Abstract
The immuno-modulating activities of seaweed (Hizikia fusiforme) extracts on murine macrophage and splenocyte were studied in vitro. Polysaccharide (HFP) exhibited the potential macrophage stimulating effects than water extract (HFW) such as NO production and enhanced pro-inflammatory cytokines on the Raw 264.7 cells and splenocytes. From the mono-sugar composition, HFP-associated fucose based on HFP of H. fusiforme acts as immune modulator.
Brown marine algae have been traditionally used food and medicinal herbs for long time in East Asian countries as China, Korea, and Japan.Citation1) Recently, number of investigators have studied beneficial biological effects on the various fields and reported the antioxidant,Citation2) anticoagulant,Citation3) antiviralCitation4) and antitumor effects.Citation5) Reportedly, their various biological activities reside in the polysaccharide (HFP) and non-polysaccharide fractionCitation2,6) on the seaweed. In a previous study, we reported that the aqueous extract of Hizikia fusiforme had enhanced the production of cytokines on the macrophage and whole spleen cells.Citation7) However, immune-modulating effect of HFP fraction from H. fusiforme has not been extensively study. Currently, some of pharmacological activities, anticomplementary activity, mitogenic activity on lymphocytes, and antitumor activities have been observed in polymer such as HFPs isolated from several food source such as fruit of Capsium annuumCitation8) and edible mushrooms.Citation9) Indeed, several species of algae have been found to be the sources of HFPs and glycoproteins with immune-stimulant, antitumoral or antiviral activity.Citation10−12) Based on these and other studies showing that many HFPs exert macrophage immune-modulatory activities,Citation9,13) we suggest that the putative immune-modulating effect of H. fusiforme of HFP may be stronger than hot water extract (HFW) of H. fusiforme on the murine macrophage as well as splenocyte. In order to address these questions, we extracted and purified HFPs from fresh seaweed H. fusiforme and analyzed their immune-modulatory effects on the murine macrophage and splenocyte including the characterization of its chemical composition.
The HFP and HFW of H. fusiforme (brown seaweed) were prepared as described previously.Citation14) The total sugar and protein were determined by phenol sulfuric acid methodCitation15) and Lowry methodCitation16) using respective standards as fucose and bovine serum albumin, respectively. The sugar composition was analyzed by a Hewlett Packard 5890 gas chromatography based on the hydrolysis method.Citation17) Murine macrophage Raw 264.7 cell was obtained from KCTC Korea, and it was cultured in DMEM supplemented 10% (v/v) heat-inactivated endotoxin-free fetal bovine serum, 2 mM glutamine and antibiotics. Splenocyte suspensions were prepared according to the previous report.Citation14) Briefly, it obtained from male C57BL/6 mice aged 6–8 weeks and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum. All cells were cultured at 37°C for 48 h in a humidified atmosphere containing 5% CO2 and then, cell numbers and viability were assessed microscopically using trypan blue exclusion and MTT methods. Specific lymphocyte mitogen, such as LPS and Concanavalin A (Con A), was used as reference at a final concentration of 2 and 1 μg/ml in splenocyte. Raw 264.7 cells were cultured in 200 μl of RPMI 1640 medium containing graded doses of HFP or LPS as positive control, and subsequently, their NO production and iNOS expression were determined for immune-modulating activities. The pro-inflammatory cytokines were measured using ELISA kits (BD Bioscience, CA, USA). Cytokine concentrations were determined by extrapolation using the appropriate standards according to the manufacturer’s protocol. The group mean was compared using a one-way analysis of variance, and each data value was expressed as the mean ±SE. The statistical difference was considered significant at p < 0.05.
The HFW and HFP were produced from 1 kg of fresh brown seaweed as 72.7 and 26.1 g, respectively (data not shown). To determine immune-modulating activities, the NO production in the murine macrophage Raw 264.7 cells treated with HFP and HFW were evaluated. As shown in Fig. (A), treatment of Raw 264.7 cells with HFW and HFP resulted in dose-dependent increase in the production of NO and cell viability showed the 84.2% cell survival at the 1 mg/ml concentration (data not shown).
Fig. 1. Immuno-modulating effects of HFP on the murine macrophage Raw 264.7 cells and splenocyte.
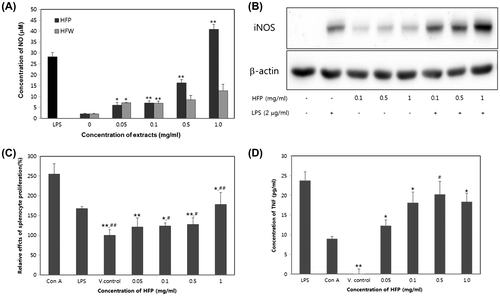
In this study, Raw 264.7 cells treated with HFP at four different concentration (0.05, 0.1, 0.5, and 1 mg/ml) combined without LPS, the highest activity was observed at 1 mg/ml which 3.2 folds stronger than HFW at the 1 mg/ml concentration. From those results, we know that macrophage was activated by HFP than HFW, and consequently, HFP showed stronger potent activator for macrophage than HFW. To confirm the iNOS expression, Western blotting was carried out after incubation with or without HFP and LPS condition in Raw 264.7 cells. Data presented in Fig. (B) show that the expression of iNOS protein by HFP combined with LPS was synergistically increased, which indicates that HFP strengthened LPS action to increase macrophage activity. However, in this study, the precise physiological significance of NO synthesis by HFP has not been demonstrated. Macrophage plays a pivotal role in host defense system, including production of NO, cytokines, antigen processing and presentation to T cell.Citation18) Several of natural HFP can modulate macrophage function of pro-inflammatory molecules such as cytokines and NO,Citation19,20) which play a critical role in host immune response. In this study, HFP showed dose-dependent increase in the production of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF (Table ).
Table 1. Production of IL-1β, IL-6, and TNF on murine macrophage Raw 264.7 cells by polysaccharide extracted from H. fusiforme.
Among them, HFP rapidly increased the TNF level compared with IL-1β and IL-6, and those TNF level was significantly (p < 0.05) increased to 1529 and 925 pg/ml at the 1 mg/ml of HFP with or without LPS treatment, respectively. IL-6 and TNF are important pro-inflammatory cytokines that can role on immune and inflammatory cells in a paracrine/autocrine manner in host defense, immune regulation and homeostasis.Citation21)
As shown in Fig. (C), splenocyte proliferation stimulated by HFP shows the dose-dependent manner and all test concentration showed the significantly increase compared to vehicle control and did not affect cytotoxicity. In this study, we used the two mitogens LPS and Con A for B and T lymphocyte. Splenocyte consists of a variety of cell populations such as T and B lymphocyte, dendritic cells, and macrophage, which have a different immune function.Citation22) As shown in Fig. (D), we treated the T and B lymphocyte activators as a Con A (T lymphocyte activator) and LPS (B lymphocyte activator), which showed 2.5- and 1.6 fold increase in the proliferation of splenocyte compared with vehicle control. Although T and B lymphocyte does not separate in this study, it generally assumed that separated splenocyte from C57BL/6 mouse was contained the T and B lymphocyte, and HFP was able to enhance immune-modulatory effect based on the T and B lymphocyte on splenocyte. Notably, 1 mg/ml of HFP-treated group showed the higher proliferation activity than the LPS at 2 μg/ml. It was not due to LPS contamination because splenocyte proliferation activity of HFP treated with polymyxin B endotoxin-removing gel was essentially the same, whereas LPS combined with polymyxin B significantly diminished proliferation (data not shown). The sugar composition of HFP is summarized in Table . The sugar components of HFP were fucose, galactose, xylose, and glucose in the molar ratios of 1.00: 0.50: 0.24: 0.21 by GC analysis, which contained mainly fucose and galactose.
Table 2. General chemical composition of HFP and HFW.
These results showed the quite similar component as a Schweiger’s report which isolated HFP from seaweed Macrocytis pyrifera consisting of 18: 1 ratio of fucose to galactose; then, he first reported that fucoidan was not pure fucan sulfate but the heteropolymer of fucose, galactose. From those results, we estimated that immune-modulatory HFP of H. fusiforme may be fucoidan based on the characterized mono-sugar content and concluded that HFP is sulfated fucopyranose and galactopyranose at near double amounts (1.0 M ratio and 0.5 M ratios), xylose and glucose, which were found as minor sugars.
In conclusion, this study proved that HFP acts an activator on the murine macrophage and splenocyte and suggests that HFP is a potent activator on H. fusiforme. However, precise mechanism of action of HFP is not studied in this study; therefore, further studies are necessary to determine the precise mechanism of action of HFP or purified active HFP from HFP involved in the expression of the various immune-modulatory effects and to elucidate the relationship between its structural characteristics and immunological activities.
Acknowledgment
This work (Grant No. C0012346) was supported by Business for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2012.
References
- Choi EY, Hwang HJ, Kim IH, Nam TJ. Protective effects of a polysaccharide from Hizikia fusiformis against ethanol toxicity in rats. Food Chem. Toxicol. 2009;47:134–139.10.1016/j.fct.2008.10.026
- Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006;44:1144–1150.10.1016/j.fct.2006.02.002
- Athukorala Y, Lee K-W, Kim S-K, Jeon Y-J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007;98:1711–1716.10.1016/j.biortech.2006.07.034
- Lee J-B, Takeshita A, Hayashi K, Hayashi T. Structures and antiviral activities of polysaccharides from Sargassum trichophyllum. Carbohydr. Polym. 2011;86:995–999.10.1016/j.carbpol.2011.05.059
- Synytsya A, Kim W-J, Kim S-M, Pohl R, Synytsya A, Kvasnička F, Čopíková J. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010;81:41–48.10.1016/j.carbpol.2010.01.052
- Okai Y, Higashi-Okai K. Identification of antimutagenic activities in the extract of an edible brown alga, Hijikia fusiforme (Hijiki) by umu gene expression system in Salmonella typhimurium (TA 1535/pSK 1002). J. Sci. Food Agric. 1994;66:103–109.10.1002/(ISSN)1097-0010
- Yoon Y, Lee E, Park J, Kim M, Lee J, Kim T, Na M, Kim J. Immunostimulatory effect by aqueous extract of Hijikia fusiforme in Raw 264.7 macrophage and whole spleen cells. Biotechnol. Bioprocess Eng. 2011;16:1099–1105.10.1007/s12257-011-0177-5
- Paik SY, Ra KS, Chang IS, Park YC, Park HS, Baik HS, Yun JW, Choi JW. Purification and characterization of complement-activating acidic polysaccharides from the fruits of capsicum annuum. J. Biochem. Mol. Biol. 2003;36:230–236.10.5483/BMBRep.2003.36.2.230
- Jeong SC, Koyyalamudi SR, Jeong YT, Song CH, Pang G. Macrophage immunomodulating and antitumor activities of polysaccharides isolated from Agaricus bisporus white button mushrooms. J. Med. food. 2012;15:58–65.
- Abdel-Fattah AF, Hussein MM-D, Salem HM. Studies of purifiation and some properties of Sargassan, a sulfated heteropolysaccharide from Sargassum linifolium. Carbohydr. Res. 1974;33:9–17.10.1016/S0008-6215(00)82935-3
- Nishino T, Yokoyama G, Dobashi K, Fujihara M, Nagumo T. Isolation, purification, and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr. Res. 1989;186:119–129.10.1016/0008-6215(89)84010-8
- Smit AJ. Medicinal and pharmaceutical uses of seaweed natural products: a review. J. Appl. Phycol. 2004;16:245–262.10.1023/B:JAPH.0000047783.36600.ef
- Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Smith PT, Ananthan R, Longvah T. Antioxidant and immunomodulatory activities of polysaccharides from the roots of Saguisorba officinalis. Int. J. Biol. Macromol. 2012;51:1057–1062.10.1016/j.ijbiomac.2012.08.019
- Jeong SC, Jeong YT, Yang BK, Song CH. Chemical characterization and immuno-stimulating properties of biopolymers extracted from Acanthopanax sessiliflorus. J. Biochem. Mol. Biol. 2006;39:84–90.10.5483/BMBRep.2006.39.1.084
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugar and relate sunstances. Anal. Chem. 1956;28:350–356.10.1021/ac60111a017
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275.
- Jones TM, Albersheim P. A gas chromatographic method for the determination of aldose and uronic acid constitutes of plant cell wall polysaccharides. Plant Physiol. 1972;49:926–936.10.1104/pp.49.6.926
- Parihar A, Eubank TD, Doseff AI. Monocytes and macrophage regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2010;2:204–215.10.1159/000296507
- Jeong SC, Cho SP, Yang BK, Jeong YT, Ra KS, Song CH. Immunomodulating activity of the exopolymer from submerged mycelia culture of Phellinus pini. J. Microbiol. Biotechnol. 2004;14:15–21.
- Jeong SC, Koyyalamudi SR, Hughes J, Khoo C, Bailey T, Marripudi K, Park JP, Kim JH, Song CH. Antioxidant and immunomodulating activities of exo-and endopolysaccharide fractions from submerged mycelia cultures of culinary-medicinal mushrooms. Int. J. Med. Mushr. 2013;15:251–266.10.1615/IntJMedMushr.v15.i3
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 2005;175:3463–3468.10.4049/jimmunol.175.6.3463
- Zhang L, Cardinal JS, Pan P, Rosborough BR, Chang Y, Yan W, Huang H, Billiar TR, Rosengart MR, Tsung A. Splenocyte apoptosis and autophagy is mediated by interferon regulatory factor 1 during murine endotoxemia. Shock. 2012;37:511–517.10.1097/SHK.0b013e318249cfa2
