Abstract
In this study, we developed a novel bioreactor to load hydrostatic pressure to promote chondrogenesis of prechondrogenic ATDC5 cells in as little as 3 days. Furthermore, we showed that loading hydrostatic pressure induced the upregulation of PKR, which is known to participate in mechanotransduction in various models.
Mechanical stimuli in the physiological range have been regarded as a significant factor to regulate the metabolism of chondrocytes in tissue engineering.Citation1) By loading the physiological range of hydrostatic pressure onto chondrocytes, the cartilage matrix anabolism is enhanced, further repairing and regenerating the damaged tissues.Citation2) Moreover, hydrostatic pressure is capable of redifferentiating dedifferentiated bovine articular cartilage,Citation3) which could solve the dedifferentiation issue encountered during tissue-engineered strategies such as autologous chondrocyte transplantation. Although hydrostatic pressure is a promising mechanical stimulus to promote chondrogenesis, its mechanism has not yet been precisely clarified.
We employed mouse ATDC5 cells, a pre-chondrogenic cell line derived from mouse teratocarcinoma cells that is widely known as an in vitro model for chondrocyte research.Citation4) Previous studies on mouse chondrocytes have reported the use of hydrostatic pressures in the range 10–70 kPa applied at 0.25–1 Hz for short (5, 15, 30, and 60 min) or long periods (1 h daily for up to 19 days).Citation5,6) In this study, a similar range of pressure, 5–37 kPa at 0.5 Hz, was loaded 1 h daily for 3 days.
The aim of this study was to investigate the mechanotransduction under hydrostatic pressure during chondrogenesis. First, a novel experimental system to load a low dynamic hydrostatic pressure in the physiological range was developed and applied for a short period of 3 days. We found that a novel target gene coding for Protein Kinase R (PKR) were upregulated by hydrostatic pressure as well as chondrogenic markers. PKR is a double-stranded RNA-dependent protein kinase that is activated through phosphorylation in response to double-stranded RNA, interferon, stress signals, and mechanical stimuli.Citation7,8) Some studies suggested that both PKR play a role as mechanosensors in chondrocytes under mechanical stimuli such as compression.Citation9) In this study, we studied the involvement of those genes as well as chondrogenesis markers under hydrostatic pressure.
ATDC5 cells were routinely cultured in high-glucose Dulbecco’s modified Eagle’s medium (GIBCO)/Ham’s F12 (GIBCO) (1:1), including 5% of fetal bovine serum (GIBCO) and 1% of antibiotic–antimycotic (GIBCO). To prepare confluent monolayers, 2 × 10Citation6 cells were subcultured on 35 mm Petri dishes in a humidified incubator at 37 °C with 5% CO2 for 3 days in cell culture medium supplemented with chondrogenic factors, namely 1% of insulin–transferrin–selenium (ITSTM + Premix; BD Biosciences) and 0.1% of ascorbic acid 2-phosphate (50 μg/ml). In this study, cells were used between passage 8, 9, and 10 (n = 5).
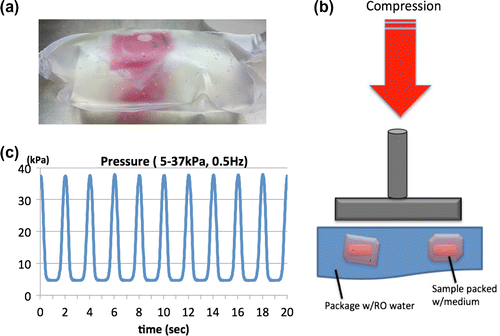
We utilized polyolefin blood transfer bags (KBP-1000CP; Kawasumi Laboratories) to pack the Petri dishes with 20 mL of medium. The samples were then placed in a big polyolefin bag and double packed with reverse osmosis (RO) water (Fig. (a)). The sealed bag was then compressed using a Shimadzu Microservo device (MMT-250 N; Shimadzu), thus loading hydrostatic pressure in the monolayer samples (Fig. (b)). As shown in Fig. (c), the pressure loaded by the device was measured by a blood pressure sensor (A0C00116; National Institute of Advanced Industrial Science and Technology) through a tube connected to the polyolefin bag. The certain range of pressure for mouse, 5–37 kPa at 0.5 Hz, was loaded 1 h daily for 3 days. As a control, the double packed samples were prepared without loading hydrostatic pressure under the same conditions using the same amount of medium and RO water.
Fig. 1. The novel hydrostatic pressure bioreactor.
(a) Double package of monolayer sample filled up with culture medium and RO water, (b) schematic diagram of the bioreactor; Shimadzu Microservo device was utilized for compression loading onto the double-packed sample, (c) pressure measured during loading by the pressure sensor.
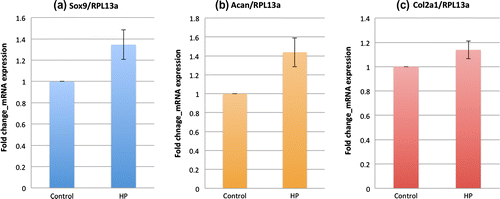
In this study, real-time PCR (RT-PCR) was carried out to examine the mRNA expression in the samples in response to hydrostatic pressure loading. After 3 days of pressurization, the samples were immediately collected and lyzed in Trizol reagent (Invitrogen) before RNA extraction and cDNA synthesis (ReverTra Ace® qPCR RT Master Mix with gDNA Remover; TOYOBO). We examined how hydrostatic pressure affects the chondrogenesis of ATDC5 cells by measuring the change in mRNA expression of sex determining region Y – box 9 (Sox9), Aggrecan (Acan) and Type II Collagen (Col2a1) that are known markers of chondrocyte differentiation. Moreover, the mRNA expression of PKR was also examined by RT-PCR. mRNA expression was normalized to RPL13a expression. We analyzed changes in a target sample relative to an untreated control samples in order to provide the fold change of mRNA expression in the sample.
Fig. shows the real-time PCR results for chondrogenic markers. In Fig. (a), the mRNA expression of Sox9, a key transcription factor for chondrocyte differentiation, was upregulated 1.35 fold (p < 0.10) by hydrostatic pressure. Loading hydrostatic pressure for 3 days also promotes other chondrogenesis markers such as Aggrecan (1.44-fold change; p < 0.10) and Type II Collagen (1.14-fold change; p < 0.20) as shown in Fig. (b) and (c), respectively.
Fig. 2. mRNA Expression of chondrogenic markers in ATDC5 cells under hydrostatic pressure measured by real-time PCR.
(a) Sox9 (b) Acan (c) Col2a1. Graphs show the fold change of chondrogenic markers mRNA expression relative to RPL13a mRNA normalized to the control mean. The bars represent the mean ± standard error. All the mRNA expressions of chondrogenic markers increased compared to control (n = 5) (p-value was obtained from student’s t-test).
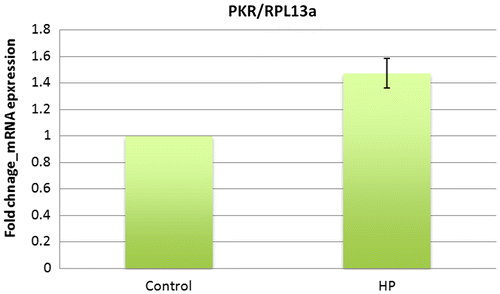
As shown in Fig. , significant changes in the mRNA expression of PKR were observed. The mRNA expression of sample loaded by hydrostatic pressure showed a 1.47 fold (p < 0.05) increase for PKR.
Fig. 3. mRNA expression of PKR in ATDC5 cells under hydrostatic pressure measured by real-time PCR.
Graphs show the fold change of PKR-mRNA expression relative to RPL13a mRNA normalized to the control mean. The bars represent the mean ± standard error. mRNA expression of PKR increased compared to control (n = 5) (p-value was obtained student’s t-test).
In this study, we set up a novel hydrostatic bioreactor using the Shimadzu device that allowed samples packed in a compressible double package filled with water to be subjected to hydrostatic pressure by compressing the package. As a result of this method, it showed a remarkable performance with regard to its precise control of pressure, which was the relatively low value of dynamic pressure, 5–37 kPa at 0.5 Hz. It also allowed users to adjust the pressure inside the double package by monitoring the pressure sensor.
Loading hydrostatic pressure by this experimental system promoted the mRNA expression of the key transcription factor Sox9 and enhancing the mRNA expressions of Aggrecan and type II collagen, well-known chondrogenesis markers, further suggesting that it promoted the chondrogenesis of ATDC5 cells in as little as 3 days. While other studies have loaded hydrostatic pressure to enhance chondrogenesis for up to 3 weeks or longer, we utilized our novel bioreactor for only 3 days, which is a sufficient period to promote the mRNA expression of all chondrogensis markers under study. We expect this system to give even stronger gene upregulation and more reproducible results if experiments are carried out for a longer period.
Previous studies have shown that PKR mediates the metabolic response of chondrocytes under mechanical stimuli such as compression.Citation9) In this study, we studied whether PKR acted as a mechanotransducer to induce chondrogenesis under hydrostatic pressure, since those proteins have been shown to be mechanosensors under other mechanical stimuli. As shown by real-time PCR, hydrostatic pressure upregulated the mRNA expression of PKR. Hence, hydrostatic pressure, one of the crucial mechanical stimuli experienced by chondrocytes in vivo, promoted the mRNA expression of PKR as well as chondrogenesis markers. From this finding, further studies may help us to understand in more detail the sensory mechanism of hydrostatic pressure by chondrocytes. However, inhibitor tests will have to be performed in order to confirm the direct interaction between PKR and Sox9. Moreover, further experiments including Western blotting will also need to be carried out to check the upregulation of these novels target genes at the protein level.
In conclusion, we have developed a novel bioreactor to load hydrostatic pressure on chondrocytes to promote chondrogenesis of ATDC5 cells. Furthermore, hydrostatic pressurizing induced the upregulation of PKR, suggesting these genes may mediate chondrogenesis by mechanically transducing hydrostatic pressure.
References
- Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86.10.1016/j.tibtech.2003.12.001
- Elder BD, Athanasiou KA. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng. 2009;15:1–12.
- Kawanishi M, Oura A, Furukawa K, Fukubayashi T, Nakamura K, Tateishi T, Ushida T. Redifferentiation of dedifferentiated bovine articular chondrocytes enhanced by cyclic hydrostatic pressure under a gas-controlled system. Tissue Eng. 2007;13:957–964.10.1089/ten.2006.0176
- Newton PT, Staines KA, Spevak L, Boskey AL, Teixeira CC, Macrae VE, Canfield AE, Farquharson C. Chondrogenic ATDC5 cells: an optimised model for rapid and physiological matrix mineralisation. Int. J. Mol. Med. 2012;30:1187–1193.
- Gardinier JD, Majumdar S, Duncan RL, Wang L. Cyclic hydraulic pressure and fluid flow differentially modulate cytoskeleton re-organization in MC3T3 osteoblasts. Cell. Mol. Bioeng. 2008;2:133–143.
- Nagatomi J, Arulanandam BP, Metzger DW, Meunier A, Bizios R. Cyclic pressure affects osteoblast functions pertinent to osteogenesis. Ann. Biomed. Eng. 2003;31:917–923.
- Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008;8:559–568.
- Williams BRG. The role of the dsRNA-activated kinase, PKR, in signal transduction. Semin. Virol. 1995;6:191–202.10.1006/smvy.1995.0024
- Lomas C, Tang XD, Chanalaris A, Saklatvala J, Vincent TL. Cyclic mechanical load causes global translational arrest in articular chondrocytes: a process which is partially dependent upon PKR phosphorylation. Eur. Cell Mater. 2011;22:178–189.
