Abstract
Phomolide C (1) was isolated from a fungus Diaporthe sp. being found at Shirakami mountainous area. Although only the planar structure of 1 has been known, our detailed NMR spectroscopic analysis and ECD studies revealed its both relative and absolute configuration.
Total structure of phomolide C (1) was established by detailed NMR analysis and ECD spectrum after dibenzoylation.
Phomolide C (1) was first isolated in 2008 from endophytic fungus Phomopsis sp. B27 at the roots of Annona squamosa L. by Chun-Hua.Citation1) Their NMR analyses led a unique 10-membered lactone with a fused tetrahydropyran framework. However, they did not discuss its stereochemistry due to unsufficient information in the NMR spectra. We met an opportunity to isolate a substance giving the identical 1H and 13C NMR spectra to 1. Our independent NMR spectral analyses led not only the same planar structure but also its relative configuration. The absolute chemistry was established by applying Nakanishi’s dibenzoate rule after conversion into the 4,5-O-dibenzoate.
During our exploration of novel and bioactive substances from fungus in the Shirakami mountainous area,Citation2–4) we found potent antifungal activity against Cochliobolus miyabeanus in the culture broth of Diaporthe sp. strain 1308-05. This fungus was isolated in 2013 from a canker lesion on the stem of a Himeaoki tree (Aucuba japonica var. borealis) growing in the Shirakami Natural Science Park of Hirosaki University, and has been deposited at Biological Resource Center of National Institute of Technology and Evaluation, Japan (ID: NBRC 110736).Citation5) Chromatographic fractionations of ethyl acetate extracts from the culture broth gave phomolide C (1) along with eujavanicols A.Citation6) The 1H and 13C NMR data of the latter compound accorded well with the data in the literature.
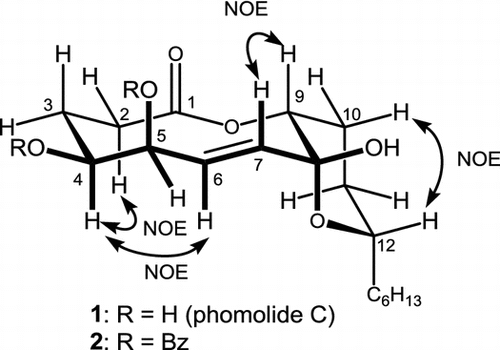
The molecular formula of phomolide C (1) was established as C18H30O6 by observing the adduct ions m/z = 365.1939 [M + Na]+ and 381.1683 [M + K]+ in the ESIMS, which was confirmed by detecting 18 resonances in the 13C NMR spectrum in CDCl3. Further spectral analyses involving the 2D spectra led the planar structure as shown in Fig. . Literature search revealed that this planar structure has been reported as phomolide C (1) by Chun-Hua in 2008.Citation1) Since they reported the data in CD3OD, we also took the spectra in CD3OD to obtain the identical data to those in the literature. They did not discuss its stereochemical properties due to insufficient NOEs in CD3OD. Thus, we studied that employing CDCl3 as the solvent.
Fig. 1. Structures of 1 and its 4,5-O-dibenzoate as well as representative NOEs observed from 1 in CDCl3.
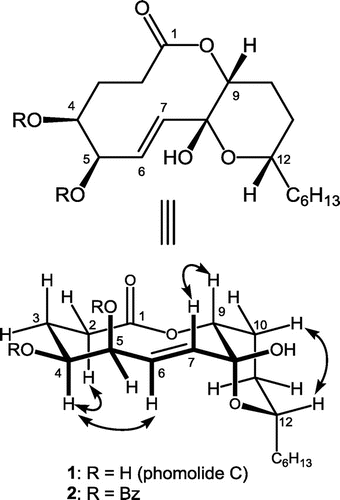
The NMR signal for H-9 (4.93 ppm) appeared as a triplet (J = 2.9 Hz), which suggested H-9 takes gauche conformation with both methylene protons at C-10. Signal profile for H-12 (3.98 ppm) was a doublet of doublet of triplet (J = 12.4, 2.9, and 6.8 Hz, respectively). Since the C12–C13 linkage would freely rotate to give a triplet (typically with 7 Hz spin coupling), the Citation3J values with H2-11 could be assigned as 12.4 and 2.9 Hz, suggesting that H-12 takes axial configuration on the C8–C12 tetrahydropyran ring. Homo decoupling at 2.88 ppm (C6–OH) resulted the H-5 signal (4.55 ppm) as quartet (J = 2.0 Hz) which revealed gauche conformations for both H-4/H-5 and H-5/H-6. The last split (2.0 Hz) of H-5 resonance was due to the long range coupling with 7-H, which was confirmed with COSY spectrum. The H-4 signal was observed at 3.71 ppm as a broaden double of triplet (J = 10.8 and 2.0 Hz, respectively), suggesting that H-4 takes axial like conformation. Thus, the relative configuration of 1 was suggested as shown in Fig. by assuming a chair-chair conformation for the 10-membered lactone moiety. In contrast to Chun-Hua’s report, the usage of CDCl3 led 1 to afford enough NOESY correlations such as at Hα-2/H-4, Hα-3/H-4, H-4/H-6, H-7/H-9, and Hβ-10/H-12, which confirmed above conclusion.
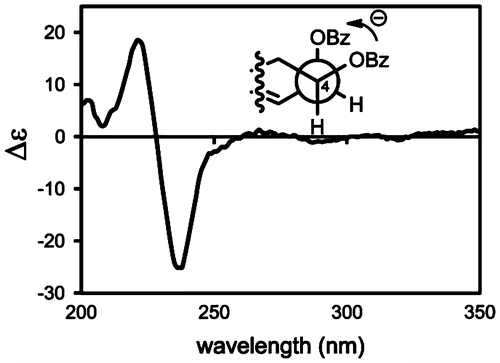
We next investigated its chirality with ECD exciton coupling method. The 4,5-O-dibenzoate (2, ESI: m/z = 1123.5004 [2 M + Na]+, APCI m/z = 533.2515 [MH-H2O]+) was prepared by treating 1 with benzoyl chloride in the presence of DMAP in CH2Cl2 for 7 days. The H-4 and H-5 were observed at 5.28 (dt, J = 11.2, 2.0 Hz) and 6.15 (brs) ppm, respectively, while the corresponding protons were resonated at 3.70 and 4.55 ppm, respectively, in 1. These higher magnetic field shifts indicated successful benzoylations at C3–OH and C4–OH. The coupling profiles of these signals were similar between 1 and 2, disclosing no considerable conformational change by the benzoylation. Dibenzoate 2 gave a pair of exciton Cotton effect with a negative Cotton effect at 237 nm (Δε − 26) and a positive one at 223 nm (Δε + 18) as show in Fig. . Since these Cotton effects accorded with the UV absorption of the benzoyl group, application of Nakanishi’s dibenzoate ruleCitation7) disclosed negative chirality for these benzoyl group, i.e. (4S,5R)-configuration. Since we have established relative configuration, the absolute chemistry of the stereogenic centers were concluded to be (4S,5R,8R,9R,12S)-form.
Finally, phomolide C (1) inhibited human colon adenocarcinoma (COLO 201) cell proliferation at 50 μg/mL, while eujavanicols A did not at 100 μg/mL. In spite of antifungal activity guided fractionation, neither 1 nor eujavanicols A inhibited the growth of the test fungus. Thus, exploration of the active species is undergoing in our laboratory.
Experimental
Isolation of Phomolide C
Diaporthe sp. being isolated at from Aucuba japonica var. borealis in the Shirakami Natural Science Park of Hirosaki University in 2013 was cultured in potato-dextrose medium [2.0 L, prepared with potato extracts (from 400 g potato), 40 g of dextrose, and water] at 27 °C for 14 days on a rotary shaker (110 rpm). After filtration, the culture broth was extracted with ethyl acetate (2.0 L) and concentrated in vacuo. The residue thus obtained was subjected to silica gel column chromatography (AcOEt/hexane = 75:25) to give a crude sample (24 mg). Preparative TLC (AcOEt:hexane = 67:33) gave phomolide C (1) (Rf = 0.3, 6 mg) and eujavanicols A (Rf = 0.45, 4 mg) both as oils. The 1H and 13C NMR spectral data of eujavanicols A in CDCl3 and 1 in CD3OD were identical to those in literature.Citation1) The NMR data of 1 in CDCl3 is described in Table .
Table 1. 1H and 13C NMR data of 1 in CDCl3.
Physical property of 1. [α]D25 −81 (c 4.6, MeOH), IR (film)νmax 2920, 2850, 1715, 1705, 1430, 1390, 1355, 1315, 1230, 1205, 1155, 1090, 1030 cm−1.
Benzoylation of 1 giving dibenzoate 2
A solution of 1 (2.0 mg) was stirred with benzoyl chloride (5.0 μL) and DMAP (5.0 mg) in CH2Cl2 (100 mL) at room temperature for seven days. After methanol was added, the mixture was further stirred for 30 min at room temperature. The mixture was poured into water (10 mL) and extracted with ether (15 mL × 2). The combined organic solution was washed with brine (15 mL), dried over MgSO4, and concentrated in vacuo. The residue was subjected to preparative silica gel TLC (AcOEt:benzene = 5:95) gave 2 (Rf = 0.5, 1.0 mg). The quantity of 2 was estimated by UV adsorption by assuming its ε value as 3 × 104 at 229 nm. 1H NMR (CDCl3) δ 0.90 (3H, t, J = 6.6 Hz), 1.87 (1H, dq, J = 14.2, 2.8 Hz), 1.95 (1H, dd, J = 13.6, 11.7 Hz), 2.18 (1H, tt, J = 14.2, 4.0 Hz), 2.34 (1H, brs, alcoholic), 2.37 (1H, dd, J = 15.2, 11.3 Hz), 2.62 (1H, dd, J = 15.4, 9.9 Hz), 2.84 (1H, dt, J = 15.2, 11.7 Hz), 3.96 (1H, m), 5.00 (t, J = 2.8 Hz), 5.28 (1H, dt, J = 11.2, 2.0 Hz), 5.63 (1H, dd, J = 15.4, 2.0 Hz), 6.03 (1H, dd, J = 15.4, 2.0 Hz), 6.15 (1H, brs), 7.36 (2H, t, J = 7.8), 7.46 (2H, t, J = 7.8 Hz), 7.49 (1H, m), 7.52 (2H, brt, J = 7.8), 7.62 (1H, t, J = 7.8 Hz), 7.92, 8.23 (each 2H, dd, J = 7.8 Hz).
Proliferation assay
The effect of compound 1 and eujavanicols A on human colon adenocarcinoma (COLO 201) cell proliferation was measured by WST-1 assay.Citation8) COLO 201 cells were cultured in RPMI medium (5 × 103 cells/well) containing these compounds in 96-well tissue culture plates with 1, 10, 100 μg/mL concentrations. After 24 h, 10 μg/mL of WST-1 reagent was added to each well and the absorbance measured at 450 nm vs. reference 650 nm using a titer plate reader.
Notes
Abbreviations: ECD, electric circular dichroism; DMAP, N,N-dimethyl-4-amiopyridine; HWHM, half width at half maximum.
References
- Lin X, Lu C-H, Shen Y-M. One new ten-membered lactone from Phomopsis sp. B27, an endophytic fungus of Annona squamosa. Chin. J. Nat. Med. 2008;6:391–394.
- Hirose A, Maeda H, Tonouchi A, Nehira T, Hashimoto M, Neomacrophorin I. Neomacrophorin I, II, and III, novel drimenyl cyclohexanes with hydroxylated butanoates from Trichoderma sp. 1212-03. Tetrahedron. 2014;70:1458–1463.10.1016/j.tet.2013.12.087
- Yasumura R, Dilip Ashtekar KD, Tonouchi A, Nehira T, Borhan B, Hashimoto M. 7-β- and 10-β-Hydroxylated congeners of CAF-603; elucidation of absolute configuration of CAF-603 family, and their SAR studies in the anti-fungal activity. Tetrahedron. 2013;69:9469–9474.10.1016/j.tet.2013.08.065
- Yasumura R, Tanaka K, Nehira T, Hashimoto M. Structural corrections of photinides A, B and their novel derivatives. Tetrahedron. 2012;68:7991–7996.
- Available from: http://www.nbrc.nite.go.jp/e/index.html.
- Nakadate S, Nozawa K, Horie H, Fujii Y, Nagai M, Hosoe T, Kawai K-i, Yaguchi T, Fukushima K. Eujavanicols A–C, decalin derivatives from Eupenicillium javanicum. J. Nat. Prod. 2007;70:1510–1512.10.1021/np078008a
- Harada N, Nakanishi K. Exciton chirality method and its application to configurational and conformational studies of natural products. Acc. Chem. Res. 1972;5:257–263.10.1021/ar50056a001
- Ishiyama M, Shiga M, Sasamoto K, Mizoguchi M, He P-G. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem. Pharm. Bull. 1993;41:1118–1122.10.1248/cpb.41.1118