Abstract
We examined whether a hyperthermophilic microbial fuel cell (MFC) would be technically feasible. Two-chamber MFC reactors were inoculated with subsurface microorganisms indigenous to formation water from a petroleum reservoir and were started up at operating temperature 80 °C. The MFC generated a maximum current of 1.3 mA 45 h after the inoculation. Performance of the MFC improved with an increase in the operating temperature; the best performance was achieved at 95 °C with the maximum power density of 165 mWm−2, which was approximately fourfold higher than that at 75 °C. Thus, to our knowledge, our study is the first to demonstrate generation of electricity in a hyperthermophilic MFC (operating temperature as high as 95 °C). Scanning electron microscopy showed that filamentous microbial cells were attached on the anode surface. The anodic microbial consortium showed limited phylogenetic diversity and primarily consisted of hyperthermophilic bacteria closely related to Caldanaerobacter subterraneus and Thermodesulfobacterium commune.
Hyperthermophilic microorganisms were for the first time proved to be capable of transferring electrons to anode and acting biocatalysts in anodes of microbial fuel cells.
Bioelectrochemical systems are a promising technology where microorganisms are used as catalysts for electrochemical reactions, with possible applications in wastewater treatment, biogas production, biosensors, and bioremediation.Citation1–5) The microbial fuel cell (MFC) is the best-studied bioelectrochemical system, designed for electricity generation with simultaneous treatment of wastewater. In a typical MFC, biocatalytic microorganisms, collectively called “exoelectrogens”, oxidize organic materials and release electrons. The resulting electrons are collected at the anode and pass through an external circuit to the cathode. On the cathode, the electrons are consumed to reduce terminal electron acceptors (such as oxygen), producing an electric current.Citation5)
Recently, it has been suggested that MFCs that are operated at elevated temperatures (>45 °C) are superior to mesophilic MFCs (operated at temperatures between 20 and 45 °C) in performance, showing higher reaction activity, a limited biomass yield, greater durability, and a wider substrate range.Citation6) The increase in operating temperature can enhance the diffusion coefficient of substratesCitation7); this improvement could reduce internal resistance and thereby improve performance of the MFC. Bioavailability of insoluble environmental pollutants that cannot be easily degraded can also be improved at elevated temperatures.Citation8) Several studies have shown that thermophilic MFCs, which are inoculated with thermophilic microorganisms (with optimal growth temperature between 45 and 80 °C), exhibit good performance at operating temperatures approximately 55–60 °C.Citation6,9–13) However, compared to mesophilic systems, MFCs with higher operation temperatures remain underexploited. For example, exoelectrogenic activity has been reported in more than 20 bacterial species, but most are mesophilic species affiliated with the phylum Proteobacteria.Citation3,14) On the other hand, only three thermophilic species, Thermincola potens strain JR,Citation15) Thermincola ferriacetica,Citation11) and Calditerrivibrio nitroreducens,Citation9) have been shown to generate electricity in an MFC.
The aim of this study was to test the possibility of a hyperthermophilic MFC based on hyperthermophilic microorganisms as the biocatalysts. Hyperthermophiles are particularly extreme thermophiles of which the growth is optimal at temperatures above 80 °C and their proteins and membranes are exceedingly stable at these extremely high temperatures. Due to the hyperthermostability of their enzymes, hyperthermophiles are of special interest in terms of industrial biotechnological applications. To the best of our knowledge, however, the current-generating capability of hyperthermophiles in an MFC has never been examined.
Compared to mesophilic or thermophilic MFCs, the number of practical applications of hyperthermophilic MFCs may be limited. Nonetheless, this type of MFC can be useful for special industrial processes involving wastewater treatment at extremely high temperatures, where even thermophiles can neither propagate nor be metabolically active. For example, chemical pulp manufacture, textile dyeing, and oil/natural-gas production processes produce wastewater or an effluent at extreme high temperatures (80–100 °C, when produced), which contains pollutants (e.g. lignin, azo dyes, and phenolic compounds, respectively). Furthermore, a high concentration of arsenic has been detected in hot-spring water and geothermal power plant wastewater. These pollutants can be effectively treated using the MFC technology.Citation16–18) However, because all previous studies were performed under mesophilic (ca. 20–37 °C) or at most thermophilic (ca. 50–60 °C) conditions, the MFC systems examined in previous studies cannot be applied to on-site treatment of those extremely hot wastewater. With the increasing importance of water resource management and waste heat utilization, a hyperthermophilic MFC enabling efficient treatment of such wastewater (or hot-spring water) at extremely high temperatures may serve as an additional option for various industrial processes. Thus, as the first step to test the technical feasibility of a hyperthermophilic MFC, a two-chamber MFC was inoculated here with hyperthermophilic microorganisms originating from a high-temperature petroleum reservoir, and the bioelectrochemical properties of this MFC were analyzed.
Materials and methods
MFC configuration
Two-chamber MFC reactors were constructed mostly as described in a previous study.Citation9) Each MFC reactor consisted of two glass bottles (300 mL) separated by a proton exchange membrane with a diameter of 4 cm (Nafion 117, DuPont Co., Wilmington, USA). The proton exchange membranes were serially boiled with 10% H2O2, deionized water, and 0.5 M H2SO4 solution, as described previously.Citation19) All the electrodes were composed of plain carbon cloth (4 × 10 cm, TMIL Ltd., Ibaraki, Japan) and were connected to the circuits via titanium wires (0.5 mm, Alfa Aesar, Karlsruhe, Germany). The internal resistance between the electrodes and titanium wires was less than 3 Ω.
MFC inoculation and startup
Formation water from a petroleum reservoir was collected from an oil field in Niigata, Japan. The reservoir is located around 2000 m beneath the surface with in situ temperature of approximately 98 °C. Microorganisms in the sample of formation water were first grown by precultivation in the presterilized anaerobic TYEG medium (JCM medium 684, http://www.jcm.riken.jp/cgi-bin/jcm/jcm_grmd?GRMD=684) at 80 °C for 2 weeks. All mandatory laboratory health and safety procedures have been complied with in the course of conducting the experimental work reported in this article. The research work with unknown microorganisms may be hazardous to health. The anaerobic TYEG medium contained 0.75 g of KH2PO4, 1.5 g of K2HPO4, 0.9 g of NaCl, 0.9 g of NH4Cl, 0.2 g of MgCl2∙6H2O, 0.5 μg of FeSO4∙7H2O, 2.0 g of glucose, 2.0 g of tryptone, 1.0 g of resazurin, 2.0 g of yeast extract, and 10 mL of Wolfe’s mineral solution per liter.Citation20,21)
For MFC inoculation, 25 mL of the preculture and 225 mL of a fresh presterilized anaerobic TYEG medium (without FeSO4∙7H2O, vitamins, and resazurin) were added to the anode chamber of each reactor as an anolyte. A bicarbonate solution (2.5 g of NaHCO3 per liter) containing 50 mM potassium ferricyanide (as the terminal electron acceptor) was used as a catholyte. To maintain anaerobic conditions, the headspace of the reactors was filled with the N2/CO2 mixture (80/20) and sealed with butyl rubber stoppers and aluminum seals. During the startup process, fixed external resistance (100 Ω) was inserted into the circuit between the anode and cathode. The reactors were operated in an incubator (HB-100, TAITEC Ltd., Tokyo, Japan) at 80 °C (during the startup process) in fed-batch mode without stirring.
Measurement and calculations
The voltage (E) across the external resistance in the circuit was monitored every 5 min by means of a data acquisition unit (Agilent 34970A, Agilent Technologies, Santa Clara, CA, USA). The MFC polarization and power-density curves were constructed by altering the external resistance from 10,000 to 30 Ω using a variable resistance box. The current (I) was calculated using Ohm’s law: I = E/R, and power density (PD) was calculated using the formula, PD = E × I/A, where R (Ω) is external resistance and A (m2) is the surface area of the anode. When assessing the MFC performance at various operating temperatures (75, 80, 85, 90, 95, and 98 °C), we first incubated the MFC at each temperature for at least 2 h until a stable current was produced. The internal resistance at each temperature was also calculated based on the slope of the polarization curve in the linear regime.Citation22)
Cyclic voltammetry (CV) analysis of the anodes was performed when the MFCs produced a relatively stable current at 80 °C. The CV was performed using a potentiostat (HSV-110, Hokuto Denko, Japan) in a standard three-electrode system. The anode and cathode acted as the working electrode and counter electrode, respectively. An Ag/AgCl reference electrode was inserted into the anode chamber as a reference electrode. Before scanning, we poised the anode at −0.6 V (versus Ag/AgCl) for 99 s (equilibrium time) and then scanned it from −0.6 to 0.3 V (versus Ag/AgCl) at a scan rate of 1 m Vs−1. The scan direction was then reversed and swept to the original value of −0.6 V (versus Ag/AgCl). An abiotic control electrode was also assessed in an uninoculated reactor under the same conditions.
Characterization of the anodic bacterial population
The morphology of the anode surface was analyzed using scanning electron microscopy (SEM). The anode from the current-producing MFC was fixed with 2.5% (w/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). For analysis of the microbial population, community DNA was extracted directly from 250 mg of the aseptically crushed anode using the Power Soil DNA Isolation Kit (Mo Bio, Carlsbad, CA, USA). The extracted DNA samples (20 ng) were used as templates for PCR to amplify bacterial 16S rRNA gene fragments using the primers 8F (5′-AGAGTTTGATYMTGGCTCAG-3′) and 1492R (5′-CGGYTACCTTGTTACGACTT-3′).Citation23) Thermocycling was performed as follows: initial denaturation of 5 min at 95 °C; 18 cycles of 1.5 min at 95 °C, 1.5 min at 53 °C, and 2.5 min at 72 °C; and final extension of 10 min at 72 °C. To minimize PCR bias, the number of PCR cycles was minimized (18 cycles), and PCR products of three independent reactions were pooled. The pooled PCR amplicons were cloned into the pCR4-TOPO vector using the TOPO TA cloning system (Invitrogen, Carlsbad, CA, USA). The plasmids were purified using the Montage Plasmid DNA Isolation Kit (Millipore, Billerica, MA, USA) and sequenced using primers T3 and T7. In total, 58 clones of the library were sequenced until Good’s coverage estimatorCitation24) reached over 97%. The assembled sequences were examined for potential chimeric artifacts using DECIPHER (http://decipher.cee.wisc.edu/FindChimeras.html) and were aligned using the Infernal aligner (version 1.1rc4) in the RDP pipeline (http://pyro.cme.msu.edu) with the closest sequence relatives from NCBI (http://www.ncbi.nlm.nih.gov/) in August 2014. The alignment was then manually improved in the MEGA software (version 4.0.2).Citation25) A total of 1235 nucleotide positions were used in the alignments. Phylogenetic trees were constructed on the basis of the Tamura–Nei model, and the evolutionary history was deduced using the neighbor-joining method.Citation26) The evolutionary distances were computed using the maximum composite likelihood method and were expressed as the number of base substitutions per site.Citation26)
Nucleotide sequence accession numbers
The nucleotide sequences obtained in this study were deposited in the GenBank database with the accession numbers KC491218 through KC491220.
Results
Electricity generation by the MFC operated at 80 °C
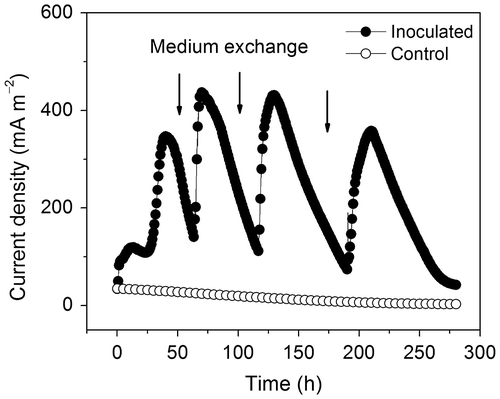
Fig. shows the electricity generation profile of an inoculated MFC operated at 80 °C (representative data from three independent reactors). The MFCs began to produce electricity soon after inoculation. The current produced by the MFCs increased to approximately 100 mA m−2 at 3 h postinoculation (hpi), then began to increase drastically at 25 hpi, and reached the first maximum value of 350 mA m−2 at 45 hpi. Such rapid increase in current generation was most likely due to the growth of electrochemically active microorganisms on the anode surface. In contrast, the uninoculated control reactors produced a weak current (at the background level), which gradually decreased to nearly 0 mA m−2. Generation of the current by the inoculated MFCs was stable at the maximum level for around 10 h and then began to decrease gradually; the latter phenomenon was probably due to depletion of electron donors. When the produced current dropped to around 100 mA m−2, both the anolyte and catholyte of the MFCs were replaced with fresh ones (arrows in Fig. ). In the inoculated MFCs, the production of the current recovered to nearly the maximum level within 4 h after the replacement of the media. Such quick recovery of the current generation was observed each time after refreshment of the media, suggesting that electricity generation in the inoculated MFCs was primarily due to microorganisms attached to the anode surface rather than the suspended cells in the media.
Fig. 1. A startup curve of a MFC inoculated with hyperthermophilic microorganisms and operated at 80 °C. The experiment was performed in triplicate, and the figure shows a representative experiment.
Electrochemical activity of the MFC anode
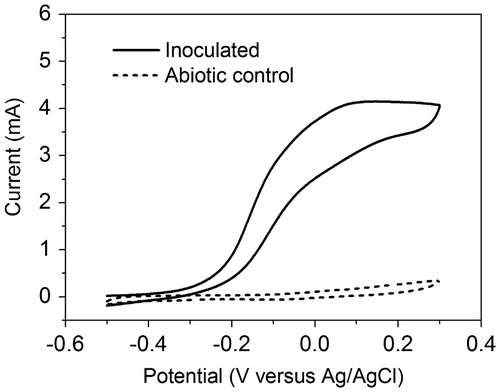
To evaluate the ability of the anodic microorganisms to catalyze a current-generating reaction, we performed CV of the anode when the MFC was producing a relatively stable current. Fig. shows a representative voltammogram of the anode of an inoculated MFC (the solid line) and an uninoculated control anode (the dashed line). The voltammogram of the MFC anode showed a typical catalytic wave that started at −0.25 V (versus Ag/AgCl) and increased sharply at the midpoint potential of −0.15 V (versus Ag/AgCl). The maximum current during the voltammetry of the MFC anode was around 4 mA, which was lower than that of thermophilic MFCs (operated at 55 °C) of identical design.Citation9) In addition, both the onset potential (−0.25 V versus Ag/AgCl) and midpoint potential (−0.15 V versus Ag/AgCl) during the voltammetry of the MFC were more positive than those of thermophilic MFCs,Citation9,11) meaning relatively weaker generation of the current by the MFCs in the present study. Nonetheless, the voltammogram of the uninoculated control showed only a background level current. Thus, the CV analysis suggested that the microorganisms attached to the anode were responsible for the current generation in the inoculated MFCs. The detailed mechanism of electron transfer between the microorganisms and the anode has yet to be determined and is beyond the scope of this study.
Fig. 2. CV analysis of the MFC anode. The experiment was performed in triplicate, and the curve shows data of a representative experiment.
We can conclude that thanks to the subsurface microorganisms as biocatalysts, an MFC with the operating temperature of >80 °C was started up successfully; this appears to be the highest operating temperature for an MFC startup reported to date.
Effects of the operating temperature on MFC performance
The effects of operating temperatures on the MFC performance were analyzed in Fig. . Fig. (A) and (B) shows the polarization and power-density curves of the MFCs at the operation temperatures from 75 to 98 °C, respectively. In the range 75–95 °C, the maximum PD of the MFCs was improved with the increase of the operating temperature, from 41 mWm−2 at 75 °C to 165 mWm−2 at 95 °C (Fig. (B)). In contrast, when the operating temperature was further increased to 98 °C, the maximum PD decreased to 156 mWm−2. Correspondingly, the internal electrical resistance decreased from 199 Ω (at 75 °C) to 80 Ω (at 95 °C) and then increased to 90 Ω at 98 °C (Table ). As the internal resistance of a MFC can be contributed by multiple factors (including activation, ohmic, and mass-transfer losses as well as losses due to biological limitations such as limited metabolic rates), the mechanism(s) of the temperature-dependent alteration of the internal resistance remains unclear. However, it was noted that, at 75 and 98 °C, the polarization and power-density curves are accompanied by a “power overshoot” phenomenon, in which both current and power decrease concomitantly at high current density regions (illustrated by a bending inwards of the curves at 75 and 98 °C in Fig. (A) and (B)).Citation27) Although the phenomenon has been reported in numerous MFC studies, its mechanism is not fully understood.Citation28) Nevertheless, it has been suggested that power overshoot is due to limited performance of the anode under sub-optimal conditions.Citation29) In addition, the increase in the operating temperature could also improve the diffusion coefficients of the substrates and protons, causing the decrease in internal resistance of the MFC and hence the improvement of MFC performance.Citation7,30,31) Thus, taken together, these results suggested that the catalytic activity of the anodic microorganisms was relatively limited at 75 °C and improved with the increase in the operating temperature up to 95 °C, but was inhibited at 98 °C (in other words, it was optimal at 95 °C). To the best of our knowledge, electricity generation by an MFC >90 °C has never been reported. The highest operating temperature of an MFC reported to date is 75 °C, for a thermophilic MFC inoculated with a marine sediment.Citation12) Although the MFC could produce a current at the density of approximately 50 mAm−2 at 75 °C, the performance was optimal at 60 °C.Citation12) Thus, the MFC developed in this study indeed represents the first demonstration of a hyperthermophilic MFC, with the optimal current-generating activity at 95 °C.
Fig. 3. Effects of the operating temperature on the performance of the hyperthermophilic microbial fuel cell. (A) Polarization curves and (B) power-density curves.
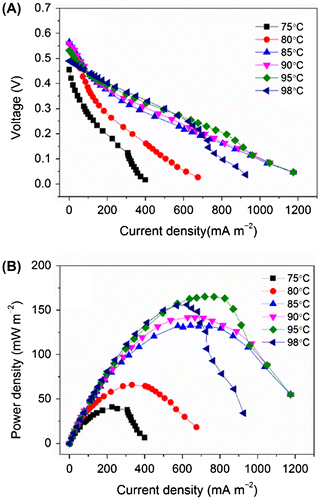
Table 1. Maximum PD and internal resistance of the hyperthermophilic microbial fuel cell at different temperatures.
SEM analysis of the anode surface
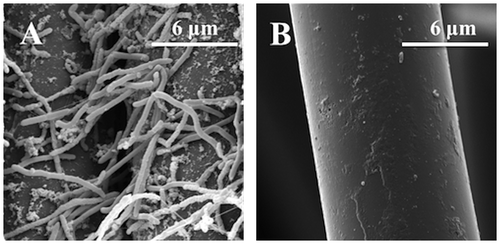
To characterize the catalytic microorganisms of the hyperthermophilic MFC, we used SEM to analyze the surface of the anode producing electricity for more than 1 month. A mostly single-layer biofilm was observed on the surface of the MFC anode (Fig. (A)). The microbial cells colonizing the surface were relatively homogeneous and filamentous in shape; some of them were elongated (>10 μm). In contrast, no microbial cells were detected on the anode of the uninoculated control (Fig. (B)), confirming that the electricity generation was due to the microorganisms attached on the anode. Compared to the anodes of mesophilic and thermophilic MFCs in other studies,Citation9,32,33) the surface biofilm here is relatively thin; this observation can explain the limited performance of the hyperthermophilic MFC.
Molecular phylogenetic analysis of the anodic bacterial population
Phylogenetic diversity of the anodic bacteria was analyzed by constructing a 16S rRNA gene clone library using the DNA samples extracted from the current-generating anode (Fig. ). No archaeal sequences were detected by PCR with primers for archaeal 16S rRNA sequences (data not shown). The bacterial library (58 clones sequenced in total) contained three phylotypes belonging to two phyla: Firmicutes (52 clones, 90% of the clones analyzed) and Thermodesulfobacteria (6 clones, 10% of the clones analyzed). In comparison with the anodic consortia of mesophilic and thermophilic MFCs,Citation16–18) the anodic consortium of the hyperthermophilic MFC showed limited diversity. Sequences related to known exoelectrogenic microorganisms were not detected. In our analysis, the Firmicutes-affiliated phylotypes HyTMFC-AB-1, and HyTMFC-AB-2 were closely related to Caldanaerobacter subterraneus (subspecies subterraneus and tengcongensis, respectively) that were isolated from thermophilic anaerobic environments (a petroleum reservoir and hot spring, respectively).Citation34,35) Both C. subterraneus subsp. subterraneus and tengcongensis are fermentative hyperthermophiles, generating acetate, H2, and CO2 as products of glucose fermentation.Citation34,35) The other phylotype HyTMFC-AB-3 was closely related to Thermodesulfobacterium commune, a hyperthermophilic dissimilatory sulfate-reducing bacterium that is also capable of dissimilatory reduction of poorly crystalline Fe(III) oxide using H2 as the electron donor.
Fig. 5. A phylogenetic tree illustrating relationships of the phylotype detected on the hyperthermophilic bioanode. The tree was constructed using the neighbor-joining method. Bootstrap values (2000 replicates) of ≥50% are shown above the nodes. The scale bar shows the number of changes per nucleotide position. Thermotoga lettingae strain TMO (CP000812.1) served as the outgroup (not shown).

Discussion
In this study, a hyperthermophilic MFC was successfully constructed using subsurface microorganisms as the inoculum. A maximum PD of 165.3 mWm−2 was obtained at the operating temperature of 95 °C. The maximum PD of the hyperthermophilic MFC was comparable to that of thermophilic MFCs inoculated with a marine sediment (207 mWm−2 at 60 °C)Citation12) and T. ferriacetica (146 mWm−2 at 60 °C).Citation11) Thus, the hyperthermophilic MFC has so far the highest startup and optimal operating temperatures (80 and 95 °C, respectively), which are respectively 20 and 35 °C higher than those reported in previous studies,Citation12,13) and thereby expands possibility for industrial applications of MFC.
It should be noted that the reactors we used here were not optimized for performance. For example, a magnetic stirrer was not used in the reactor (this drawback may limit the mass transfer of substrate from the bulk liquid to biofilm, resulting in the relatively poor development of the surface biofilm), and a proton exchange membrane (Nafion 117) was used in our reactor (this approach may reduce the diffusion rate of protons, particularly at high temperatures). Thus, the performance of our MFC can be improved via optimization of the reactor design in future studies.
This study provides the first evidence of hyperthermophilic consortium capable to oxidize organic substrates and transfer electrons to an anode. The composition of the anodic bacterial consortium suggests that current generation in the MFC can be driven by hyperthermophilic dissimilatory metal-reducing bacteria (T. commune). It has been reported that several dissimilatory metal-reducing bacterial species have the ability to transfer electrons extracellularly to a solid electrode, either via direct contact through outer-membrane c-type cytochromes or indirectly through redox mediator(s).Citation3) Indeed, T. commune contains c-type cytochromesCitation36); the latter have been shown to mediate electron transfer to electrodes in T. potens strain JR.Citation13) Therefore, it is possible that the current is produced by T. commune using H2 as the electron donor, which is syntrophically produced by C. subterraneous-related species via fermentation.
It should be noted that this project was intended to be a proof-of-concept study of a hyperthermophilic MFC; accordingly, detailed mechanisms of this type of current generation are beyond the scope of our study. The microbial mechanism(s), including the type of electron transfer, will be analyzed by isolating the bacteria from the hyperthermophilic MFC or using cultured representative bacteria in a future study. Because the hyperthermophilic MFC can be operated at high temperatures ranging from 75 to 98 °C, this device may be useful for some industrial processes under extreme conditions: paper pulp, textile, and petroleum industries as well as geothermal water management.
Conflict of interest
The authors declare no competing financial interests.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) under Grant-in-Aid for Scientific Research (A) [grant number 20246128, to K.S.], a Grant-in-Aid for Young Scientists (B) [grant number 23780074, to H.K.].
Acknowledgments
The Engineering for Sustainable Carbon Cycle (INPEX Co.) Social Cooperation Program is a joint project of the University of Tokyo and INPEX Co. This work was supported by the Japan Society for the Promotion of Science (JSPS) under Grant-in-Aid for Scientific Research (A) (#20246128, to K.·S.) and a Grant-in-Aid for Young Scientists (B) (#23780074, to H.·K.). The authors thank Dr. Hiroshi Sagara (The Institute of Medical Science, the University of Tokyo) for SEM analysis and Prof. Ken Takai (JAMSTEC) for insightful suggestions.
References
- Rozendal RA, Hamelers HVM, Euverink GJW, Metz SJ, Buisman CJN. Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int. J. Hydrogen Energy. 2006;31:1632–1640.10.1016/j.ijhydene.2005.12.006
- Ditzig J, Liu H, Logan BE. Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). J. Hydrogen Energy. 2007;32:2296–2304.10.1016/j.ijhydene.2007.02.035
- Logan BE. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009;7:375–381.10.1038/nrmicro2113
- Chang IS, Moon H, Jang JK, Kim BH. Improvement of a microbial fuel cell performance as a BOD sensor using respiratory inhibitors. Biosens. Bioelectron. 2005;20:1856–1859.10.1016/j.bios.2004.06.003
- Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K. Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 2006;40:5181–5192.10.1021/es0605016
- Jong BC, Kim BH, Chang IS, Liew PW, Choo YF, Kang GS. Enrichment, performance, and microbial diversity of a thermophilic mediatorless microbial fuel cell. Environ. Sci. Technol. 2006;40:6449–6454.10.1021/es0613512
- Liu Y, Climent V, Berná A, Feliu JM. Effect of temperature on the catalytic ability of electrochemically active biofilm as anode catalyst in microbial fuel cells. Electroanalysis. 2011;23:387–394.10.1002/elan.201000499
- Niehaus F, Bertoldo C, Khler M, Antranikian G. Extremophiles as a source of novel enzymes for industrial application. Appl. Microbiol. Biotechnol. 1999;51:711–729.10.1007/s002530051456
- Fu Q, Kobayashi H, Kawaguchi H, Wakayama T, Maeda H, Sato K. A thermophilic gram-negative nitrate-reducing bacterium, Calditerrivibrio nitroreducens , exhibiting electricity generation capability. Environ. Sci. Technol. 2013;47:12583–12590.10.1021/es402749f
- Ha PT, Lee TK, Rittmann BE, Park J, Chang IS. Treatment of alcohol distillery wastewater using a bacteroidetes-dominant thermophilic microbial fuel cell. Environ. Sci. Technol. 2012;46:3022–3030.10.1021/es203861v
- Marshall CW, May HD. Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica. Energy Environ. Sci. 2009;2:699.10.1039/b823237g
- Mathis BJ, Marshall CW, Milliken CE, Makkar RS, Creager SE, May HD. Electricity generation by thermophilic microorganisms from marine sediment. Appl. Microbiol. Biotechnol. 2008;78:147–155.10.1007/s00253-007-1266-4
- Wrighton KC, Thrash JC, Melnyk RA, Bigi JP, Byrne-Bailey KG, Remis JP, Schichnes D, Auer M, Chang CJ, Coates JD. Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2011;77:7633–7639.10.1128/AEM.05365-11
- Fedorovich V, Knighton MC, Pagaling E, Ward FB, Free A, Goryanin I. Novel electrochemically active bacterium phylogenetically related to Arcobacter butzleri, isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2009;75:7326–7334.10.1128/AEM.01345-09
- Wrighton KC, Agbo P, Warnecke F, Weber KA, Brodie EL, DeSantis TZ, Hugenholtz P, Andersen GL, Coates JD. A novel ecological role of the Firmicutes identified in thermophilic microbial fuel cells. ISME J. 2008;2:1146–1156.10.1038/ismej.2008.48
- Li Z, Zhang X, Lin J, Han S, Lei L. Azo dye treatment with simultaneous electricity production in an anaerobic–aerobic sequential reactor and microbial fuel cell coupled system. Bioresour. Technol. 2010;101:4440–4445.10.1016/j.biortech.2010.01.114
- Luo H, Liu G, Zhang R, Jin S. Phenol degradation in microbial fuel cells. Chem. Eng. J. 2009;147:259–264.10.1016/j.cej.2008.07.011
- Sakdaronnarong CK, Thanosawan S, Chaithong S, Sinbuathong N, Jeraputra C. Electricity production from ethanol stillage in two-compartment MFC. Fuel. 2013;107:382–386.10.1016/j.fuel.2012.10.030
- Li J, Fu Q, Liao Q, Zhu X. Persulfate: a self-activated cathodic electron acceptor for microbial fuel cells. J. Power Sources. 2009;194:269–274.10.1016/j.jpowsour.2009.04.055
- Patel B, Morgan HW, Daniel RM. A simple and efficient method for preparing and dispensing anaerobic media. Biotechnol. Lett. 1985;7:277–278.10.1007/BF01042377
- Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 1979;43:260–296.
- Fan Y, Sharbrough E, Liu H. Quantification of the internal resistance distribution of microbial fuel cells. Environ. Sci. Technol. 2008;42:8101–8107.10.1021/es801229j
- Grabowski A, Nercessian O, Fayolle F, Blanchet D, Jeanthon C. Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol. Ecol. 2005;54:427–443.10.1016/j.femsec.2005.05.007
- Good I, Toulmin G. The number of new species, and the increase in population coverage, when a sample is increased. Biometrika. 1956;43:45–63.10.1093/biomet/43.1-2.45
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599.10.1093/molbev/msm092
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11030–11035.10.1073/pnas.0404206101
- Nien PC, Lee CY, Ho KC, Adav SS, Liu L, Wang A, Ren N, Lee DJ. Power overshoot in two-chambered microbial fuel cell (MFC). Bioresour. Technol. 2011;102:4742–4746.10.1016/j.biortech.2010.12.015
- Hong Y, Call DF, Werner CM, Logan BE. Adaptation to high current using low external resistances eliminates power overshoot in microbial fuel cells. Biosens. Bioelectron. 2011;28:71–76.10.1016/j.bios.2011.06.045
- Winfield J, Ieropoulos I, Greenman J, Dennis J. The overshoot phenomenon as a function of internal resistance in microbial fuel cells. Bioelectrochemistry. 2011;81:22–27.10.1016/j.bioelechem.2011.01.001
- Jadhav G, Ghangrekar M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009;100:717–723.10.1016/j.biortech.2008.07.041
- Min B, Román ÓB, Angelidaki I. Importance of temperature and anodic medium composition on microbial fuel cell (MFC) performance. Biotechnol. Lett. 2008;30:1213–1218.10.1007/s10529-008-9687-4
- Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3968–3973.10.1073/pnas.0710525105
- Parameswaran P, Bry T, Popat SC, Lusk BG, Rittmann BE, Torres CI. Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ. Sci. Technol. 2013;47:4934–4940.10.1021/es400321c
- Fardeau ML, Magot M, Patel BKC, Thomas P, Garcia JL, Ollivier B. Thermoanaerobacter subterraneus sp nov., a novel thermophile isolated from oilfield water. Int. J. Syst. Evol. Microbiol. 2000;50:2141–2149.10.1099/00207713-50-6-2141
- Xue YF, Xu Y, Liu Y, Ma YH, Zhou PJ. Thermoanaerobacter tengcongensis sp nov., a novel anaerobic, saccharolytic, thermophilic bacterium isolated from a hot spring in Tengcong, China. Int. J. Syst. Evol. Microbiol. 200;151:1335–1341.
- Hatchikian E, Papavassiliou P, Bianco P, Haladjian J. Characterization of cytochrome c3 from the thermophilic sulfate reducer Thermodesulfobacterium commune. J. Bacteriol. 1984;159:1040–1046.