Abstract
The ascomycete Pyricularia oryzae (teleomorph: Magnaporthe oryzae) causes one of the most serious diseases known as rice blast. The Nijmegen breakage syndrome protein (NBS1) is essential for DNA repair; thus, we studied the P. oryzae NBS1 homolog (PoNBS1). A PoNBS1 null mutant exhibited high sensitivity to DNA damage-inducing agents. The mutant also exhibited the retarded hyphal growth, and induced abnormal conidial germination and shape, but showed normal appressorium formation. The phenotypes of the null mutant were complemented by introducing the cDNA of PoNBS1 driven by a TrpC promoter of Aspergillus nidulans. In addition, the null mutant similarly complemented with the PoNBS1 cDNA lacking the FHA domain that had a normal phenotype except for hyphal growth. These results suggest that PoNBS1 is involved in DNA repair and normal development in P. oryzae. Moreover, the FHA domain of PoNBS1 participates in normal hyphal growth.
PoNBS1, a Pyricularia oryzae homolog of the Nijmegen breakage syndrome protein (NBS1) gene, is involved in DNA repair and normal development in P. oryzae.
The ascomycete Pyricularia oryzae (teleomorph: Magnaporthe oryzae) causes the disease known as rice blast, which is one of the most serious diseases that affect rice. Moreover, the blast fungus P. oryzae substantially reduces the global rice harvest by 10 to 30%.Citation1) The infection process of P. oryzae begins with the attachment of conidia to the plant surface and the conidia subsequently germinate and the germ tube elongates. Furthermore, P. oryzae recognizes the chemical and physical properties of the plant surface and it differentiates into a specialized infection structure called the appressorium, which is required for plant penetration. The appressorium differentiation process requires a single round of mitosis.Citation2,3) Saunders et al. Citation2) revealed that S-phase entry was essential for the initiation of appressorium formation by pharmacological study using the treatment of hydroxyurea (HU), which completely blocked DNA replication, and the molecular biological study using conditional inactivation mutant of Never-in-Mitosis 1 (nim1I327E), which allowed nuclear division to proceed without DNA replication. This result indicates that S-phase arrest leads to unsuccessful appressorium formation. Harmful DNA damage is recognized by the DNA damage response pathway, thereby causing the activation of cell cycle checkpoints that lead to S-phase arrest and DNA repair.Citation4,5) Therefore, we hypothesized that a DNA repair mechanism may be involved in appressorium formation.
Previously, we constructed a differential cDNA library that represented the early stage of appressorium formation using a cDNA subtraction strategy.Citation6) In this study, we used this cDNA library to identify the genes involved in the DNA repair mechanism during appressorium formation. Therefore, we identified a candidate gene, which was presumed to be a homolog of the Nijmegen breakage syndrome (NBS) protein (NBS1) of P. oryzae (PoNBS1) because the protein encoded by PoNBS1 had a forkhead-associated (FHA) domain at the N-terminus and a conserved C-terminal region.Citation7,8) The NBS1 mutation is responsible for the NBS in humans that is an autosomal recessive disorder and characterized by microcephaly, growth retardation, immunodeficiency, and predisposition to malignancies.Citation9) Cells from NBS patients exhibited sensitivity to ionizing radiation, an increased level of chromosomal aberrations, and defects in DNA repair as well as checkpoint control.Citation7,10) In addition, NBS1 is a part of the Mre11-Rad50-Nbs1 (MRN) complex, which senses double-strand breaks (DSB), recruits, and activates ataxia-telangiectasia-mutated (ATM) kinase to amplify internal signals that regulate the cell cycle checkpoints, and regulates chromatin remodeling in mammalian cells.Citation11) Moreover, NBS1 has additional roles in DNA end-associated processes, such as homologous recombination (HR), non-homologous end joining (NHEJ), meiosis, and telomere maintenance.Citation12–16) Previous reports indicate that NBS1 has essential roles in DNA repair. Therefore, we created the PoNBS1 null mutant and its complementary strains, which carried PoNBS1 with or without its FHA domain, and we investigated the functions of PoNBS1 and its FHA domain using these transformants.
Materials and methods
Strains and growth conditions
P. oryzae wild-type strain P2 and all of the transformants generated in this study are described in Table . These strains were grown on oatmeal agar [OMA: 5% oatmeal (w/v), 0.5% sucrose (w/v), and 1.5% agar (w/v)] plates or yeast glucose agar [YGA: 0.5% yeast extract (w/v), 2.0% glucose (w/v), and 1.5% agar (w/v)] plates at 28 °C in darkness. To extract DNA or RNA, these strains were cultured in yeast-glucose [YG: 0.5% yeast extract (w/v) and 2.0% glucose (w/v)] liquid medium for three days with shaking.
Table 1. Wild type and transgenic mutant strains of P. oryzae used in this study.
DNA and RNA isolation and Southern blot hybridization
To isolate DNA and RNA, P. oryzae grown in YG liquid medium was harvested and ground to a powder in liquid nitrogen. Genomic DNA was extracted from P. oryzae as described previously.Citation17) Total RNA was extracted from mycelia with ISOGEN, according to the manufacturer’s instructions (Nippon Gene, Toyama, Japan). RNA was treated with DNase I (Promega, Madison, WI) before RT-PCR. First-strand cDNA synthesis was conducted using Oligo(dT)12–18 Primer (Invitrogen, Carlsbad, CA) with Ready-To-Go You Prime First-Strand Beads (GE Healthcare, Little Chalfont, UK), according to the manufacturer’s instructions.
Southern blot hybridization of P. oryzae was performed according to the digoxigenin (DIG) application manual for filter hybridization (Roche Diagnostics K·K., Tokyo, Japan). Genomic DNA was digested with BamHI or SphI and hybridized with DIG-labeled DNA probes: FL probe and HPH-C probe. The FL and HPH-C probes were synthesized using a PCR DIG Probe Synthesis Kit (Roche), according to the manufacturer’s protocol using the B51FLup/B51FLdown and hphprobe2F/hphprobe2R primer pairs (Table ), respectively.
Table 2. Polymerase chain reaction primers used in this study.
Construction of plasmid vectors
The upstream and downstream flanking sequences of PoNBS1 were amplified with the primer pairs B51FLup/B51FLdown and B51FRup/B51FRdown (Table ). The upstream flanking sequence (1.8-kb) was cloned between the SpeI and EcoRV sites of the pCSN43 vector, which contains the hygromycin phosphotransferase gene (HPH) expression cassette (HPH-C).Citation18) The resulting vector was digested with KpnI and ligated with the downstream flanking sequence (1.7-kb), which was digested by KpnI. This final gene replacement construct was designated as the pDB51 vector.
The pBF101 vector, which contains the blasticidin S deaminase gene (BSD) from Aspergillus terreus between the TrpC promoter and terminator sequences from A. nidulans was used for the complementation assay.Citation19) PoNBS1 cDNA was amplified with the primer pair: GP3up and GB513down (Table ). The cDNA from PoNBS1 without the FHA domain was amplified with the primer pair: B51LOFHAup and GB513down (Table ). The cDNA samples amplified from PoNBS1 and PoNBS1 without FHA domain were digested with HindIII and PstI. Furthermore, the cDNA was cloned between the TrpC promoter and terminator sequences of pBF101, which were digested with HindIII and PstI, to yield pCB51 and pCB51dF, respectively. Therefore, pCB51 and pCB51dF lacked the BSD expression cassette.
Fungal transformation
P. oryzae was transformed according to previously described methods.Citation19,20) To replace the target gene, P. oryzae wild-type strain P2 was transformed by introducing pDB51. The transformants were suspended in YG20S agar [0.5% yeast extract (w/v), 2% glucose (w/v), 20% sucrose (w/v), and 1.5% agar (w/v)] containing 100 μg/mL of hygromycin B (Wako Pure Chemical Industries, Osaka, Japan) and cultured at 28 °C for two to three days. The plates were overlaid with YGA medium containing 200 μg/mL of hygromycin B. The plates were incubated at 28 °C for three days and the transformants were selected, which were then transferred to new YGA plates containing 100 μg/mL of hygromycin B and were cultured. These transformants were confirmed by Southern blot analysis. The disrupted mutant was named D4.
pBF101 was used with pCB51 or pCB51dF to cotransform the protoplasts of D4 in the complementation experiments. pBF101 was used to confer resistance to blasticidin S. The transformants were transferred to BN20S agar [0.16% yeast nitrogen base w/o amino acids and ammonium sulfate (w/v), 0.1% ammonium nitrate (w/v), 0.5% glucose (w/v), 20% sucrose (w/v), and 1.5% agar (w/v)] plates containing 5 μg/mL of blasticidin S hydrochloride (Funakoshi, Tokyo, Japan) and cultured at 28 °C for three to four days. Next, the plates were overlaid with BN agar [0.16% yeast nitrogen base w/o amino acids and ammonium sulfate (w/v), 0.1% ammonium nitrate (w/v), 0.5% glucose (w/v), 0.5% sucrose (w/v), and 1.5% agar (w/v)] medium containing 10 μg/mL of blasticidin S hydrochloride and cultured at 28 °C for three days. The transformants were selected and transferred to new BN plates containing 5 μg/mL of blasticidin S hydrochloride, and then cultured. These transformants were confirmed by genomic PCR using the primer pairs; GP3up/GB513down and B51LOFHAup/GB513down. The genomic PCR was performed with Taq DNA polymerase (New England Biolabs Japan Inc., Tokyo, Japan), according to the manufacturer’s instructions.
Analysis of colony growth in DNA damage-inducing conditions
Six-day-old mycelia from YGA plates were transferred to new YGA plates containing various concentrations of DNA damage-inducing agents, i.e. methyl methanesulfonate (MMS) or HU, and incubated at 28 °C for eight days in dark conditions. The hyphal growth of P. oryzae was determined by measuring the diameter of the colonies.
Observations of the conidial morphology, conidial germination, and appressorium formation
Conidial germination and appressorium formation were analyzed as described previously,Citation17) with minor modifications. To induce the production of conidia, five-day-old mycelia was cultured on OMA plates at 25 °C for three days under black light (FL20S・BLB, Panasonic Corporation, Osaka, Japan), after removing aerial hyphae. The conidia were collected and resuspended at 3 × 104 conidia/mL in distilled water. Next, the cell numbers within conidia were counted using light microscopy. The conidia were cultured on microscope cover slips (Fisher Scientific, Waltham, MA) at 25 °C. The total numbers of germinated conidia and appressoria were counted at 6 and 24 h after inoculation of the conidia. Over 100 conidia were observed in each experiment and all of the experiments were repeated at least three times.
GenBank accession numbers of genes
The sequence data for the genes described in this study were submitted to GenBank, as follows: PoNBS1 (EHA57248), SpNbs1 (BAC80248), ScXrs2 (AAB64805), HsNBS1 (BAA28616), MmNibrin (BAA76298), and Dmnbs1 (NP_651973).
Results
Isolation of PoNBS1 from the differential cDNA library
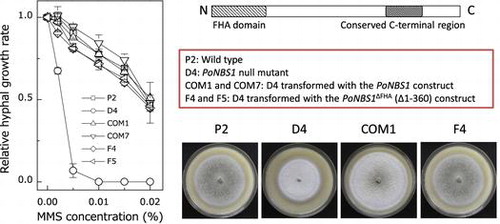
Using the differential cDNA library, we identified the gene involved in DNA repair, which was expressed during appressorium formation.Citation6) We identified a P. oryzae cDNA clone (named B51) that putatively encoded a protein of 881 amino acids, where the domains were characteristic of NBS1, i.e. an FHA domain at the N-terminus and a conserved C-terminal region (Fig. (A)). The FHA domain of NBS1 is estimated to be a phospho-specific protein–protein interaction motif and the conserved C-terminal region of NBS1 binds to the MRN complex, ATM and ataxia-telangiectasia mutated related (ATR) protein kinase, which are proteins involved in DNA repair.Citation7,8) Therefore, we designated the B51 clone as PoNBS1. Next, we aligned the protein sequence of the FHA domain with previously characterized NBS1 homologs using CLUSTALW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and BoxShade (http://www.ch.embnet.org/software/BOX_form.html). The alignment showed that the FHA domain of PoNBS1 was highly conserved compared with the previously characterized NBS1 proteins (Fig. (B)).
Fig. 1. PoNBS1 contains conserved domains that are characteristic of the NBS1 protein.
Notes: (A) Domain structure of the typical NBS1 protein. The FHA domain is believed to be a phospho-specific protein–protein interaction motif. NBS1 binds to an MRN complex, ATM and ATR, which are proteins involved in DNA repair, at the conserved C-terminal region; (B) Amino acid alignment of the FHA domain from PoNBS1 and previously characterized NBS1 proteins. The sequences were aligned using CLUSTALW2 and processed with Boxshade. The residues highlighted in black boxes indicate the conserved amino acids. Sp, Schizosaccharomyces pombe; Sc, Saccharomyces cerevisiae; Hs, Homo sapiens; Mm, Mus musculus; and Dm, Drosophila melanogaster.
Generation of PoNBS1 mutants
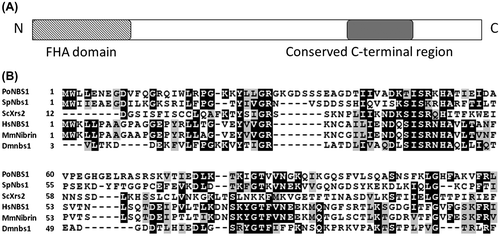
To analyze the functions of PoNBS1 and its FHA domain, we constructed the P. oryzae mutants shown in Table . The knockout mutant of PoNBS1 was generated via a homology-dependent replacement strategy and we designated the disrupted mutant as D4 (Fig. (A)). The Southern hybridization analysis detected a single 10.1-kb band in wild-type strain P2 and a single 3.7-kb band in D4 using the FL probe (Fig. (B), left). In addition, we detected no band in P2 and a single 4.7-kb band in D4, when using the HPH-C probe (Fig. (B), right). These results suggest that the HPH-C construct was replaced in the PoNBS1 region of the genome in D4. To facilitate genetic complementation, a construct containing the full-length cDNA of PoNBS1 or the cDNA of PoNBS1 that lacked the FHA domain (amino acid residues 1–120) was transfected into D4. To confirm the insertion of these cDNAs in D4, we performed genomic PCR using the primer pairs GP3up/GB513down to determine the full-length PoNBS1 cDNA-complemented strains and B51LOFHAup/GB513down to determine the PoNBS1△FHA cDNA-complemented strains (Fig. (C)–(E)). The genomic PCR using the primer pair GP3up/GB513down obtained a 3.0-kb product using the P2 genome and a 2.7-kb product using the COM1 and COM7 genomes (Fig. (D)). These results agreed with the predicted sizes of the genomic DNA and cDNA from PoNBS1, respectively. The genomic PCR using the primer pair B51LOFHAup/GB513down obtained a 2.4-kb product using all strains, except for the D4 genome (Fig. (E)). These results agreed with the predicted size of the cDNA from PoNBS1△FHA. Therefore, we were able to obtain the following strains: COM1 and COM7, the complemented mutants; F4 and F5, the FHA domain of PoNBS1 deletion mutants.
Fig. 2. Generation of PoNBS1 mutants.
Notes: (A) Schematic diagrams showing the strategy used for gene replacement in the PoNBS1 loci of the P. oryzae genome. The pDB51 construct was generated via ligation of the HPH-C, the amplified FL (upstream flanking sequence of PoNBS1) fragment and FR (downstream flanking sequence of PoNBS1) fragment to facilitate targeted gene deletion using the gene replacement approach. The restriction enzyme sites are abbreviated as follows: B, BamHI; S, SphI. (B) Confirmation of PoNBS1 disruption by Southern blot analysis. Genomic DNA extracted from the wild type (P2) and the PoNBS1-disrupted mutant (D4). DNA was digested with either BamHI (left) or SphI (right). The blots were probed with the FL (left) and HPH-C (right) fragments shown in (A). The results showed that the replacement of PoNBS1 with the knockout construct carrying HPH-C was accomplished successfully. M: position of the Lambda-HindIII DNA size standard. (C) The arrows indicate the primer positions for the genomic PCR. (D) Genomic PCR of the wild type and PoNBS1 mutants using the primer pair GP3up/GB513down. The 3.0-kb band corresponds to the genomic PoNBS1 gene and the 2.7-kb band corresponds to the PoNBS1 cDNA. M: position of the Lambda-HindIII DNA size standard. (E) Genomic PCR of the wild type and PoNBS1 mutants using the primer pair B51LOFHAup/GB513down. The 2.4-kb band corresponds to the PoNBS1△FHA cDNA. M: position of the Lambda-HindIII DNA size standard.
Mutation of PoNBS1 increased the sensitivity to DNA damage
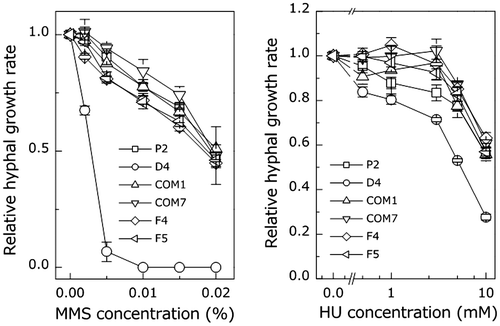
One of the hallmarks of NBS1 is its sensitivity to mutagenic agents that lead to DNA damage. MMS causes the methylation of DNA at the N-7 position in guanine and N-3 in adenine bases, thereby inducing single-strand breaks caused by methylation and DSB caused by stalled replication forks.Citation21) HU inhibits ribonucleotide reductase and depletes the deoxyribonucleotide (dNTP) pool, thereby causing the stalling of replication fork.Citation22) Thus, MMS and HU are used widely as potent DNA damage-inducing agents. To investigate whether PoNBS1 has a DNA repair function in P. oryzae, we examined the effects of MMS and HU on hyphal growth in the wild type and PoNBS1 mutants (Fig. ). Hyphal growth was decreased by MMS or HU in a dose-dependent manner in all strains. Furthermore, D4 exhibited the highest degree of growth inhibition in the presence of MMS or HU among the strains tested. The hyphal growth of D4 was decreased significantly by MMS and decreased slightly by HU. The complemented strains COM1 and COM7 restored hyphal growth to the P2 phenotype. Moreover, F4 and F5, which possessed FHA domain deletions, differed little in terms of their sensitivity to MMS or HU compared with P2 and the complemented strains (Fig. ). These results suggest that PoNBS1 is required for DNA repair, and that it acts in the same manner as NBS1. Our results also indicate that the function of the FHA domain of PoNBS1 is not required for the DNA repair after damage by mutagenic agents.
PoNBS1 is required for appropriate hyphal growth
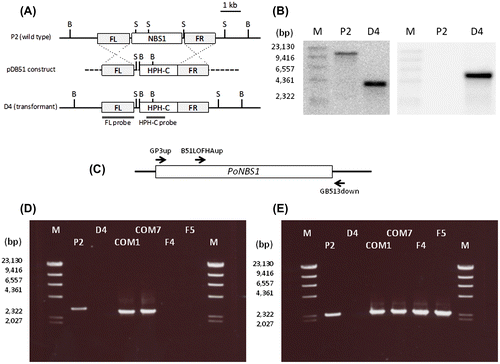
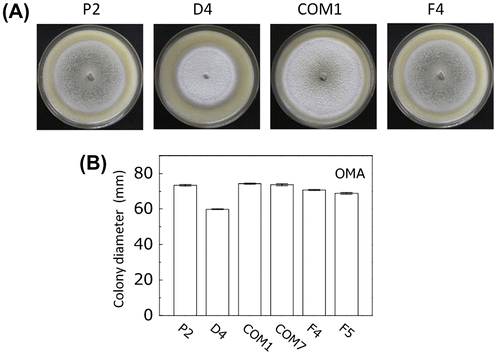
In our more detailed analysis of PoNBS1 in P. oryzae, we investigated the vegetative growth of the wild type and PoNBS1 mutants on OMA plates for 13 days (Fig. ). Based on the statistical analysis (Supplemental Fig. 1), we found that D4 exhibited reduced radial growth compared with the other strains, whereas the family-wise comparison detected no significant differences between P2, COM1, and COM7. Moreover, F4 and F5 exhibited relatively weak hyphal growth restoration. These results suggest that PoNBS1 promotes appropriate hyphal growth, and that the FHA domain of PoNBS1 functions partially during hyphal growth.
Fig. 4. Comparison of vegetative growth by the wild type and PoNBS1 mutants.
Notes: (A) Colony morphology of the wild type and PoNBS1 mutants. The P. oryzae strains were cultured on OMA plates for 13 days. Scale bar = 1 cm. (B) Quantification of hyphal growth by the wild type and PoNBS1 mutants grown on OMA plates for 13 days, as shown in (A). Each data point represents the mean ± SE (n ≥ 3).
PoNBS1 is involved in conidial septation
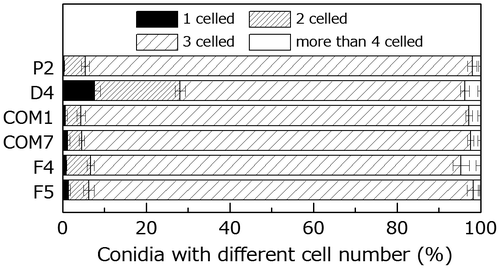
We assessed the functions of PoNBS1 in conidium development using microscopic analysis. P. oryzae conidia are typically three-celled and pear-like in shape. The disruption of PoNBS1 led to an increased proportion of one- or two-celled conidia, whereas no obvious differences in the conidial morphology were observed in P2, COM1, COM7, F4, and F5 (Fig. ). These results suggest that the conidia of D4 exhibited an abnormal phenotype in terms of their septation (Fig. ). Moreover, we examined the distribution of nuclei in P2 and D4 (Supplemental Fig. 2). Although this research is still in a preliminary stage, our results suggest that the number of nuclei tended to be lower in the three-celled conidia of D4. Therefore, our results indicate that PoNBS1 is required for correct asexual reproduction in P. oryzae, whereas the FHA domain of PoNBS1 is not required.
PoNBS1 has a functional role in the germination of conidia
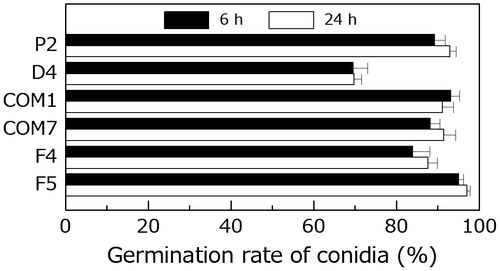
To determine whether PoNBS1 is involved in the germination of conidia, freshly harvested wild-type and PoNBS1 mutant conidia were incubated for 6–24 h on a hydrophobic surface. At 6 h post-inoculation, 89% of the P2 conidia had germinated, whereas only 69% of the D4 conidia germinated (Fig. ). The percentage of germinated conidia in D4 did not increase up to 24 h post-inoculation (Fig. ). This suggests that the decrease in germinated conidia in D4 was not attributable to the delayed germination of conidia. By contrast, the percentages of germinated conidia in COM1, COM7, F4, and F5 differed little compared with P2, regardless of the observation time (Fig. ). These results suggest that PoNBS1 has specific functions in the germination of conidia, whereas the FHA domain of PoNBS1 is not involved.
Normal appressorium formation in the PoNBS1 mutants
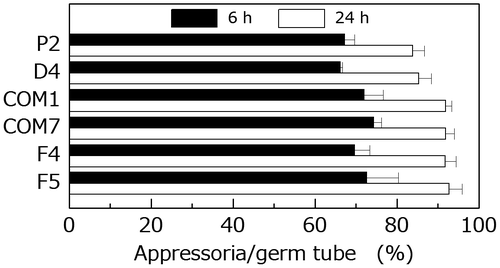
Mutation in PoNBS1 may prevent entry into the S-phase cell cycle checkpoint in the same manner as mutation in NBS1 in various other eukaryotes.Citation23–25) Activation of the S-phase cell cycle checkpoint inhibits appressorium formationCitation2); thus, we predicted that D4 would have the same or a higher percentage of conidia-forming appressoria than P2. As expected, the frequency of appressorium formation in D4 differed little from that in P2 (Fig. ). F4 and F5 also exhibited the same frequency of appressorium formation as P2, COM1, and COM7 (Fig. ).
Discussion
NBS1 is reported to be important in various biological processes related to DNA repair.Citation7,12) In this study, we identified and characterized PoNBS1 in P. oryzae. The FHA domain sequences in the N-terminus of NBS1 are well conserved in eukaryotes, including P. oryzae (Fig. (B)). The FHA domain is a modular phosphopeptide recognition domain, which is found in over 200 proteins.Citation26,27) Most of the proteins that contain the FHA domain are localized in the nucleus, and they are involved in cell cycle progression, DNA repair, and the control of transcription.Citation26,27) The FHA domain of NBS1 is related to genome integrity and foci formation as well as participating in the intra-S-phase and the G2/M phase checkpoint, although the FHA domain is not involved in the MRN complex formation.Citation28–32) Therefore, we investigated the functions of the FHA domain of PoNBS1 in this study.
To determine whether PoNBS1 acts in the same manner as NBS1, we examined whether PoNBS1 mutants exhibited the same response as NBS1 mutants to DNA damage-inducing agents. We examined the sensitivity of the PoNBS1 mutants to MMS and HU, and we found that the null mutation of PoNBS1 resulted in an increased sensitivity to DNA damage-inducing agents (Fig. ). This suggests that PoNBS1 is involved in DNA repair, and that it functions in the same manner as NBS1 in P. oryzae. In addition, the strains where PoNBS1 lacked its FHA domain did not differ in terms of their sensitivity to MMS and HU compared with the wild-type and the PoNBS1-complemented strains of P. oryzae (Fig. ). This result is consistent with previous studies using other eukaryotes.Citation31,32) NBS1 interacts with ATR, which is involved in single-strand break repair,Citation33–35) and MMS induces single-strand breaks.Citation21) The putative ATR-binding region of NBS1 is not located in its FHA domainCitation33); thus, the FHA domain of PoNBS1 is presumably dispensable for the repair of DNA damage induced by MMS.
The hyphal growth rates of the PoNBS1 mutants were slow compared with the wild type (Fig. ), which are consistent with previous studies, e.g. NBS1 mutants of Schizosaccharomyces pombe also exhibited delayed growth.Citation15) NBS1 is involved in DSB repair,Citation12) which can occur at any time during the life cycle of P. oryzae,Citation36) thereby suggesting that the delayed hyphal growth of the PoNBS1 mutants was caused by a deficit in the normal DSB repair system. A previous studyCitation37) suggests that NHEJ is employed more commonly for DSB repair in fungi, with an asexual lifecycle. However, in P. oryzae and many other fungi, the mutations in Ku80 and Lig4, which are involved in NHEJ, did not change the normal hyphal growth, but instead they increased the frequency of HR.Citation38–40) As shown in Fig. , the FHA domain of PoNBS1 is required partially for normal hyphal growth. The FHA domain is assumed to function during NHEJ in Saccharomyces cerevisiaeCitation31); thus, the FHA domain of PoNBS1 may have a minor role in normal hyphal growth via NHEJ.
In this study, the PoNBS1 mutants had a relatively high percentage of conidia, which possessed fewer conidial septa (Fig. ). In A. nidulans, the NBS1 homolog ScaA is involved in the septum formation.Citation41) The reduced cell number in conidia may be attributable to abnormal septum formation because of the PoNBS1 mutation. The one- and two-celled conidia comprised 28% of the total conidia in D4 (Fig. ), which was similar to the percentage of ungerminated conidia in D4 (Fig. ). Moreover, the one- or two-celled conidia were likely to have difficulty germinating (data not shown), and the decreased germination of D4 conidia may have been because of the malformation of conidia. In addition, we obtained preliminary data which showed that the PoNBS1 mutation caused a decrease in the number of nuclei in conidia (Supplemental Fig. 2). This unusual nuclear distribution because of a mutation in PoNBS1 may have been attributable to a decrease in the germination of D4 conidia.
The formation of appressoria in P. oryzae demonstrated the progression through the S-phase of the cell cycle.Citation2) We showed that D4 exhibited normal appressorium formation (Fig. ) and the NBS1 mutation allowed the cell cycle to proceed irrespective of DNA damage. Thus, we suggest that the PoNBS1 mutation may have promoted progression into the S-phase of cell cycle. However, a detailed analysis of the relationship between PoNBS1 and the cell cycle during appressorium formation demands further research.
In this study, our analysis of PoNBS1 using the PoNBS1 mutants showed that PoNBS1 is involved in DNA repair and normal development in P. oryzae. In P. oryzae, appressorium formation is essential for rice infection in natural conditions; thus, clarifying the roles of PoNBS1 in appressorium formation may facilitate rice blast control in the future.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1015951.
Acknowledgments
We thank Yuuya Kakumu and Yuuki Koizumi for technical assistance. We also thank Shin Fukui and Yo Shinoda for comments regarding statistical analysis.
References
- Skamnioti P, Gurr SJ. Against the grain: safeguarding rice from rice blast disease. Trends Biotechnol. 2009;27:141–150.10.1016/j.tibtech.2008.12.002
- Saunders DGO, Aves SJ, Talbot NJ. Cell cycle-mediated regulation of plant infection by the rice blast fungus. Plant Cell. 2010;22:497–507.10.1105/tpc.109.072447
- Veneault-Fourrey C, Barooah M, Egan M, Wakley G, Talbot NJ. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science. 2006;312:580–583.10.1126/science.1124550
- Goodarzi AA, Jeggo PA. The repair and signaling responses to DNA double-strand breaks. Adv. genet. 2013;82:1–45.10.1016/B978-0-12-407676-1.00001-9
- Caldecott KW. DNA single-strand break repair. Exp. Cell. Res. 2014. http://dx.doi.org/10.1016/j.yexcr.2014.08.027
- Kamakura T, Xiao JZ, Choi WB, Kochi T, Yamaguchi S, Teraoka T, Yamaguchi I. cDNA subtractive cloning of genes expressed during early stage of appressorium formation by Magnaporthe grisea. Biosci. Biotechnol. Biochem. 1999;63:1407–1413.10.1271/bbb.63.1407
- Kobayashi J, Antoccia A, Tauchi H, Matsuura S, Komatsu K. NBS1 and its functional role in the DNA damage response. DNA Repair. 2004;3:855–861.10.1016/j.dnarep.2004.03.023
- Williams RS, Williams JS, Tainer JA. Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem. Cell Biol. 2007;85:509–520.10.1139/O07-069
- Chrzanowska KH, Gregorek H, Dembowska-Baginska B, Kalina MA, Digweed M. Nijmegen breakage syndrome (NBS). Orphanet J. Rare Dis. 2012;7:http://www.ojrd.com/content/7/1/13.
- vanderBurgt I, Chrzanowska KH, Smeets D, Weemaes C. Nijmegen breakage syndrome. J. Med. Genet. 1996; 33: 153–156.10.1136/jmg.33.2.153
- Zhou BBS, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439.
- Saito Y, Fujimoto H, Kobayashi J. Role of NBS1 in DNA damage response and its relationship with cancer development. Transl. Cancer Res. 2013;2:178–189.
- Dimitrova N, de Lange T. Cell cycle-dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of nonhomologous end joining (NHEJ) in G(1) and resection-mediated inhibition of NHEJ in G(2). Mol. Cell. Biol. 2009;29:5552–5563.10.1128/MCB.00476-09
- Takata H, Tanaka Y, Matsuura A. Late S phase-specific recruitment of Mrel11 complex triggers hierarchical assembly of telomere replication proteins in Saccharomyces cerevisiae. Mol. Cell. 2005;17:573–583.10.1016/j.molcel.2005.01.014
- Ueno M, Nakazaki T, Akamatsu Y, Watanabe K, Tomita K, Lindsay HD, Shinagawa H, Iwasaki H. Molecular characterization of the Schizosaccharomyces pombe nbs1(+) gene involved in DNA repair and telornere maintenance. Mol. Cell. Biol. 2003;23:6553–6563.10.1128/MCB.23.18.6553-6563.2003
- Tsukamoto Y, Taggart AKP, Zakian VA. The role of the Mre11-Rad50-Xrs2 complex in telomerase-mediated lengthening of Saccharomyces cerevisiae telomeres. Curr. Biol. 2001;11:1328–1335.10.1016/S0960-9822(01)00372-4
- Kamakura T, Yamaguchi S, Saitoh K, Teraoka T, Yamaguchi I. A novel gene, CBP1 , encoding a putative extracellular chitin-binding protein, may play an important role in the hydrophobic surface sensing of Magnaporthe grisea during appressorium differentiation. Mol. Plant Microbe Interact. 2002;15:437–444.10.1094/MPMI.2002.15.5.437
- Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E. Use of a bacterial Hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet. Newslett. 1989;36:79–81.
- Kimura M, Kamakura T, Tao QZ, Yamaguchi I. Cloning of the blasticidin S deaminase gene (BSD) from Aspergillus terreus and its use as a selectable marker for Schizosaccharomyces pombe and Pyricularia oryzae. Mol. Gen. Genet. 1994;242:121–129.10.1007/BF00391004
- Ishii A, Kumasaka M, Nagashima Y, Nakajima Y, Kuramochi K, Sugawara F, Narukawa M, Kamakura T. A eukaryotic molecular target candidate of roxithromycin: fungal differentiation as a sensitive drug target analysis system. Biosci. Biotechnol. Biochem. 2013;77:1539–1547.10.1271/bbb.130210
- Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman ASH, Helleday T. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005;33:3799–3811.10.1093/nar/gki681
- Koc A, Wheeler LJ, Mathews CK, Merrill GF. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J. Biol. Chem. 2004;279:223–230.10.1074/jbc.M303952200
- Lim DS, Kim ST, Xu B, Maser RS, Lin JY, Petrini JHJ, Kastan MB. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature. 2000;404:613–617.
- Chahwan C, Nakamura TM, Sivakumar S, Russell P, Rhind N. The fission yeast Rad32 (Mre11)-Rad50-Nbs1 complex is required for the S-Phase DNA damage checkpoint. Mol. Cell. Biol. 2003;23:6564–6573.10.1128/MCB.23.18.6564-6573.2003
- D’Amours D, Jackson SP. The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes Dev. 2001;15:2238–2249.10.1101/gad.208701
- Durocher D, Henckel J, Fersht AR, Jackson SP. The FHA domain is a modular phosphopeptide recognition motif. Mol. Cell. 1999;4:387–394.10.1016/S1097-2765(00)80340-8
- Durocher D, Jackson SP. The FHA domain. FEBS Lett. 2002;513:58–66.10.1016/S0014-5793(01)03294-X
- Difilippantonio S, Celeste A, Kruhlak M, Lee Y, Difilippantonio MJ, Feigenbaum L, Jackson SP, McKinnon PJ, Nussenzweig A. Distinct domains in Nbs1 regulate irradiation-induced checkpoints and apoptosis. J. Exp. Med. 2007;204:1003–1011.10.1084/jem.20070319
- Wu LM, Luo KT, Lou ZK, Chen JJ. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11200–11205.10.1073/pnas.0802885105
- Lee AYL, Liu E, Wu X. The Mre11/Rad50/Nbs1 complex plays an important role in the prevention of DNA rereplication in mammalian cells. J. Biol. Chem. 2007;282:32243–32255.10.1074/jbc.M705486200
- Shima H, Suzuki M, Shinohara M. Isolation and characterization of novel xrs2 mutations in Saccharomyces cerevisiae. Genetics. 2005;170:71–85.10.1534/genetics.104.037580
- Tauchi H, Kobayashi J, Morishima K, Matsuura S, Nakamura A, Shiraishi T, Ito E, Masnada D, Delia D, Komatsu K. The forkhead-associated domain of NBS1 is essential for nuclear foci formation after irradiation but not essential for hRAD50 center dot hMRE11 center dot NBS1 complex DNA repair activity. J. Biol. Chem. 2001;276:12–15.10.1074/jbc.C000578200
- Stiff T, Cerosaletti K, Concannon P, O’Driscoll M, Jeggo PA. Replication independent ATR signalling leads to G2/M arrest requiring Nbs1, 53BP1 and MDC1. Hum. Mol. Genet. 2008;17:3247–3253.10.1093/hmg/ddn220
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008;9:616–627.10.1038/nrm2450
- Nam EA, Cortez D. ATR signalling: more than meeting at the fork. Biochem. J. 2011;436:527–536.10.1042/BJ20102162
- Ndindeng S, Miki S, Abe A, Asano K, Sone T. EGFP-Rhm51 foci enable the visualization and enumeration of DNA double-strand breaks in Magnaporthe oryzae. J. Gen Plant Pathol. 2010;76:377–381.10.1007/s10327-010-0271-0
- Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010;79:181–211.10.1146/annurev.biochem.052308.093131
- Ishibashi K, Suzuki K, Ando Y, Takakura C, Inoue H. Nonhomologous chromosomal integration of foreign DNA is completely dependent on MUS-53 (human Lig4 homolog) in Neurospora. Proc. Natl. Acad. Sci. U.S.A. 2006;103:14871–14876.10.1073/pnas.0604477103
- Kück U, Hoff B. New tools for the genetic manipulation of filamentous fungi. Appl. Microbiol. Biotechnol. 2010;86:51–62.10.1007/s00253-009-2416-7
- Villalba F, Collemare J, Landraud P, Lambou K, Brozek V, Cirer B, Morin D, Bruel C, Beffa R, Lebrun M-H. Improved gene targeting in Magnaporthe grisea by inactivation of MgKU80 required for non-homologous end joining. Fungal Genet. Biol. 2008;45:68–75.10.1016/j.fgb.2007.06.006
- Semighini CP, Fagundes MRVK, Ferreira JC, Pascon RC, Goldman MHD, Goldman GH. Different roles of the Mre11 complex in the DNA damage response in Aspergillus nidulans. Mol. Microbiol. 2003;48:1693–1709.10.1046/j.1365-2958.2003.03539.x