Abstract
Ionizing radiation and oxidizing agent like H2O2 were used to degrade chitosan (CS) and its derivatives; N-maleoylchitosan (NMCS), and N-phthaloylchitosan (NPhCS). The structure changes were detected using gel permeation chromatography (GPC). The results revealed that ionizing radiation degraded CS, MNCS, NPhCS and altered their molecular weights and antioxidant activity. The higher the irradiation dose, the lower the molecular weight and the higher antioxidant activity. The addition of irradiated CS and NMCS to minced chicken resulted in highly significant reduction in malondialdehyde (MDA) content (50 and 70%, respectively) if compared with the control. The irradiated NMCS toxicity study did not show strong proliferative effect at small concentrations or cytotoxic effects at higher concentrations. The obtained results suggested that CS and NMCS could be used as natural antioxidant for improving the oxidative deterioration of minced chicken during refrigerated storage.
Irradiated NMCS has higher scavenging activity. NMCS could be used as antioxidant. Irradiated NMCS did not show proliferative or cytotoxic effects.
Lipid oxidation, leading to rancidity, is one of the major reasons of meat quality deterioration. To avoid oxidative deterioration of meat and to extend shelf life, several food additives with antioxidative properties from natural source could be used. Nowadays, antioxidants are an important group of food additives as health protecting factors due to the ability to preserve foodstuffs by retarding oxidative deterioration and/or discoloration caused by the oxidation of fats which consequently decrease nutritional quality and safety due to the formation of secondary, potentially toxic, compounds.
In recent years, polysaccharides have been demonstrated to scavenge free radicals in vitro and used as antioxidants for the prevention of oxidative damage in foods.Citation1) The antioxidant activity of polysaccharides depends upon several structural parameters, such as the molecular weight,Citation2,3) type and position of functional groups, (such as hydroxyl, sulfate, amine, carboxyl, and phosphate), type of saccharide, and glycosidic branching.Citation4)
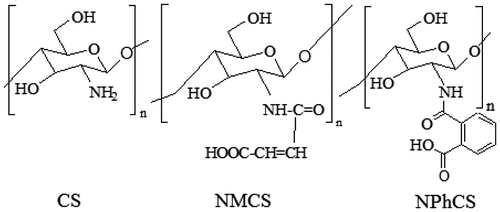
Chitosan is a cationic polysaccharide copolymer, one of the most abundant natural polymers obtained from crustacean shells, insects, molluscan organs, and fungi.Citation5) It exhibits a wide variety of biological activities, including potential food preservative of natural origin.Citation6) Recently, the modification and preparation of low molecular weight fractions or oligosaccharides of natural polymers and identification of their antioxidant properties were investigated.Citation7,8) The chemical modifications of chitosan by introducing new functional groups not only enlarge its applications, but also enhance its water solubility and improve its antioxidant activity. Water-soluble CS derivatives were synthesized by the chemical modification of chitosan with maleic anhydride or phthalic anhydride to prepare N-maleoylchitosan (NMCS) and N-phthaloylchitosan (NPhCS). The degree of substitution of NMCS and NPhCS was calculated as 0.57 and 0.31, respectively.Citation9)
The present work aims to study the effectiveness of irradiated chitosan and its derivatives to improve the oxidative deterioration of minced chicken during refrigerated storage. Water-soluble chitosan derivatives will be synthesized by introducing functional groups onto chitosan backbone by chemical modifications and followed by exposing them to gamma irradiation at different doses in the solid form and in presence of oxidizing agent could enhance the degradation and improve its antioxidant property. Scavenging effect on DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals, reducing power and the ferrous ion chelating activity assays could be used to evaluate the antioxidant activity of oligosaccharides.
Experimental
Materials
Chitosan (CS), degree of deacetylation not less than 85% of high molecular weight, Aldrich. 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt (Ferrozine) were bought from Sigma Chemicals. Maleic- and phthalic anhydride were bought from NICE laboratory reagents. Hydrogen peroxide (H2O2) 30%, Loba chemie, India. EDTA, ferric chloride, and ferrous chloride were supplied from BDH. Potassium ferricyanide was supplied from Riedel laboratory reagents. Trichloroacetic acid and thiobarbituric acid were supplied from SUVCHEM laboratory chemicals. Other reagents and solvents were of analytical grade.
Chicken breasts were collected from a local poultry processing plant immediately after evisceration. Samples were transported to the laboratory in an ice box and stored at 4 °C for no longer than 2 h before use. Samples were separated from the bones and minced in an electrical mincer. Twenty gram samples were sealed in polyethylene bags after the addition of 0.1% of the tested antioxidants and put on the refrigerator (4 ± 1 °C) shelf until analysis.
Synthesis and characterization of chitosan derivatives
N-maleoyl chitosan (NMCS) and N-phthaloyl chitosan (NPhCS) were synthesized and characterized in detail in a previous study by Abd El-Rehim et al.Citation9) The structure of CS, NMCS, and NPhCS was illustrated as shown in Fig. .
Irradiation process
Chitosan (CS), N-maleoyl-chitosan (NMCS), or N-phthaloyl-chitosan (NPhCS) in the solid form were treated by 10% (v/w) H2O2 and well mixed, then irradiated by γ-rays at different doses of 25, 50, 75, and 100 kGy at dose rate of 3.52 kGy/h.
Analytical methods
The number average molecular weights of the degraded polysaccharides were determined by gel permeation chromatography (GPC) 1100 Agilent instrument. The molecular weights were determined from a calibration curve using polyethylene oxide standards of known molecular weights. Analysis by a UV spectrophotometer was carried out using Jasco V-560, Japan, in the range from 190 to 900 nm.
Antioxidant activity measurements
Measurement of free radical scavenging activity on DPPH radical was determined according to the method described by Yamaguchi et al.Citation10) The reducing power of chitosan was determined by the method of Yen and DuhCitation11) The absorbance was measured at 700 nm. Chelating ability was determined according to the method shown by Dinis et al.Citation12) Ascorbic acid was used for comparison as antioxidant materials.
Thiobarbituric acid reactive substances
Lipid peroxidation was assessed through the determination of secondary oxidation products, (Malondialdehyde, MDA, CHO–CH2–CHO), formed during 10 days of refrigerated storage (4 ± 1 °C), by a spectrophotometric method described by Vyncke.Citation13) Thiobarbituric acid reactive substances (TBARS) was performed on days 0, 3, and 10 days of refrigerated storage on 3 separate samples from each group. On 0 day, the TBARS was performed only for the control samples. The results were expressed as mg MDA/kg sample which were then analyzed statistically to determine the significance between the groups.
Fungal toxicity of NMCS
NMCS (2 mg/mL) was added to malt extract agar medium (malt extracts 20 g, glucose 20 g, and peptone 1 g/L) after sterilization; the dissolved NMCS was added right before pouring the media into sterilized plates. After the plates were left to set, 4 mm disks were taken from Phanerocheate chrysosporium ATCC 34541 culture, the disks were punched using the broad end of a sterile tip and placed in the center of each plate. Malt extract agar medium was used as a positive growth control. All plates were left to incubate at 30 °C; the radial growth was measured every 24 h to monitor growth changes in each set. Triplicate plates were used for both the control-and sample-amended plates. The mycelial growth inhibition was calculated using the following equation:
The mycelial growth inhibition = (Gc − Gs/Gc) × 100
where Gc is the mycelial growth for Phanerocheate chrysosporium in centimeter (control plates), and Gs is the mycelial growth in sample-amended plates. The results obtained are presented as percentage of growth inhibition.Citation14)
Cytotoxicity of NMCS
i) Cell Culture. Human colorectal adenocarcinoma cell line (Caco-2) which was purchased from ATCC, USA, was used to evaluate the cytotoxic effect of the tested samples. Cells were routinely cultured in Eagle’s Minimum Essential Medium which was supplemented with 20% fetal bovine serum, 2 mM L-glutamine, containing 100 units/mL penicillin G sodium, 100 units/mL streptomycin sulfate, and 250 μg/mL amphotericin B. Cells were maintained at sub-confluency at 37ºC in humidified air containing 5% CO2. For sub-culturing, monolayer cells were harvested after trypsin/EDTA treatment at 37 °C. Cells were used when confluence had reached 75%. Tested solid sample was dissolved in dimethyl sulfoxide and then diluted thousand times in the assay. All cell culture material was obtained from Cambrex BioScience (Copenhagen, Denmark). All chemicals were from Sigma–Aldrich, USA, except mentioned. All experiments were repeated three times, unless mentioned.
ii) Antitumor Activity. Cytotoxicity of tested extract was measured against Caco-2 cells using the MTT Cell Viability Assay. MTT (3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide) assay is based on the ability of active mitochondrial dehydrogenase enzyme of living cells to cleave the tetrazolium rings of the yellow MTT and form a dark blue insoluble formazan crystals which is largely impermeable to cell membranes, resulting in its accumulation within healthy cells. Solubilization of the cells results in the liberation of crystals, which are then solubilized. The number of viable cells is directly proportional to the level of soluble formazan dark blue color. The extent of the reduction of MTT was quantified by measuring the absorbance at 570 nm.Citation15)
Data analysis
An average value of the replicate analyses was used in calculations of sample variation and significance testing. All statistical analysis was performed with SPSS (SPSS Inc., USA). Differences at p < 0.05 were considered to indicate statistical significance.
Results and discussion
Effect of γ-irradiation on chitosan derivatives
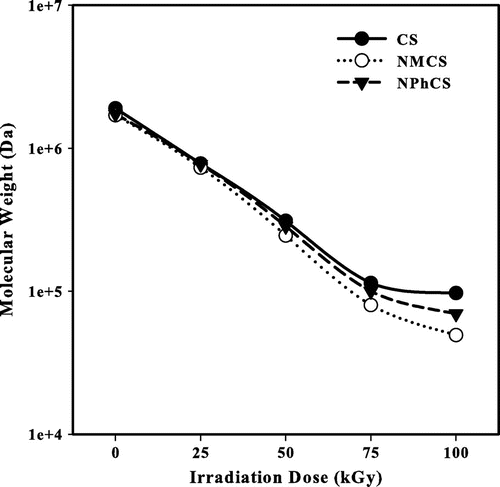
Fig. shows the changes in the number average molecular weights of CS and its derivatives irradiated by γ-rays at different doses in the presence of 10% H2O2 (v/w). With increasing the irradiation dose, the degradation increases and the number average molecular weight of CS and its derivatives decrease. Using 30 kGy irradiation dose, the number average molecular weights of CS, NMCS, and NPhCS decreases from 1.9 × 106, 1.7 × 106, and 1.73 × 106 to 5.8 × 105, 5.5 × 105, 5.6 × 105 Da, respectively. Meanwhile, increasing the irradiation dose to 100 kGy, these values became 9.7 × 104, 4.93 × 104, and 6.95 × 104 Da, respectively. Polysaccharides are well known as typical degradable materials, reducing their molecular weights by ionizing radiation.Citation16) Hydrogen peroxide as chemical initiator when used in small concentration causes breaking of 1,4-β-D-glucoside bonds and rapid decrease in the molecular weight.Citation17,18) The combination of oxidizing agents and ionizing radiation accelerates the degradation process of polysaccharides.Citation19)
Fig. 2. The changes in the number average molecular weight measured by GPC of (●) Cs, (○) NMCS, (▼) and NPhCS treated by 10% H2O2 (v/w) and exposed to gamma irradiation.
FT-IR spectrum of CS Fig. ((A), curve a) shows absorption bands at 3440, 1638, 1598, 1387, 1156, 1097 cm−1 corresponds to –OH and –NH2 groups, stretching C=O of amide group, N–H bend, NH of amide, asymmetric bridge-O-stretch, and skeletal vibration involving the C–O stretch, respectively. For the irradiated CS, Fig. ((A), curves b and c), the intensity of the absorption peak at 1602 cm−1 assigned to the N–H bend vibration of –NH2 increases and shift to low wavenumber. This may be due to the decreasing of inter- or intra-molecular hydrogen bonding between the –OH and –NH2 groups. The band at 1100 cm−1 that corresponds to the ether bond in the pyranose ring has no significant change, which indicates that the stability of the β-glycosidic bonds in the molecular chains of chitosan.
Fig. 3. FT-IR spectra of (A) CS, (B) NMCS, and (C) NPhCS treated by 10% H2O2 (v/w) where (a) unirradiated polymers, (b) irradiated ones at 50 kGy, and (c) irradiated ones at 100 kGy.
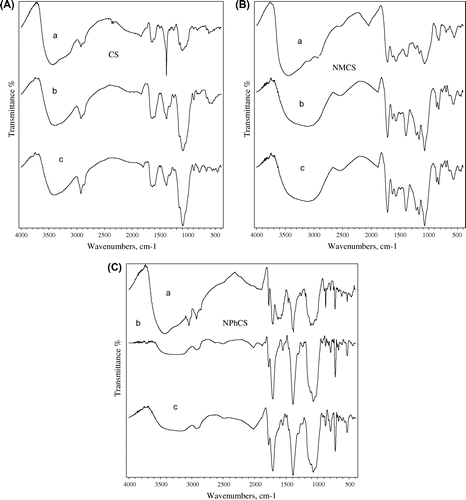
FT-IR spectrum of NMCS Fig. ((B), curve a) shows absorption band at 3400 cm−1 is assigned to the hydrogen bonding of –OH and –NH bands. The bands at 2935 and 1598 cm−1 correspond to C–H stretching vibration and N–H bend vibration, respectively. Also, the new bands at 1719, 1632 and 1547 cm−1 are attributed to (C=O) of acid groups, –NHC=O– and –CH=CH– groups, respectively. FT-IR spectrum of NPhCS Fig. ((C), curve a) shows the absorption bands at 2941 and 3074 cm−1 due to C–H stretch of the –CH=CH– groups and C–H stretch of aromatic system, respectively. The new peaks at 1719, 1773, and 1547 cm−1 are characteristic for the carbonyl group (C=O) of carboxylic acid, residual anhydride, and –CH=CH– groups, respectively. The FT-IR spectra of NMCS and NPhCS after gamma irradiation conditions are shown in Fig. ((B), curves b and c) and Fig. ((C), curves b and c). It was found that most of the characteristic bands of NMCS and NPhCS almost remained after gamma irradiation conditions. It can be concluded that the radiation degradation of CS and its derivatives lead to lower molecular weights by oxidation leaves main polysaccharide chain structure almost remained during degradation process.
Evaluation of antioxidant activity of CS and its derivatives
The antioxidant activity of any compound has been attributed to various mechanisms, among which are prevention of chain initiation, binding of transition metal ion catalysts, decomposition of peroxides, reductive capacity, and radical scavenging.Citation20)
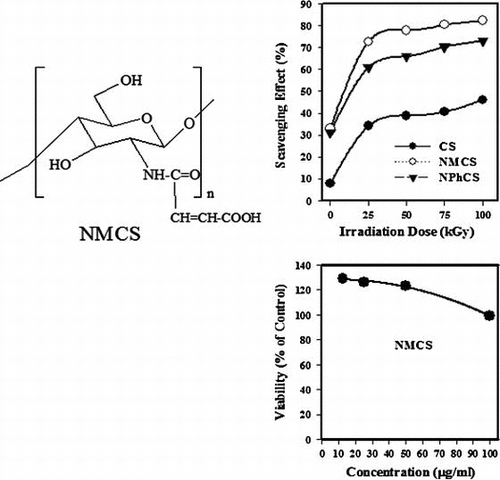
Fig. shows the scavenging effect (%) on DPPH radicals of CS, NMCS, and NPhCS exposed to different irradiation doses in the presence of 10% H2O2 (v/w). It was clear from the results that the scavenging effect (%) increased with increasing the irradiation dose (Fig. (A)) due to the decrease in the molecular weights of polysaccharide. The order of the scavenging effect was NMCS > NPhCS > CS and so irradiated NMCS had the highest antioxidant activity if compared with CS, NPhCS, and ascorbic acid. This finding was corresponding with that reported by other researchersCitation21) that the scavenging ability of CS might be enhanced after N-alkylation of disaccharide. The scavenging effect (%) on DPPH radicals of unirradiated CS, NMCS, NPhCS increased from 7.9, 32.9, and 31.1 (%), to 34.2, 72.67, and 60.89 (%), respectively, when irradiated with 25 kGy, and to 38.8, 77.8, 65.9 (%), respectively, with increasing the irradiation dose to 50 kGy which then leveled off even with further increase in the dose. The scavenging effect (%) on DPPH radicals increased with increasing the concentration of the irradiated CS, NMCS, and NPhCS to 1 mg/mL; thereafter, it tends to level off even with further increase in the concentration (Fig. (B)).
Fig. 4. The scavenging effect (%) on DPPH radicals of (●) CS, (○) NMCS, (▼) NPhCS, and (x) Ascorbic acid.
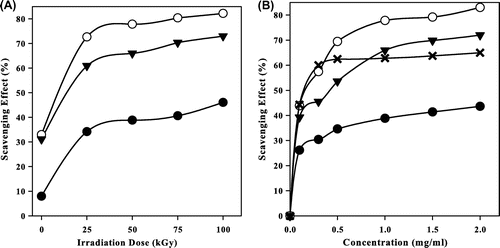
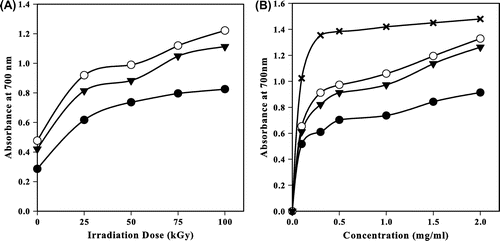
Fig. shows the reducing power of irradiated CS, NMC, and NPhCS in the presence of 10% H2O2 (v/w). It was observed that the reducing power increased with increasing the irradiation dose up to 50 kGy (Fig. (A)), then it leveled off with any further increase in the irradiation dose. NMCS possessed the highest reducing power. The reducing power of 50-kGy-treated CS, NMCS, and NPhCS increased as their concentration increased (Fig. (B)).
Fig. 5. Reducing power of (●) CS, (○) NMCS, (▼) NPhCS and (x) Ascorbic acid.
Metallic cations might be used as catalysts during the oxidation assays. Transition metal ions can initiate lipid peroxidation and start a chain reaction, which lead to the deterioration of flavor and taste in food.Citation22) Iron as transition metal ions can stimulate lipid peroxidation by the Fenton reaction and also accelerate peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals that can themselves abstract hydrogen and perpetuate the chain reaction of lipid peroxidation.Citation23) Since ferric ions (Fe3+) can be reduced by the antioxidants to a catalytically active ion (Fe2+) that provokes the antioxidant to behave as prooxidant. The Fe2+-chelating ability could be monitored spectro-photometrically by absorbance of the ferrous iron–ferrozine complex at 562 nm.
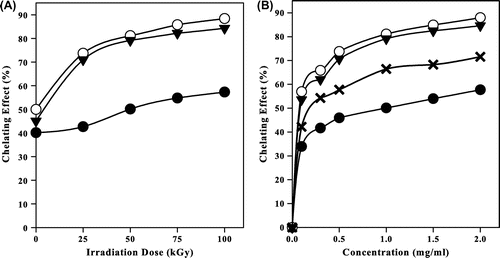
Fig. represents the chelating effect of irradiated CS, NMCS, and NPhCS in the presence of 10% H2O2 (v/w). It was clear that the chelating ability (%) increased with increasing the irradiation dose (Fig. (A)). It also increased with increasing the concentration of the irradiated CS and its derivatives to 1 mg/ml (Fig. (B)) and then leveled off even with further increase in the concentration. The chelating effect (%) of unirradiated CS, NMCS, and NPhCS was 40.1, 49.9, and 45 (%), respectively. At 25 kGy, the values increased to 42.6, 73.6, and 70.9 (%), respectively, meanwhile at 100 kGy, it reached 57.2, 88.3, and 84.2 (%), respectively.
Fig. 6. Chelating effect (%) of (●) CS, (○) NMCS, (▼) NPhCS, and (x) Ascorbic acid.
Chemical and radiation modification of chitosan may partly destroy the inter-molecular and intra-molecular hydrogen bonds. The low solubility of CS is attributed to the crystallinity and intra- and inter-molecular hydrogen bonding within the polymer chains. Improvement of reactivity of CS can be employed by chemical modifications of CS. For example, Wan et al. prepared phosphorylated CS membranes using chemical modification of chitosan membranes through breaking some inter- and intra-molecular hydrogen bonding by introduction of bulky phosphate groups and formation of polyphosphates in the membrane to increase the hydrophilicity, to decrease its crystallinity, and to increase ionic conductivity.Citation24) Also, ionizing radiation can break the interaction between the chitineous substances molecules, decrease their crystallinity % and molecular weight, and enhance their solubility. These changes are expected to increase the antioxidant activity of chitineous substances.Citation9)
As a result, the reactivity is improved. The size and type of the groups introduced to CS may affect the degree of destruction of these bonds. Also, NH2, –COOH, and –CONH– free functional groups of low-molecular weight CS and its derivatives help to form chitosan–Fe2+ complexes.Citation25,26)
γ-Irradiated CS and NMCS as antioxidants for minced chicken
The effect of storage time on extent of the minced chicken lipid peroxidation was investigated. Extent of lipid oxidation of minced chicken was determined by quantifying the MDA concentration formed during 3 and 10 days of refrigerated storage (4 ± 1 °C) Table . It was clear that there was a dramatic increase in the MDA in control samples during storage (from 0.587 at 0-time to 1.234 mg MDA/kg after 10 days). Use of irradiated CS and NMCS resulted in significantly reduction of lipid peroxidation and MDA concentration in minced chicken compared with the control one. The MDA concentration in samples containing 100-kGy-irradiated NMCS was 0.262 and 0.454 mg MDA/kg and samples containing 100-kGy-irradiated CS was 0.333 and 0.550 mg MDA/kg after 3 and 10 days of storage, respectively. Meanwhile, the MDA concentration in the control samples was 0.835 and 1.234 mg MDA/kg after 3 and 10 days of storage, respectively. This means that the addition of 100-kGy-irradiated CS and NMCS at 0.1 (wt %) concentration to minced chicken resulted in a highly significant decrease in malonaldehyde (MDA) content (50 and 70%, respectively), after 10 days storage at 4 ± 1 °C compared with the control. This finding was similar to that reported by Darmadji and IzumimotoCitation6) that the TBA value (expressed as mg MDA/kg) of beef increased sharply in control samples after 10 days of storage at 4 °C as compared with those containing 1% chitosan. In beef patties, Dzudie et al.Citation27) reported that the increase in TBA values was attributed to the accumulation and formation of MDA throughout storage either chemically or microbiologically which indicated oxidative deterioration of lipid into the end products TBARS during storage. Georgantelis et al.Citation28) reported that beef burger containing chitosan did not exceed the threshold values of MDA after 180th day of frozen storage.
Table 1. TBARS (mg MDA/kg sample) in minced chicken during 10 days of refrigerated storage (4 ± 1 °C).
These results suggested that such natural antioxidants may be used as natural preservative for improving the oxidative deterioration of minced chicken during refrigerated storage.
Toxicity of NMCS
Despite the high number of published studies on chitosan and its derivatives, very few biotech companies are using this material. The term “Chitosan” represents a large group of structurally different chemical entities that may show different biodistribution, biodegradation, and toxicological profile.Citation29) It was reported that Chitosan with 0.25, 0.5, 0.75, and 1% concentrations from tiger prawn shell waste was not toxic for BHK-21 cell culture when using parameter CD50.Citation30)
For the NMCS, fungal toxicity and cell culture methods were used to evaluate the NMCS toxicity. Fig. (A) represents the percentage of fungal inhibition zone during 144 h as calculated from the fungal radial growth around the media containing NMCS. The result showed that in the first day, the initial fungal inhibition zone reached to 42%. The inhibition zone decreases and disappeared after 144 h. The decrease in inhibition zone reflects an adaptation of the fungus to the polymer.
Fig. 7. (A). Effect of NMCS on percentage of inhibition radial growth of mycelial and (B) Cytotoxic effect of different concentration of NMCS using MTT assay.
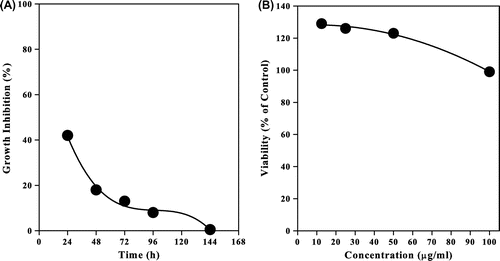
Fig. (B) showed the effect of NMCS on the proliferation of Caco-2 cells after 48 h of incubation. It was found that the treatment of Caco-2 cells with NMCS did not show strong proliferative effect at small concentrations or cytotoxic effects at higher concentration regarding their IC50 643.5 μL/mL and 253.7 μg/mL, respectively.
Conclusion
The modification of CS by chemical treatment and/or ionizing radiation improved its antioxidant activity. The lower molecular weight of CS, NMCS, and NPhCS, the higher the antioxidant activity was obtained. The addition of 100-kGy-irradiated CS and NMCS to minced chicken resulted in a highly significant decrease in MDA content. The irradiated NMCS toxicity study did not show strong proliferative effect at small concentrations or cytotoxic effects at higher concentrations. The obtained results suggested that CS and NMCS could be used as natural antioxidants for preservation of minced chicken during refrigerated storage.
Acknowledgment
This work is supported by The International Atomic Energy Agency (IAEA) under Research Contract No. 14425/RO Regular Budget Fund.
Additional information
Funding
References
- Kim KW, Thomas RL. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007;101:308–313.10.1016/j.foodchem.2006.01.038
- Je JY, Park PJ, Kim SK. Free radical scavenging properties of hetero-chitooligosaccharides using an ESR spectroscopy. Food Chem. Toxicol. 2004;42:381–387.10.1016/j.fct.2003.10.001
- Wang J, Jiang X, Mou H, Guan H. Anti-oxidation of agar oligosaccharides produced by agarase from a marine bacterium. J. Appl. Phycol. 2004;16:333–340.10.1023/B:JAPH.0000047944.40463.e6
- Melo MRS, Feitosa JPA, Freitas ALP, de Paula RCM. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polym. 2002;49:491–498.10.1016/S0144-8617(02)00006-1
- Jang MK, Kong BG, Jeong YI, Lee CH, Nah JW. Physicochemical characterization of _α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci., Part A: Polym. Chem. 2004;42:3423–3432.10.1002/(ISSN)1099-0518
- Darmadji P, Izumimoto M. Effect of chitosan in meat preservation. Meat Sci. 1994;38:243–254.10.1016/0309-1740(94)90114-7
- Sun L, Wang C, Shi Q, Ma C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009;45:42–47.10.1016/j.ijbiomac.2009.03.013
- Xing R, Liu S, Yu H, Guo Z, Li Z, Li P. Preparation of high-molecular weight and high-sulfate content chitosans and their potential antioxidant activity in vitro. Carbohydr. Polym. 2005;61:148–154.10.1016/j.carbpol.2005.04.007
- Abd El-Rehim HA, El-Sawy NM, Hegazy EA, Soliman EA, Elbarbary AM. Improvement of antioxidant activity of chitosan by chemical treatment and ionizing radiation. Int. J. Biol. Macromol. 2012;50:403–413.
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-Diphenyl-2-picrylhydrazyl. Biosci. Biotechnol., Biochem. 1998;62:1201–1204.10.1271/bbb.62.1201
- Yen GC, Duh PD. Antioxidative properties of methanolic extracts from peanut hulls. J. Am. Oil Chem. Soc. 1993;70:383–386.10.1007/BF02552711
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994;315:161–169.10.1006/abbi.1994.1485
- Vyncke WC. Direct determination of the thiobarbituric acid extract of fish as a mean of oxidative rancidity. Fette Seifen Anstrichmittel. 1970;72:1084–1087.10.1002/(ISSN)1521-4133
- Murugesan K, Yang IH, Kim YM, Jeon JR, Chang YS. Enhanced transformation of malachite green by laccase of Ganoderma lucidum in the presence of natural phenolic compounds. Appl. Microbiol. Biotechnol. 2009;82:341–350.10.1007/s00253-008-1819-1
- Hansen MB, Nielsen SE, Berg kJ. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. 1989;119:203–210.10.1016/0022-1759(89)90397-9
- Leonhardt J, Arnold G, Baer M, Langguth H, Gey M, Hgbert S. Radiation degradation of cellulose. Radiat. Phys. Chem. 1985;25:899–904.
- Tian F, Liu Y, Hu K, Zhao BY. Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr. Polym. 2004;57:31–37.10.1016/j.carbpol.2004.03.016
- El-Sawy NM. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr. Polym. 2010;79:555–562.10.1016/j.carbpol.2009.09.002
- Abd El-Rehim HA, El-Sawy NM, Farag IA, Elbarbary AM. Synergistic effect of combining ionizing radiation and oxidizing agents on controlling degradation of Na-alginate for enhancing growth performance and increasing productivity of zea maize plants. Carbohyd. Polym. 2011;86:1439–1444.
- Vishwajeet K, Anil L, Krishnamurty Vijay K. Screening of antioxidant activity from Triticum aestivum extracts. Int. J. Pharm. Tech. Res. 2010;2:1256–1259.
- Lin HY, Chou CC. Antioxidative activities of water-soluble disaccharide chitosan derivatives. Food Res. Int. 2004;37:883–889.10.1016/j.foodres.2004.04.007
- Gordon MH. The mechanism of antioxidant action in introduction. In: Hudson BJF, editor. Food antioxidants. New York, NY: Elsevier Applied Science; 1990. p. 1–18.
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med. 1991;91:814–822.
- Wan Y, Creber KAM, Peppley B, Bui VT. Characterization and ionic conductive properties of phosphorylated chitosan membranes. Macromol. Chem. Phys. 2003;204:850–858.10.1002/macp.200390056
- Guzman J, Saucedo I, Revilla J, Navarro R, Guibal E. Copper sorption by chitosan in the presence of citrate ions: influence of metal speciation on sorption mechanism and uptake capacities. Int. J. Biol. Macromol. 2003;33:57–65.10.1016/S0141-8130(03)00067-9
- Xing R, Liu S, Guo Z, Yu H, Wang P, Li C. Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorg. Med. Chem. 2005;13:1573–1577.10.1016/j.bmc.2004.12.022
- Dzudie T, Kouebou CP, Essia-Ngang JJ, Mbofung CMF. Lipid sources and essential oils effects on quality and stability of beef patties. J. Food Eng. 2004;65:67–72.10.1016/j.jfoodeng.2003.12.004
- Georgantelis D, Blekas G, Katikou P, Ambrosiadis I, Fletouris DJ. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 2007;75:256–264.10.1016/j.meatsci.2006.07.018
- Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Delivery Rev. 2010;62:3–11.10.1016/j.addr.2009.09.004
- Ariani MD, Yuliati A, Adiarto T. Toxicity testing of chitosan from tiger prawn shell waste on cell culture. Dent J. 2009;42:15–20.