1 extract in ethanol/chloroform/D2O. The concentration of Chinese leek-1 extract is 1.34 g/L. (B) Change in absorbance of DPBF at 413 nm during the reaction of DPBF with 1O2 in the absence and presence of sample (α-tocopherol and Chinese leek-1 extracts) in ethanol/chloroform/D2O at 35 °C. [DPBF]t=0 = 6.42 × 10−5 M and [EP]t=0 = 4.33 × 10−4 M. The values of [α-Toc]t=0 and [Chinese leek-1]t=0 are shown in panel B. (C) Change in absorbance of DPBF, where the correction of baseline due to Chinese leek-1 extract was performed. (D) Plot of ln (absorbance) vs. t. (E) Plot of Sblank/SChinese leek-1 vs. [Chinese leek-1]. (F) Plot of / vs. [Chinese leek-1].](/cms/asset/774b1c84-9a02-43c2-9290-4e22516aa8aa/tbbb_a_972329_f0002_b.gif)
As listed in Table (A) and (B), a fair agreement between the (S) and
(t1/2) values was observed for 14 kinds of vegetable extracts among 16 vegetable ones and all 7 kinds of fruits. Agreement was not sufficient only in the cases of eggplant-1, daikon radish-1 and -2, having slow 1O2-quenching rates (i.e. low 1O2-quenching activity). However, the result of the present investigation indicates that measurements of the rate constants (
(S) and
(t1/2)) based on the analyses of the first-order rate constant (S) and the half-life (t1/2) of the decay curve of DPBF, respectively, are applicable to general food extracts having more than two orders of magnitude different 1O2-quenching rates. Average values of the relative rate constants (
(S)/
(S)) obtained for 16 vegetable and 7 fruit extracts in mixed solvent are shown as a bar graph in Fig. (A).
Fig. 3. Comparison of (A) the relative rate constants ( (S)/
(S)) and (B) the relative SOAC values for 16 vegetable and 7 fruit extracts in ethanol/chloroform/D2O solution.
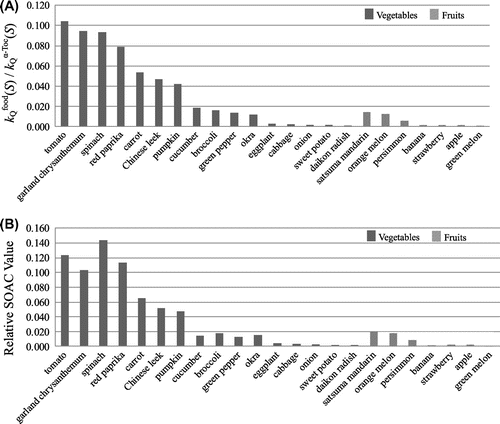
As summarized in Table , the differences in the total carotenoid concentrations in food-1 and -2 extracts were less than by 10% for 13 kinds (and by 15% for 18 kinds) of vegetable and fruit extracts among 23 food extracts. Consequently, we may expect similar rate constants for these food-1 and -2 extracts. In fact, good agreement of the (S) (and
(t1/2)) values between food-1 and -2 extracts was observed for 10 vegetables (such as tomato, spinach, cucumber, and cabbage) among 16 vegetables and 4 fruits (such as orange melon, strawberry, and apple) among 7 fruits, as listed in Table . The difference between the rate constants of food-1 and -2 extracts was less than 10% for these extracts.
10–20% differences between the rate constants of food-1 and -2 extracts were observed for three food extracts (pumpkin, orange melon, and banana). Larger (20–40%) differences were observed for four food extracts (green pepper, okra, sweet potato, and daikon radish), containing lower total concentrations of carotenoids and, thus, showing smaller rate constants (see Tables and ). The difference may be due to the inhomogeneous distribution of carotenoids included in freeze-dried powder sample and/or the experimental errors which occur in the process of solvent extraction, as described in a previous section. Furthermore, if the (S) (and
(t1/2)) values observed are small, these values will accompany larger experimental errors, as will be described in Discussion section.
Measurements of the relative SOAC values for 23 kinds of food extracts
Relative SOAC values were measured for 16 vegetable and 7 fruit extracts having different 1O2-quenching activity. Consequently, detailed analysis was performed for each food extract to obtain a reliable SOAC value.
For example, as measurements of the SOAC values were performed for one concentration of α-Toc and four concentrations of pumpkin-1 ([pumpkin-1]), we may determine four sets of relative SOAC values (0.0603, 0.0526, 0.0479, and 0.0458), using Equation (Equation5(5) ), as listed in Table 2(A). An average SOAC value (av 0.0516) (given on a weight basis (g/L)) for pumpkin-1 was listed in Table (A), where the result indicates that the intake of 1.00 g of pumpkin-1 extract has the singlet oxygen quenching activity equal to that of 0.0516 g of pure α-Toc. The relative SOAC value (av 0.0516) obtained for pumpkin-1 showed a fair agreement with the ratio (
(S)/
(S) = 0.0458) of the quenching rate constant of pumpkin-1 to that of α-Toc, as expected from Equation (Equation5
(5) ). Similarly, four kinds of SOAC values (and an average SOAC value) were measured for 23 food extracts. The results obtained are summarized in Table and Supplementary Table S1.
We may obtain the reliable SOAC value, when the difference between the half-lives of sample and blank ( −
) (i.e. the value of a numerator in Equation (Equation5
(5) )) is larger than ~5 min, as reported in previous works.Citation25–27) In fact, as listed in Table 2(A), the values of
−
(i = 1–4) obtained for pumpkin-1 extract satisfy this condition. Similar results were obtained for 11 food extracts having comparatively large
(S) values, showing similar SOAC values for samples 1–4 (see Table S1).
On the other hand, if the 1O2-quenching activity of food extracts is low (i.e. the (S) value is small), it is not easy to prepare the samples which satisfy the above condition (
−
> 5 min) for all samples 1–4, as Equation (Equation5
(5) ) indicates. The SOAC values determined under the following three different conditions ((i)
−
≥ 5 min, (ii) 5 min >
−
≥ 3 min, and (iii) 3 min >
−
) are listed in Table S1 as black, red and blue figures, respectively.
For example, in eggplant-2 extract, ( −
) values for samples 1, 2, 3, and 4 are 4.0, 4.7, 5.4, and 7.4 min, respectively (see Table 2(B)). In such a case, SOAC values decrease remarkably in the order of 0.0074 (for sample 1) > 0.0058 (for 2) > 0.0033 (for 3) > 0.0027 (for 4) with increasing the concentrations of samples and approach to a constant value. We can obtain similar SOAC values (0.0033 and 0.0027) for samples 3 and 4 with high concentrations, respectively. In such a case, an average (0.0030) of the SOAC values (0.0033 for 3 and 0.0027 for 4) obtained under the condition (i) (
−
> 5 min) was used as a SOAC value for eggplant-2.
Similar behavior was observed for green pepper, okra, and cabbage. The average SOAC values obtained for samples which satisfy the condition (i) are listed in Table (column 5) and Table S1 (column 6). As expected from Equation (Equation5(5) ), the average SOAC values obtained showed a fair agreement with the ratios (
(S)/
(S)) (see Table ), indicating that the above analysis is reasonable.
Furthermore, in the case of strawberry-1 extract, the SOAC values decreased in the order of 0.0055 (for sample 1) > 0.0034 (for 2) > 0.0023 (for 3) > 0.0020 (for 4), as listed in Table 2(C), where all 4 SOAC values for samples 1–4 were determined under the conditions (ii) and (iii) (i.e. −
< 5 min). Similar results were obtained for six food extracts (onion, sweet potato, daikon radish, banana, apple, and green melon) having low 1O2-quenching activity (see Table and Table S1). In such a case, the SOAC value obtained for sample 4 (with the highest concentration among samples 1–4) was tentatively used as the SOAC value for food extract. Generally, these SOAC values are considered to be in some degree larger than the true SOAC values (see Table and Table S1).
As listed in Table , a fair agreement between the relative rate constant ( (S)/
(S)), and the average SOAC value was obtained for 21 food extracts (except for spinach and red paprika extracts), as expected from Equation (Equation5
(5) ). The result indicates that the method of the analysis used for estimating the SOAC value is reasonable. The relative (average) SOAC values obtained for 16 vegetable and 7 fruit extracts in mixed solvent are shown as a bar graph in Fig. (B), together with that for the relative rate constants (
(S)/
(S)) (see Fig. (A)).
Discussion
Comparison between  (S) (obsd.) and
(S) (obsd.) and  (S) (calcd.) values for 23 kinds of food extracts
(S) (calcd.) values for 23 kinds of food extracts
It is well known that various AOs coexist in vegetables, fruits, and edible oils.Citation15–20) In the present work, the (S) (L g−1 s−1) values for 23 food extracts were measured by the Sblank/Sfood vs. [food] (g/L) plot, using Equation (Equation6
(6) ).
(6)
As reported in previous works,Citation21–27) (S) values of carotenoids show 2–5 orders of magnitude larger than those (
(S)) of general phenolic AOs, including tocopherol homologues, ubiquinol-10, catechins, and caffeic acids. The result suggests that the contribution of carotenoids to the 1O2-quenching activity of food extracts is considered to be very important. In fact, food extracts which include high total concentrations of carotenoids show large
(S) values, as listed in Table and Table .
As listed in Table , seven carotenoids (Car-i [i = 1–7]) are included in 22 food extracts except for onion among 23 food extracts. Consequently, the (S) values for 23 food extracts were calculated, using Equation (Equation7
(7) ), that is,
, where the
(S) (L g−1 s−1) values are the rate constants for seven carotenoids in mixed solvent.Citation25,26) [Car-i] (unit: mg/100 g) (see Table ) is the concentration of seven carotenoids included in food extracts. The
(S) (calcd.) values calculated for 23 food extracts are listed in Table .
(7)
We may expect that the (S) (obsd.) (A) values are larger than the corresponding
(S) (calcd.) (B) values, that is, the values of ratio (B)/(A) in Table are ≤1, because not only seven carotenoids, but also the other carotenoids are included in food extracts.Citation15−17,31) Many phenolic AOs included in food extracts will also contribute to 1O2-quenching. Furthermore, chlorophyll a (λmax = 430 nm) is included in green vegetables, as observed for Chinese leek-1 extract (see Fig. (A)). Chlorophyll a shows higher 1O2-quenching rate (kQ (S) = 7.3 × 108 M−1 s−1 in benzene) than α-Toc (1.31 × 108 M−1 s−1 in mixed solvent), suggesting the contribution of chlorophyll a to the kQ (S) (obsd.) values of green vegetable extracts.Citation32)
As listed in Table , good agreement between observed (S) values (=1.60 × 104 and 1.63 × 104 L g−1 s−1) and calculated
values (=1.67 × 104 and 1.66 × 104 L g−1 s−1) was obtained for carrot-1 and -2 extracts, respectively. The result indicates that the total 1O2-quenching activity of carrot-1 and -2 extracts may be well explained by only considering the contribution of 3 kinds of carotenoids (α-Car, β-Car, and Lut) included in carrot extracts. The contribution of the other AOs is small and negligible. Similarly, fair agreement between
(S) (obsd.) and
(S) (calcd.) values was obtained for four food extracts (tomato, Chinese leek, green pepper, and orange melon), which include comparatively high concentrations of carotenoids (see Table ).
On the other hand, the (S) (obsd.) values observed for the other 16 food extracts are larger than the corresponding
(S) (calcd.) values, as listed in Table , indicating that the other AOs included also contribute to the
(S) (obsd.) values. In fact, the values of ratio (B)/(A) observed for five brightly colored vegetable extracts (garland chrysanthemum, pumpkin, cucumber, broccoli, and eggplant) are 0.70 > (B)/(A) > 0.50. Generally, the values of ratio (B)/(A) observed for five vegetable extracts (okra, cabbage, onion, sweet potato, and daikon radish extracts), which include low concentrations of carotenoids and show comparatively small
(S) (obsd.) values, are 0.313 > (B)/(A) ≥ 0.000. Similarly, 6 fruit extracts except for orange melon also show small values of ratio (0.429 > (B)/(A) > 0.115).
For example, seven carotenoids are not included in onion-1 and -2 extracts. However, onion-1 and -2 extracts show 1O2-quenching activities ( (S) (obsd.) = 3.09 × 102 and 5.88 × 102 L g−1 s−1), respectively. Onion includes considerable amounts of quercetin which is well known as a representative flavone derivative.Citation33) The
(S) (obsd.) value of quercetin reported is 4.57 × 108 M−1 s−1 (=1.51 × 106 L g−1 s−1 in ethanol).Citation34) Quercetin included in onion will contribute to the above 1O2-quenching rate.
On the other hand, in the cases of spinach-1 and -2 and red paprika-1 and -2, the values of the ratio (B)/(A) are > 1.00 (see Table (A)). As listed in Table , the total carotenoid concentrations included in spinach-1 and -2 (197.41 and 195.18 mg/100 g) and red paprika-1 and -2 (193.68 and 168.30 mg/100 g) are the highest ones among 23 kinds of food extracts studied in the present work. The result suggests that the interactions between seven carotenoids and among seven carotenoids and many molecules included in spinach and red paprika extracts may hinder the determination of correct kQ (S) (obsd.) values. In fact, the total carotenoid concentration included in red paprika (40.88 mg/100 g) reported in a previous workCitation26) is much lower than the above values (193.68 and 168.30 mg/100 g), and the value of the ratio (B)/(A) reported is 0.255, as listed in Table . However, the reason is not clear at present. Detailed study will be necessary to clarify the reason why the values of the ratio (B)/(A) obtained for spinach-1 and -2 and red paprika-1 and -2 are >1.00.
Comparison between the  (S) (obsd.) (and SOAC) values observed for carrot, tomato, and red paprika in the present and previous works
(S) (obsd.) (and SOAC) values observed for carrot, tomato, and red paprika in the present and previous works
Measurements of (S) (obsd.) and SOAC values were performed for carrot, tomato, and red paprika in a previous work.Citation26) As listed in Table (A), the
(S) (obsd.) and the
(S) (obsd.) values obtained for tomato-1 and carrot-1 in the present work are 1.72 and 1.08 times larger, respectively, than the corresponding those reported. On the other hand, the
(S) (obsd.) value obtained for red paprika-1 in the present work is 0.767 times smaller than the value reported. Similar relations were obtained for the SOAC values for these extracts. Furthermore, the
(S) (calcd.) and the
(S) (calcd.) values calculated for tomato-1 and carrot-1 in the present work are 1.79 and 1.18 times larger, respectively, than the corresponding those reported in a previous work. The results indicate that the 1O2-quenching activity of food extracts depends on the concentrations of carotenoids included.
It is well known that carotenoid contents change remarkably during ripening. For example, Deli et al. Citation31) investigated quantitatively the changes in the 34 kinds of carotenoid and chlorophyll pigments of the red paprika during maturation by means of a HPLC technique. The concentrations of the carotenoid and chlorophyll pigments were analyzed in six consecutive stages of ripeness: green, pale green, brownish, brown, red, and deep red. The total carotenoid content increased ca. 66-fold, and the chlorophyll content reduced to zero during ripening. Total carotenoid (mg/100 g of dry weight) increased from 19.60 of green to 1297.12 mg/100 g of deep red, depending on the stage of maturation. Consequently, we may expect that the (S) (obsd.) value of red paprika at “deep red” stage is 66 times as large as the value of red paprika at “green” stage.
As listed in Table , total carotenoid content (193.68 mg/100 g) of red paprika-1 is 4.74 times as large as that (40.88 mg/100 g) of red paprika reported.Citation26) However, the (S) (obsd.) value obtained is 0.767 times as small as that reported. Similar results were obtained for red paprika-2, indicating that the results of the measurements of the
(S) (obsd.) values performed for red paprika-1 and -2 are reliable. High concentrations of carotenoids are included in red paprika-1 and -2 extracts, as listed in Table (A). As discussed in a previous section, the interactions between seven carotenoids and among seven carotenoids and many molecules included in red paprika extracts may hinder the determination of correct kQ (S) (obsd.) values. However, detailed reason is not clear at present, why the kQ (S) (obsd.) values of red paprika-1 and -2 including higher concentrations of total carotenoids are smaller than that of red paprika (reported) including lower concentration of total carotenoids. It will be interesting to measure the changes of (i) carotenoid content, (ii) the
(S) (obsd.) value, and (iii) SOAC value of red paprika extracts at six stages of maturation (green, pale green, brownish, brown, red, and deep red), to solve a riddle.
Method to obtain reliable SOAC values for general food extracts having different singlet oxygen quenching activity
Recently, measurements of the 1O2-quenching rates (kQ (S)) and the relative SOAC values were performed for 8 carotenoids, 16 phenolic AOs (tocopherol derivatives, ubiquinol-10, caffeic acids, and catechins), and vitamin C in mixed solvent at 35 °C.Citation25–27) It has been clarified that the SOAC method is useful to evaluate the 1O2-quenching activity of lipophilic- and hydrophilic-AOs having five orders of magnitude different rate constants from 1.38 × 1010 M−1 s−1 (2.57 × 107 L g−1 s−1) for lycopene to 2.71 × 105 (1.40 × 103 L g−1 s−1) for ferulic acid (FA). The SOAC value (av 123) for lycopene was also five orders of magnitude larger than that (av 0.00228) for FA, as expected from Equation (Equation5(5) ).
For instance, the kQγ-Toc (S) value (8.44 × 107 M−1 s−1) of γ-tocopherol (γ-Toc) was determined by Sblank/Sγ-Toc vs. [γ-Toc] plot using Equation (Equation3(3) ). As the measurements were performed for one concentration of α-Toc and four concentrations of γ-Toc (samples 1–4), we can determine four sets of relative SOAC values, using Equation (Equation5
(5) ) (see Table 2(A) in Ref. Citation27)). The relative SOAC values (0.685, 0.666, 0.764, 0.710, av 0.706) obtained for γ-Toc are similar to each other and agree well with the ratio of the quenching rate constant of γ-Toc to that of α-Toc (kQγ-Toc (S)/
(S) = 0.644).
On the other hand, in the case of FA, the rate constant ( (S)) (2.71 × 105 M−1 s−1) is 2–3 orders of magnitude smaller than that of γ-Toc. Consequently, we had to use 2–3 orders of magnitude higher concentrations to obtain reliable
(S) and SOAC values, as anticipated from Equations (Equation3
(3) ) and (Equation5
(5) ), respectively (see Table 2(B) in Ref. Citation27)). We could not obtain the reliable SOAC value, when the difference between the half-lives of AO and blank (
−
) was smaller than ~5 min. The relative SOAC values (0.00257, 0.00219, 0.00217, 0.00221, av 0.00228) obtained for FA are similar to each other and agree well with the ratio (
(S)/
(S) = 0.00207). The (
−
) values observed were > 5 min for all of the samples 1–4. On the other hand, if the relative SOAC value was given on a weight basis (g/L), the SOAC value for FA is calculated to be av 0.00507.
As listed in Table and Table S1, the relative SOAC values (tenta 0.0022–0.0009) tentatively determined for onion, sweet potato, daikon radish, banana, strawberry, apple, and green melon are smaller than that (av 0.00507) for FA. For example, as shown for strawberry, the difference between the half-lives of samples 1–4 and blank ( −
) was smaller than ~5 min (see Table 2(C)). Consequently, we could not obtain reliable SOAC values for the above food extracts. In the present work, samples 1–4 were prepared by dissolving 1.00 g of freeze-dried powder sample from vegetable (or fruit) in the 25.0 mL mixed solvent, as described in Materials section. If measurements were performed for the solutions including higher concentrations of food extracts, we may obtain reliable SOAC values for the above food extracts.
Summary
Recently, the SOAC assay method to assess the 1O2-quenching activity of carotenoids and phenolic AOs, which are included in foods and plants was proposed.Citation25–27) However, the examples of the application of the SOAC assay method are limited to three vegetable extracts (tomato, carrot, and red paprika), which include high concentrations of carotenoids and show large SOAC value (i.e. large (S) value). In the present work, relative SOAC values were measured for 23 kinds of vegetable and fruit extracts having different 1O2-quenching activity, and detailed analyses were performed to ascertain the validity of the application of the SOAC method to general food extracts. Furthermore, comparison of the
(S) values observed for the above food extracts with the sum of the product
of the
(S) values reported for each carotenoid and the [Car-i] values included in food extracts was performed. From the results, it has been ascertained that the SOAC method developed is applicable to general food extracts to evaluate the 1O2-quenching activity.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org./10.1080/09168451.2014.
972329_Supplemental_Material.docx
Download MS Word (25.1 KB)Acknowledgments
We are very grateful to Professors Junji Terao of Tokushima University, Shin-ichi Nagaoka of Ehime University, and Takahiro Inakuma of Tezukayama University for their continuous encouragement throughout this work. We are also very grateful to Dr Aya Ouchi of Ehime University for her kind help in the measurements of the rate constants of food extracts.
References
- Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993;14:303–311.10.1016/0891-5849(93)90027-R
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absobance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food. Chem. 2001;49:4619–4626.10.1021/jf010586o
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food. Chem. 2004;52:4026–4037.10.1021/jf049696w
- Omata Y, Saito Y, Yoshida Y, Niki E. Simple assessment of radical scavenging capacity of beverages. J. Agric. Food. Chem. 2008;56:3386–3390.10.1021/jf703771v
- Takashima M, Horie M, Shichiri M, Hagihara Y, Yoshida Y, Niki E. Assessment of antioxidant capacity for scavenging free radicals in vitro: a rational basis and practical application. Free Radical Biol. Med. 2012;52:1242–1252.10.1016/j.freeradbiomed.2012.01.010
- Davies MJ, Truscott RJW. Photo-oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol., B. 2001;63:114–125.10.1016/S1011-1344(01)00208-1
- Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003;305:761–770.10.1016/S0006-291X(03)00817-9
- Murphy ME, Sies H. Visible-range low-level chemiluminescence in biological systems. Methods Enzymol. 1990;186:595–610.10.1016/0076-6879(90)86155-O
- Kanofsky JR. Singlet oxygen production by biological systems. Chem. Biol. Interact. 1989;70:1–28.10.1016/0009-2797(89)90059-8
- Miyamoto S, Martinez GR, Martins APB, Medeiros MHG, Di Mascio P. Direct evidence of singlet molecular oxygen production in the reaction of linoleic acid hydroperoxide with peroxynitrite. J. Am. Chem. Soc. 2003;125:4510–4517.10.1021/ja029262m
- Miyamoto S, Nantes IL, Faria PA, Cunha D, Ronsein GE, Medeiros MHG, Di Mascio P. Cytochrome c-promoted cardiolipin oxidation generates singlet molecular oxygen. Photochem. Photobiol. Sci. 2012;11:1536–1546, and references are cited therein.10.1039/c2pp25119a
- Girotti AW. Photodynamic lipid peroxidation in biological systems. Photochem. Photobiol. 1990;51:497–509.10.1111/php.1990.51.issue-4
- Devasagayam TPA, Steenken S, Obendorf MSW, Schulz WA, Sies H. Formation of 8-(hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry. 1991;30:6283–6289.10.1021/bi00239a029
- Piette J. New trends in photobiology: Biological consequences associated with DNA oxidation mediated by singlet oxygen. J. Photochem. Photobiol., B. 1991;11:241–260.10.1016/1011-1344(91)80030-L
- Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J. Am. Diet. Assoc. 1993;93:284–296.10.1016/0002-8223(93)91553-3
- Holden JM, Eldridge AL, Beecher GR, Buzzard IM, Bhagwat S, Davis CS, Douglass LW, Gebhardtc S, Haytowitz D, Schakel S. Carotenoids content of U. S. foods: An update of database. J. Food Compos. Anal. 1999;12:169–196.10.1006/jfca.1999.0827
- Aizawa K, Inakuma T. Quantitation of carotenoids in commonly consumed vegetables in Japan. Food Sci. Technol. Res. 2007;13:247–252.10.3136/fstr.13.247
- Sookwong P, Nakagawa K, Murata K, Kojima Y, Miyazawa T. Quantitation of tocotrienol and tocopherol in various rice brans. J. Agric. Food. Chem. 2007;55:461–466.10.1021/jf0621572
- Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007;103:1494–1501.10.1016/j.foodchem.2006.08.014
- Hu J-N, Zhu X-M, Adhikari P, Li D, Kim I-H, Lee K-T. Determination of tocopherol contents in refined edible oils using an HPLC method. J. Food Sci. Nutr. 2009;14:260–264.10.3746/jfn.2009.14.3.260
- Foote CS, Denny RW. Chemistry of singlet oxygen VII. Quenching by β-carotene. J. Am. Chem. Soc. 1968;90:6233–6235.10.1021/ja01024a061
- Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989;274:532–538.10.1016/0003-9861(89)90467-0
- Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamin E and C, β-carotene, and other carotenoids. Ann. N.Y. Acad. Sci. 1992;669:7–20.10.1111/nyas.1992.669.issue-1
- Di Mascio P, Sundquist AR, Devasagayam TPA, Sies H. Assay of lycopene and other carotenoids as singlet oxygen quenchers. Method Enzym. 1992;213:429–438.10.1016/0076-6879(92)13144-M
- Ouchi A, Aizawa K, Iwasaki Y, Inakuma T, Terao J, Nagaoka S, Mukai K. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity (SOAC) assay method. J. Agric. Food. Chem. 2010;58:9967–9978.10.1021/jf101947a
- Aizawa K, Iwasaki Y, Ouchi A, Inakuma T, Nagaoka S, Terao J, Mukai K. Development of singlet oxygen absorption capacity (SOAC) assay method. 2. Measurements of the SOAC values for carotenoids and food extracts. J. Agric. Food. Chem. 2011;59:3717–3729.10.1021/jf104955a
- Mukai K, Ouchi A, Takahashi S, Aizawa K, Inakuma T, Terao J, Nagaoka S. Development of singlet oxygen absorption capacity (SOAC) assay method. 3. Measurements of the SOAC values for phenolic antioxidants. J. Agric. Food. Chem. 2012;60:7905–7916.10.1021/jf302021r
- Wu X, Gu L, Holden JM, Haytowitz DB, Gebhardt SE, Beecher G, Prior RL. Development of a database for total antioxidant capacity in foods: a preliminary study. J. Food Compos. Anal. 2004;17:407–422.10.1016/j.jfca.2004.03.001
- Young RH, Wehrly K, Martin RL. Solvent effects in dye-sensitized photooxidation reactions. J. Am. Chem. Soc. 1971;93:5774–5779.10.1021/ja00751a031
- Thomas MJ, Foote CS. Chemistry of singlet oxygen XXVI. Photooxygenation of phenols. Photochem. Photobiol. 1978;27:683–693.10.1111/php.1978.27.issue-6
- Deli J, Molnár P, Matus Z, Tóth G. Carotenoid composition in the fruits of red paprika (Capsium annuum var. lycopersiciforme rubrum) during ripening; biosynthesis of carotenoids in red paprika. J. Agric. Food. Chem. 2001;49:1517–1523.10.1021/jf000958d
- Tanielian C, Wolf C. Mechanism of physical quenching of singlet molecular oxygen by chlorophylls and related compounds of biological interest. Photochem. Photobiol. 1988;48:277–280.10.1111/php.1988.48.issue-3
- Hertog MGL, Feskens EGM, Lollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011.10.1016/0140-6736(93)92876-U
- Nagai S, Ohara K, Mukai K. Kinetic study of the quenching reaction of singlet oxygen by flavonoids in ethanol solution. J. Phys. Chem. B. 2005;109:4234–4240.10.1021/jp0451389