Abstract
Taste enables organisms to determine the properties of ingested substances by conveying information regarding the five basic taste modalities: sweet, salty, sour, bitter, and umami. The sweet, salty, and umami taste modalities convey the carbohydrate, electrolyte, and glutamate content of food, indicating its desirability and stimulating appetitive responses. The sour and bitter modalities convey the acidity of food and the presence of potential toxins, respectively, stimulating aversive responses to such tastes. In recent years, the receptors mediating sweet, bitter, and umami tastes have been identified as members of the T1R and T2R G-protein-coupled receptor families; however, the molecular mechanisms underlying sour taste detection have yet to be clearly elucidated. This review covers the molecular mechanisms proposed to mediate the detection and transmission of sour stimuli, focusing on polycystic kidney disease 1-like 3 (Pkd1l3), Pkd2l1, and carbonic anhydrase 4 (Car4).
Graphical Abstract
Schematic drawing illustrating conformational structures of polycystic kidney disease 1-like 3 (Pkd1l3) and Pkd2l1. This review covers the molecular mechanisms proposed to mediate the detection and transmission of sour stimuli, focusing on Pkd1l3, Pkd2l1, and carbonic anhydrase 4.
Key words:
I. Introduction
Taste system enables organisms to evaluate the properties of ingested substances by conveying information regarding the five basic taste modalities: sweet, bitter, umami (savory), sour, and salty. The sweet, umami, and salty taste modalities convey the nutritious value and sodium content of food, indicating its desirability and stimulating appetitive responses. In contrast, the bitter and sour modalities convey the presence of potential toxins and the acidity of food, respectively, stimulating aversive responses to such tastes.Citation1)
Taste compounds, when taken into the oral cavity, are detected by taste receptors localized in the taste pores at the apical ends of taste buds.Citation2,3) In the oral cavity, taste buds are abundantly distributed in four major gustatory regions: the circumvallate papillae (CvP), foliate papillae (FoP), and fungiform papillae (FuP), all of which are found on the tongue, and the soft palate.Citation4–9) Each taste bud forms an onion-like shape and is composed of 50–100 taste receptor cells (TRCs). Based on the intensity of staining and the ultrastructure of the cytoplasm, as observed by electron microscopy, the TRCs in each taste bud are classified into four morphological types. Type-I (dark), type-II (light), and type-III (intermediate) taste cells are elongated and spindle shaped, whereas type-IV taste cells are round; the latter are located at the bottom of the taste buds and are thought to be progenitor cells of other types of TRCs.Citation10) Recent studies have revealed that each type of TRC plays a different role in taste detection. Type-I cells appear to be “glial-like,” morphologically wrapping around other TRCs and producing enzymes that degrade ATP released by type-II cells.Citation11) Type-II cells are responsible for the detection of sweet, bitter, or umami tastesCitation12,13); they do not form conventional synapses and appear to release ATP as a transmitter, which in turn appears to activate closely associated nerve afferents expressing P2X receptorsCitation14,15) and adjacent taste cells expressing P2Y receptors.Citation16,17) Type-III cells that mediate sour taste transduction are “presynaptic,”Citation18,19) forming synaptic contacts with the intragemmal nerve fibers, and are thought to use serotonin (5-HT) as a neurotransmitter.Citation17,20)
In recent years, the receptors mediating sweet, bitter, and umami tastes have been identified as members of the T1R and T2R G protein-coupled receptor (GPCR) families; however, the molecular mechanisms underlying sour taste detection are yet to be clearly elucidated.Citation2,3,10,21,22)
II. Molecular basis of acid sensing
There have been a number of attempts to identify the physiological acid sensor and the signal transduction pathway that mediate acid sensing in taste cells; however, a master acid sensor in mammalian TRCs has not been identified. The search for a master sensor is complicated by the fact that virtually every protein contains amino acid residues capable of binding protons, resulting in pH-dependent effects on the functions of most channels, transporters, and signal transduction molecules, further highlighting the importance of maintaining an appropriate acid–base balance in most cells. Therefore, it is difficult to show that the effects of pH on a specific protein are related to the function of a physiological acid sensor without sophisticated and complicated in vivo experiments.
While there is widespread acidification of TRCs after exposure to an acid stimulus, only a subpopulation of TRCs showed stimulus-related Ca2+ signals.Citation23) These are mediated by action potential generation, leading to membrane depolarization and the subsequent activation of voltage-gated Ca2+ channels via a reduced cytosolic pH buffer capacity or the expression of proton-sensitive transduction molecules. These cells were subsequently identified as presynaptic type-III cells,Citation24) and intracellular protons have been proposed to be the proximate stimuli for acid sensing.Citation19)
In particular, the ability of both protons and protonated organic acids to mediate the activation of sour-sensing TRCs by seemingly separate pathways results in two separate but related stimuli contributing to the same sensory outcome, both of which require investigation. Further complicating the ability to determine the proximal mechanisms involved in the detection of sour tastes is the apparent activation of both bitter- and sour-sensing TRCs by high concentrations of salt,Citation25) which suggests that either the local pH around sour-sensing TRCs or their sensory mechanisms are affected by the salt content of stimuli.
Perhaps owing to these confounding factors, a large number of candidate molecules involved in acid sensing have been identified, the most significant of which are polycystic kidney disease 1-like 3 (Pkd1l3) and/or Pkd2l1, acid-sensing ion channels (ASICs), hyperpolarization-activated cyclic nucleotide-gated channels, two-pore domain K+ channels (K2Ps), and carbonic anhydrase 4 (Car4). Two main criteria must be met for the identification of an acid sensor; the molecule must be expressed in the appropriate (type-III) TRCs, and the absence of the molecule in sour-sensing TRCs in vivo must eliminate or greatly diminish the sour taste modality without having a major effect on the other modalities. Currently, only Pkd2l1 and Car4 have been shown to fulfill the requirements for sour taste detection in vivo using gene knockout (KO) mice.Citation26,27)
Pkd2l1/Pkd1l3
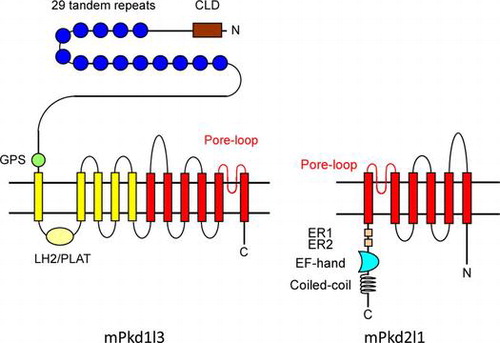
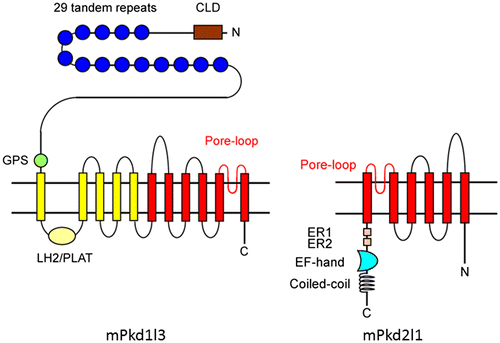
Pkd2l1 is a member of the TRP channel superfamily and has six transmembrane (TM) domains and a putative pore region between the fifth and sixth TM domains (TM5 and TM6) (Fig. ). This structure is similar to that of other TRP channel family members, many of which play important roles in signal transduction in various sensory systems, including vision, smell, pheromone, hearing, touch, osmolarity, thermosensation, and taste.Citation28) Pkd1l3 is a large protein with a very long N-terminal extracellular domain, followed by 11 TM domains that include a 6-TM TRP-like channel domain at the C-terminus (Fig. ). Due to structural differences, Pkd1l3 is not classified as a TRP channel, but is distantly related to TRP channels based on amino acid sequences.
Fig. 1. Schematic drawing illustrating conformational structures of Pkd1l3 and Pkd2l1.
Notes: Mouse Pkd1l3 is predicted to have 11 transmembrane domains, and contains an N-terminal CLD, 29 S/P-rich repeats, a GPS, and a LH2/PLAT domain. Note that human PKD1L3 does not contain S/P-rich repeats, even in its genomic sequences. Pkd2l1 is predicted to have six transmembrane domains and contains a putative Ca2+ binding EF hand motif and predicted coiled-coil domain in its C-terminal cytoplasmic tail. CLD, C-type lectin domain; S/P-rich repeats, serine/proline-rich repeats; GPS, G-protein-coupled receptor proteolytic site; PLAT, polycystin-1-lipoxygenase-alpha toxin; LH2, lipoxygenase homology 2; ER, endoplasmic recticulum retention signal; EF hand, calcium-binding domain. Portions of this Figure were modified from Fig. in Delmas et al.Citation44)
Pkd1l3 and Pkd2l1 are robustly co-expressed in the same subset of type-III TRCs of the CvP and FoP. This subset is distinct from the sweet-, bitter-, and umami-sensing type-II cells.Citation18,29) In addition, Pkd2l1, but not Pkd1l3, is expressed in TRCs of the FuP and the palate.Citation18,29)
Transgenic mice in which Pkd2l1-expressing cells were genetically ablated using the diphtheria toxin A fragment (DTA) were generated.Citation18) In these animals, electrophysiological recordings of chorda tympani (CT) nerves reveal a complete lack of response to sour stimuli, while a variety of taste stimuli representing sweet, salty, umami, and bitter are unaffected, indicating that these Pkd2l1-expressing type-III cells function as sour-sensing TRCs.
Studies in a heterologous expression system using human embryonic kidney (HEK) 293T cells demonstrate an interaction between PKD1L3 and PKD2L1 via their transmembrane domains, and that this interaction is required for their functional cell surface expression.Citation29,30) In addition, the PKD2L1 protein does not localize to the taste pore but is distributed throughout the cytoplasm in the TRCs of CvP and FoP in Pkd1l3 KO mice.Citation30) Functional analyses using Ca2+ imaging and patch-clamp recordings also show that HEK293T cells transfected with Pkd1l3 and Pkd2l1 specifically responded to a variety of sour compounds, including citric acid, hydrochloric acid, malic acid, and acetic acid, whereas they did not respond to other taste-quality classes.Citation29)
Pkd1l3 and/or Pkd2l1 KO mice were taken and analyzed using taste nerve recordings. In Pkd2l1 KO mice, CT nerve responses to sour stimuli are significantly less than those in wild-type mice,Citation26) even though Pkd1l3 is not expressed in the areas innervated by CT. In contrast, the CT nerve responses in Pkd1l3 KO mice and glossopharyngeal (GL) nerve responses in single- and double-KO (both Pkd1l3 and Pkd2l1) mice are comparable to the responses observed in wild-type mice.Citation26,31) These results indicate that Pkd1l3 is not required for acid detection in TRCs, Pkd2l1 is required at least in part for acid sensing in TRCs innervated by the CT nerve (the FuP and palate TRCs), and that there is a different sour-sensing mechanism for TRCs innervated predominantly by the GL nerve (the FoP and CvP TRCs).
Subsequent studies have revealed that the Pkd1l3/Pkd2l1 channel has a unique property referred to as an “off-response,” whereby it is activated after the removal of an acid stimulus, even though an initial acid exposure is essential.Citation32) In addition, Ca2+ imaging and patch-clamp recordings using native taste cells show off-responses upon acid stimulation in type-III cells isolated from the CvP (which co-expresses Pkd1l3 and Pkd2l1), but not in cells isolated from the FuP (which expresses Pkd2l1, but not Pkd1l3) of glutamate decarboxylase 67-green fluorescent protein (GFP) knock-in mice, in which type-III cells are labeled with GFP.Citation33) A similar analysis using taste cells isolated from Pkd1l3 and/or Pkd2l1 KO mice may clarify whether and how these two molecules are involved in the “off-response” in taste cells. Future studies are necessary to identify on-response receptors other than PKDs that are involved in sour taste detection, and the physiological function of the Pkd1l3/Pkd2l1-mediated off-response.
CO2 sensing by carbonic anhydrase (CA)
One special case of acidic taste is the sensing of CO2 by taste cells. In mammals, carbonation elicits both somatosensory and chemosensory responses in the oral cavity. CT nerve recordings show dose-dependent sensory responses to aqueous and gaseous CO2.Citation27) To identify TRCs that sense CO2, taste nerve recordings were performed on transgenic mice in which sweet- or sour-sensing TRCs were specifically ablated using cell-specific DTA expression. PKD2L1-DTA mice lacking sour-sensing type-III cells do not show CT nerve responses to various concentrations of CO2, demonstrating that CO2 is detected by the same TRCs that detect citric acid, acetic acid, and hydrochloric acid.
To identify a candidate receptor for carbonic acid, the gene expression profiles of sour-sensing TRCs in wild-type mice were compared with those of TRCs of PKD2L1-DTA mice using a microarray technique. Car4, a member of the carbonic anhydrase (CA) family, is specifically expressed in sour-sensing TRCs. CAs are implicated in CO2 detection in various sensory systems including olfactionCitation34) by reversibly catalyzing the conversion of CO2 to carbonic acid. Immunohistochemical staining using an anti-Car4 antibody reveals the expression of the Car4 protein specifically in sour-sensing TRCs.
To verify the possibility that Car4 functions as a bona fide CO2 sensor in the taste system, CT nerve responses to CO2 stimuli were examined using Car4 KO mice and benzolamide, a CA inhibitor. In both cases, the CT nerve response to CO2, but not to citric acid, was significantly less than the response observed in wild-type mice. Thus, the detection of CO2 is mediated by Car4 expressed in type-III cells in mice, catalyzing the formation of carbonic acid, which can then be detected as a sour stimulus by the acid-sensing taste cells. Even though the Car4 pathway is a highly specialized mechanism for the sensing of CO2 as sour, it is a rare example of one specific gene product being associated with the sensing of a sour-tasting chemical. However, it is not clear whether Car4 functions as a CO2 sensor in humans.
III. Neural wiring of acid-sensing systems
In general, taste buds are connected to the central nervous system (CNS) via cranial nerves (CN) VII (facial nerve) and IX (GL nerve). Taste buds in the FuP are scattered on the anterior region of the tongue, and together with those in the soft palate are innervated by the CT and greater superficial petrosal (GSP) nerves, respectively. These nerves are branches of CN VII, and their cell bodies accumulate in the geniculate ganglion (GG). In contrast, taste buds in the CvP and FoP are located in the posterior region of the tongue and are mostly innervated by CN IX, which has cell bodies in the nodose–petrosal ganglion (NPG). Taste information, as detected by taste buds at the periphery, is transmitted to peripheral gustatory neurons in the geniculate, petrosal, and nodose ganglia and is further conveyed to central gustatory neurons, including the nucleus of the solitary tract (NST), parabrachial nucleus (PbN), thalamus, and primary gustatory cortex in the insula.Citation4–9)
The wheat germ agglutinin (WGA) transgene has been used as a transneuronal tracer to label neural pathways that originate from genetically modified cells that express WGA in a variety of nervous systems, including the gustatory system.Citation35–39) To visualize the gustatory pathway that originates from sour-sensing TRCs in the posterior region of the tongue, transgenic mouse lines expressing WGA in the type-III taste cells of the CvP and FoP under the control of the mouse Pkd1l3 promoter/enhancer have been established.Citation40) Pkd1l3 exhibits specific expression in the TRCs of the CvP and FoP but not in the FuPCitation18,29) or in the solitary chemoreceptor cells of the nasal epithelium.Citation35) Pkd1l3-driven WGA has not only confirmed innervation of the CvP and FoP by neurons of CN IX in the NPG, but has also revealed a small number of cells innervated by neurons of CN VII in the GG via the CT, thereby verifying previous electrophysiological reports of these connections in rats.Citation41) The transgenic mice revealed the sour gustatory pathway from the Pkd1l3-positive sour-sensing TRCs in the posterior region of the tongue, particularly the peripheral gustatory neurons in the NPG and GG to the central neurons in the NST. This pathway is distinct from the trigeminal neural pathway, which mediates nociception.
IV. Perspectives
Although the identity of the sour taste sensor is still not known, some inferences regarding its characteristics can be drawn. Convergent experimental data indicate that the sour detection machinery is housed within Pkd2l1-expressing, pre-synaptic, type-III TRCs or a subset of these cells. There is also evidence that dissociated protons and protonated acids both stimulate the depolarization of these sour-sensing TRCs, supporting the hypothesis that intracellular acidification is the proximate stimulus of sour taste.
Among the current candidate molecules, Pkd2l1 is the only molecule so far that has been shown by in vivo KO experiments to be involved in sour-taste sensing. It could function, at least in the FuP, as one of the underlying sour-sensing mechanisms in those cells.
Many questions still require attention regarding the sour sensor, including the identity of the sensor itself, but also additional questions that remain may facilitate its identification. Chang et al. demonstrated the presence of a Zn2+-sensitive, H+-selective channel that is sufficient to depolarize sour-sensing TRCs.Citation42) If those data are accurate, this proton channel could be the key to understanding acid sensing in TRCs, both by elucidating the mechanism underlying strong acid tastes, and as a means for perturbation, since blocking or removing it may help determine the role of protonated acids.
Another gap in our knowledge is the in vivo function of the other proposed candidate mechanisms for sour tasting, as many of them play important roles outside of their proposed role in detecting sour tastes. Since we have a reliable genetic marker for sour-sensing TRCs (Pkd2l1), sour TRC-specific KO experiments will enable the determination of their involvement in sour sensing. In addition, the intriguing “off-response” mediated by Pkd1l3/Pkd2l1 in TRCs in the CvP requires further investigation, as its presence implies a physiological function that we do not yet understand.
There is also evidence that multiple mechanisms may be involved in acid sensing in sour-sensing TRCs. For instance, there are two basic sour stimuli, protons and protonated acids, and ASIC2 is involved in acid sensing in rats; however, it is not expressed in sour-sensing TRCs in mice. These findings indicate that there is no universal or master acid sensor in these TRCs, but rather two or more concurrent pathways that contribute to the acid-sensing process.
This also seems to be the case in humans. Huque et al. examined FuP tissues in two patients suffering from sour ageusia and in normal controls by performing an RT-PCR analysis to identify candidate sour-sensing molecules (ASIC, PKD, and epithelial sodium channel (ENaC) members).Citation43) Both patients displayed abnormally high detection thresholds for citric acid (representing sour taste) but normal detection thresholds for each of the other taste modalities. These patients were categorized as having profound (but not complete) loss of sour taste. FuP cells from both patients lacked all ASICs as well as both Pkd1l3 and Pkd2l1, while ∂-ENaC appeared to be normally expressed. In contrast, in normal sour tasters, the FuP cells expressed ASICs and Pkd1l3/2l1. The simultaneous lack of ASICs and PKDs in both patients with sour ageusia hints at a two-hit mechanism causing their ageusia, and further supports the hypothesis that multiple pathways are involved in sour sensing.
Taken to the extreme, this could mean that the unique presynaptic nature of type-III sour-sensing TRCs, i.e. their ability to be depolarized, generate action potentials, and release neurotransmitters, makes them the only cells that respond to the broad-based cytosolic acidification of the lingual epithelium caused by sour tastants. In short, they are the only TRCs capable of transmitting this depolarizing acidification, and H+, PKD, ASIC, HCN, and K2P channels expressed in these cells work concurrently to depolarize the TRC, and increase the sensitivity of sour-sensing TRCs to their intended stimuli.
On the other hand, the apparent existence of multiple pathways may simply reflect that the candidate molecules are simply accessory molecules, and the “master” acid sensor is simply yet to be identified. Since the most direct mechanism currently proposed for TRC acidification is via the proton channel, the discovery of such a channel and its genetic manipulation could allow us to assess the contribution of protons and protonated acids to sour-sensing TRC activation and perhaps determine whether it is the “master” acid sensor.
Acknowledgements
This research was performed at the Taste Science Laboratory, Department of Applied Biological Chemistry, Graduate School and Life Sciences at The University of Tokyo and at Duke University. I would like to express my sincere gratitude to Dr Keiko Abe, Dr Soichi Arai, and Dr Hiroaki Matsunami for their guidance and continuous encouragement. I also thank all my collaborators and the members of Taste Science Laboratory for their pertinent advice and expert experimental support.
Additional information
Funding
Notes
Abbreviations: ASIC, acid-sensing ion channel; CA, carbonic anhydrase; Car4, carbonic anhydrase 4; CN, cranial nerves; CNS, central nervous system; CT, chorda tympani; CvP, circumvallate papillae; DTA, diphtheria toxin A fragment; ENaC, epithelial sodium channel; FoP, foliate papillae; FuP, fungiform papillae; GAD, glutamate decarboxylase; GL, glossopharyngeal; GFP, green fluorescent protein; GPCR, G-protein-coupled receptor; GSP, greater superficial petrosal; HCN, hyperpolarization-activated cyclic nucleotide-gated channel; HEK, human embryonic kidney; K2P, K two-pore domain K+ channel; KO, knockout; NPG, nodose–petrosal ganglion; NST, nucleus of the solitary tract; PbN, parabrachial nucleus; Pkd1l3, polycystic kidney disease 1-like 3; Pkd2l1, polycystic kidney disease 2-like 1; TM, transmembrane; TRC, taste receptor cell.
This review was written in response to the author’s receipt of the Japan Society for Bioscience, Biotechnology, and Agrochemistry Award for the Encouragement of Young Scientists in 2012.
References
- Baeyens F, Vansteenwegen D, De Houwer J, Crombez G. Observational conditioning of food valence in humans. Appetite. 1996;27:235–250.10.1006/appe.1996.0049
- Ishimaru Y. Molecular mechanisms of taste transduction in vertebrates. Odontology. 2009;97:1–7.10.1007/s10266-008-0095-y
- Ishimaru Y, Matsunami H. Transient receptor potential (TRP) channels and taste sensation. J. Dent. Res. 2009;88:212–218.10.1177/0022034508330212
- Saper CB. Hypothalamic connections with the cerebral cortex. Prog. Brain Res. 2000;126:39–48.10.1016/S0079-6123(00)26005-6
- Nakamura K, Norgren R. Sodium-deficient diet reduces gustatory activity in the nucleus of the solitary tract of behaving rats. Am. J. Physiol. 1995;269:R647–R661.
- Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav. Neurosci. 1995;109:997–1008.10.1037/0735-7044.109.5.997
- Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav. Neurosci. 1995;109:939–954.10.1037/0735-7044.109.5.939
- Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J. Neurophysiol. 1995;73:2144–2162.
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244.10.1016/j.cell.2009.10.001
- Chaudhari N, Roper SD. The cell biology of taste. J. Cell Biol. 2010;190:285–296.10.1083/jcb.201003144
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J. Comp. Neurol. 2006;497:1–12.10.1002/(ISSN)1096-9861
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J. Comp. Neurol. 2004;468:311–321.10.1002/(ISSN)1096-9861
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J. Neurosci. 2006;26:3971–3980.10.1523/JNEUROSCI.0515-06.2006
- Yang R, Montoya A, Bond A, Walton J, Kinnamon JC. Immunocytochemical analysis of P2X2 in rat circumvallate taste buds. BMC Neurosci. 2012;13:51.10.1186/1471-2202-13-51
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499.10.1126/science.1118435
- Hayato R, Ohtubo Y, Yoshii K. Functional expression of ionotropic purinergic receptors on mouse taste bud cells. J. Physiol. 2007;584:473–488.10.1113/jphysiol.2007.138370
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J. Neurosci. 2009;29:13909–13918.10.1523/JNEUROSCI.2351-09.2009
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938.10.1038/nature05084
- Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 2008;586:2903–2912.10.1113/jphysiol.2008.151233
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Roper SD. Mouse taste buds release serotonin in response to taste stimuli. Chem. Senses. 2005;30:i39–i40.10.1093/chemse/bjh102
- Roper SD. Signal transduction and information processing in mammalian taste buds. Pflugers Arch-Eur. J. Physiol. 2007;454:759–776.10.1007/s00424-007-0247-x
- Kinnamon SC. Taste receptor signalling: from tongues to lungs. Acta Physiol. (Oxf). 2012;204:158–168.10.1111/apha.2011.204.issue-2
- Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J. Physiol. 2003;547:475–483.10.1113/jphysiol.2002.033811
- Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J. Neurosci. 2003;23:2608–2617.
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature. 2013;494:472–475.10.1038/nature11905
- Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, Ninomiya Y. Sour taste responses in mice lacking PKD channels. PLoS ONE. 2011;6:e20007.10.1371/journal.pone.0020007
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445.10.1126/science.1174601
- Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum. Genomics. 2011;5:108–116.10.1186/1479-7364-5-2-108
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Nat. Acad. Sci. USA. 2006;103:12569–12574.10.1073/pnas.0602702103
- Ishimaru Y, Katano Y, Yamamoto K, Akiba M, Misaka T, Roberts RW, Asakura T, Matsunami H, Abe K. Interaction between PKD1L3 and PKD2L1 through their transmembrane domains is required for localization of PKD2L1 at taste pores in taste cells of circumvallate and foliate papillae. FASEB J. 2010;24:4058–4067.10.1096/fj.10-162925
- Nelson TM, LopezJimenez ND, Tessarollo L, Inoue M, Bachmanov AA, Sullivan SL. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem. Senses. 2010;35:565–577.10.1093/chemse/bjq070
- Inada H, Kawabata F, Ishimaru Y, Fushiki T, Matsunami H, Tominaga M. Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 2008;9:690–697.10.1038/embor.2008.89
- Kawaguchi H, Yamanaka A, Uchida K, Shibasaki K, Sokabe T, Maruyama Y, Yanagawa Y, Murakami S, Tominaga M. Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells. J. Biol. Chem. 2010;285:17277–17281.10.1074/jbc.C110.132944
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957.10.1126/science.1144233
- Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol. Cell. Neurosci. 2008;38:505–517.10.1016/j.mcn.2008.04.011
- Ohmoto M, Maeda N, Abe K, Yoshihara Y, Matsumoto I. Genetic tracing of the neural pathway for bitter taste in t2r5-WGA transgenic mice. Biochem. Biophys. Res. Commun. 2010;400:734–738.10.1016/j.bbrc.2010.08.139
- Yoshihara Y. Visualizing selective neural pathways with WGA transgene: combination of neuroanatomy with gene technology. Neurosci. Res. 2002;44:133–140.10.1016/S0168-0102(02)00130-X
- Yoshihara Y, Mizuno T, Nakahira M, Kawasaki M, Watanabe Y, Kagamiyama H, Jishage K, Ueda O, Suzuki H, Tabuchi K, Sawamoto K, Okano H, Noda T, Mori K. A genetic approach to visualization of multisynaptic neural pathways using plant lectin transgene. Neuron. 1999;22:33–41.10.1016/S0896-6273(00)80676-5
- Damak S, Mosinger B, Margolskee RF. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008;9:96.10.1186/1471-2202-9-96
- Yamamoto K, Ishimaru Y, Ohmoto M, Matsumoto I, Asakura T, Abe K. Genetic tracing of the gustatory neural pathway originating from Pkd1l3-expressing type III taste cells in circumvallate and foliate papillae. J. Neurochem. 2011;119:497–506.10.1111/jnc.2011.119.issue-3
- Yamamoto T, Kawamura Y. Dual innervation of the foliate papillae of the rat: an electrophysiological study. Chem. Senses. 1975;1:241–244.10.1093/chemse/1.3.241
- Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc. Nat. Acad. Sci. USA. 2010;107:22320–22325.10.1073/pnas.1013664107
- Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS ONE. 2009;4:e7347.10.1371/journal.pone.0007347
- Delmas P, Padilla F, Osorio N, Coste B, Raoux M, Crest M. Polycystins, calcium signaling, and human diseases. Biochem. Biophys. Res. Commun. 2004;322:1374–1383.10.1016/j.bbrc.2004.08.044
