Abstract
Photosystem II (PSII), which catalyzes photosynthetic water oxidation, is composed of more than 20 subunits, including membrane-intrinsic and -extrinsic proteins. The extrinsic proteins of PSII shield the catalytic Mn4CaO5 cluster from exogenous reductants and serve to optimize oxygen evolution at physiological ionic conditions. These proteins include PsbO, found in all oxygenic organisms, PsbP and PsbQ, specific to higher plants and green algae, and PsbU, PsbV, CyanoQ, and CyanoP in cyanobacteria. Furthermore, red algal PSII has PsbQ′ in addition to PsbO, PsbV, and PsbU, and diatoms have Psb31 in supplement to red algal-type extrinsic proteins, exemplifying the functional divergence of these proteins during evolution. This review provides an updated summary of recent findings on PSII extrinsic proteins and discusses their binding, function, and evolution within various photosynthetic organisms.
Proposed location of the extrinsic proteins in the photosystem II supercomplex of higher plants
Photosystem II (PSII) is one of the key protein complexes involved in the redox intensive reactions of photosynthesis. PSII converts light energy into the electrochemical potential energy required to split water into H+, electrons, and molecular oxygen.Citation1) The PSII core complex is composed of more than 20 subunits, with CP47, CP43, D1, D2, Cyt b559 α- and β-subunits, and PsbI comprises the minimum complex needed for primary charge separation in vitro.Citation2) Recent X-ray structural analysis of the PSII complex at a resolution of 1.9 Å has revealed the location of most subunits, pigments, and redox cofactors in cyanobaceria.Citation3) Light excitation of the primary donor P680, comprising a special pair of chlorophyll-a dimers in PSII, results in electron transfer to a nearby pheophytin followed by electron transfer to the acceptor quinones (QA and QB). The resulting primary cation radical of P680+ receives electrons via a redox-active tyrosine of D1 from the Mn4CaO5 cluster, which, in turn, accumulates the four oxidizing equivalents required for the complete oxidation of water.Citation4)
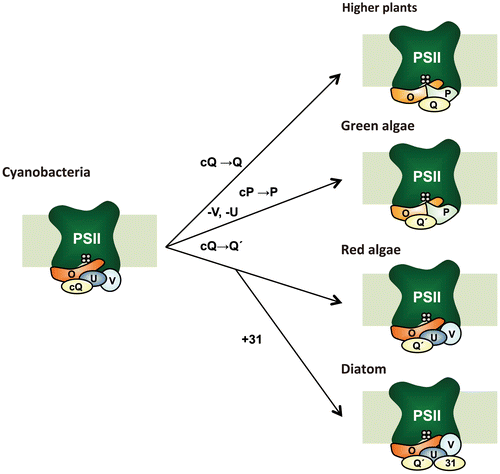
Although the mechanism of water oxidation and the basic subunit structure of the PSII core are highly conserved across oxygenic photosynthetic organisms ranging from cyanobacteria to flowering plants, several peripheral PSII subunits differ amongst them.Citation5) In particular, the composition of the extrinsic subunits of PSII surrounding the catalytic Mn4CaO5 cluster has undergone a large evolutionary changeCitation6) (Fig. ). Green eukaryotes, such as higher plants and green algae, have a set of three extrinsic proteins—PsbO, PsbP, and PsbQ—that bind to the luminal surface of PSII. In cyanobacterial PSII, PsbV, and PsbU are present instead of PsbP and PsbQ.Citation5) Futhermore, cyanobacteria have PsbP and PsbQ homologs (CyanoP and CyanoQ, respectively),Citation7) but these proteins are not yet included in the current crystal structures.
Fig. 1. Evolutionary changes in the extrinsic proteins of photosystem II (PSII).
Notes: During the course of evolution from ancient cyanobacteria to eukaryotes, only PsbO (O) has been retained by all photosynthetic organisms. In higher plants and green algae, PsbV (V) and PsbU (U) were lost while PsbP (P) and PsbQ or PsbQ′ (Q or Q′) have been derived from CyanoP (cP) and CyanoQ (cQ), respectively. Green algal PsbQ is indicated as PsbQ′(Q′) based on sequence homology. CyanoP has been excluded from the model because its stable association with PSII has not been observed. In red algae and diatoms, PsbQ′ (Q′) has developed from CyanoQ; in addition, Psb31 (31) has arisen in diatoms. These models are designed to show differences of the PSII extrinsic proteins in various photosynthetic organisms but by no means show the exact location and interaction of these extrinsic subunits within PSII. See text for further details.
PsbP and PsbQ proteins in green plants are thought to have evolved from their cyanobacterial homologs, with considerable genetic and functional modifications having occurred to generate the present eukaryotic forms.Citation8,9) In red algae and diatoms, PsbQ′, a 20-kDa homolog of CyanoQ, is bound to PSII as an extrinsic subunit in addition to PsbO, PsbU, and PsbV.Citation10,11) It should be noted that the primary sequence of green-algal PsbQ is more closely related to PsbQ′ in red algae and diatoms.Citation9) Diatoms possess an additional specific extrinsic subunit, Psb31, and recent structural analysis suggests that Psb31 might be a homolog of PsbQ.Citation12,13) Although high-resolution structures of individual PSII extrinsic subunits have been reported from eukaroytes,Citation14–16) their binding sites and topologies have not been determined because crystallographic information derived from prokaryotic cyanobacterial PSII cannot be fully applied to eukaryotic PSII.
Numerous studies have revealed the functions, structures, and evolution of PSII extrinsic proteins in various photosynthetic organisms, and extensive reviews have been published on those topics.Citation5,6,8,17) In this review, I summarize the most recent findings, including those from the laboratory to which I belong, which call into question our understanding of the role of PSII extrinsic proteins. The review is organized according to the type of photosynthetic organism, with the summarized findings in each section used to suggest future studies for the elucidation of PSII extrinsic protein functions.
I. PSII extrinsic proteins in cyanobacteria
1. Location in the PSII complex
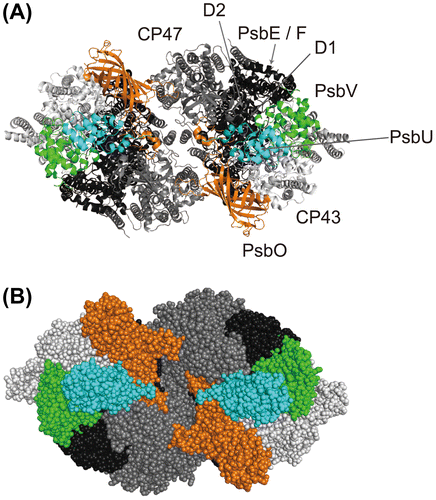
Figure , illustrating the crystal structure of PSII from Thermosynechococcus vulcanus, shows the exact location of cyanobacterial PSII extrinsic subunits PsbO, PsbU, and PsbV on the luminal surface of PSII.Citation3) Each extrinsic protein has multiple interactions with both membrane-intrinsic and -extrinsic subunits of PSII, effecting the stabilization of the complex as a whole. In brief, PsbO interacts with CP43, CP47, D1, D2, and PsbU subunits, PsbU interacts with CP47, PsbO, and PsbV, and PsbV interacts with CP43, D1, and PsbU. Detailed information about the amino acid residues involved in these interactions has been summarized and examined by Bricker et al.Citation5) Although none of these proteins provide a ligand to the catalytic Mn4CaO5 cluster, they have critical roles: they protect the metal cluster from reductants outside the PSII complex and optimize the required ionic environments, such as those of Ca2+ and Cl−, in the oxygen-evolving complex (OEC). It is of note that crystal structures and theoretical calculations suggest another role for the extrinsic proteins—namely, the maintenance of access channels for substrate water to the Mn4CaO5 cluster and exit channels for the products (molecular oxygen and protons).Citation18,19) A number of amino acid residues in the extrinsic proteins have been predicted to be associated with these channels, but their actual roles still need to be investigated experimentally.
Fig. 2. Structure of the photosystem II dimer of Thermosynechococcus vulcanus showing the location of the extrinsic subunits.
Notes: (A) A cartoon model illustrating the photosystem as viewed from the luminal space of the thylakoid membrane. The crystal structure of Umena et al. (PDB ID: 3ARC) is used.Citation3) The membrane-extrinsic PsbO is shown in orange, PsbU is shown in cyan, and PsbV is shown in green. Membrane-intrinsic subunits included in RC, CP47, and CP43 subcomplexes are shown in black, dark gray, and light gray, respectively. (B) Sphere representations for each subunit. All figures were prepared using the software package PyMol (http://www.pymol.org).
2. Functions in the dynamic life cycle of PSII
PSII is not a static structure and undergoes constant assembly, degradation, and repair.Citation20) PSII extrinsic proteins should thus also participate in a cycle of binding and release during the PSII dynamic life cycle, reflected in the suggestion that PSII assembly and repair proceeds in a highly coordinated manner with distinct intermediate subcomplexes.Citation21,22) In cyanobacteria, studies using Synechocystis sp. PCC 6803 have suggested that the early steps of PSII biogenesis take place in plasma membranes, particularly near the specialized membrane region called the PratA-defined membrane (PDM).Citation23) The PDM is defined by the presence of the PSII assembly factor PratA (for processing-associated tetratricopeptide repeat protein) and the precursor of the D1 protein (pD1).Citation24) The later steps of PSII maturation as well as the PSII damage-repair cycle, which includes assembly of the OEC and association of the extrinsic subunits, are suggested to occur in thylakoid membranes.
During PSII de novo biogenesis, the first accumulating intermediate subcomplex is the RC subcomplex consisting of D1, D2, Cyt b559 α- and β-subunits, and PsbI subunits.Citation25) The RC subcomplex is subsequently associated with the CP47 subcomplex to form the RC47 complex,Citation26) which is further associated with the CP43 subcomplex, on which all of the ligands for the catalytic Mn4CaO5 cluster are rendered complete.Citation21,27,28) After C-terminal processing of the D1 protein, the Mn4CaO5 cluster is assembled in a light-dependent process known as photoactivation, with the binding of the extrinsic subunits shielding and stabilizing the Mn4CaO5 cluster on the luminal side of PSII.Citation29,30) In Fig. , each assembly subcomplex in the cyanobacterial PSII dimer (PDB_ID: 3ARC) is shown in a different color.Citation3) The extrinsic PsbO and PsbV proteins sit on the borders of different subcomplexes while PsbU serves as a bridge between the extrinsic proteins. Furthermore, a substantial part of the PsbO subunit, that of one PSII monomer, extends over to the CP47 extrinsic loop of the second monomer in the overall PSII dimer. Consequently, binding of the extrinsic proteins stabilizes the association of assembly subcomplexes and further solidifies the entire dimeric complex across its dimeric symmetry.Citation5)
For repair of photodamaged PSII, the inactive PSII complexes must be partially disassembled to allow for the degradation of damaged D1 protein and its replacement by a de novo synthesized copy.Citation21,31) The CP43 subcomplex has been suggested to first detach from the inactive PSII complex, with the OEC, including the extrinsic subunits, concomitantly disassembled.Citation32) The exact mechanism of the dissociation of the extrinsic subunits from the PSII core has not been well characterized. Damaged D1 protein in the resulting RC47 complex is removed by FtsH metalloproteases, and newly synthesized pD1 is immediately integrated.Citation33,34) The reassembly steps after the formation of the RC47 complex, such as association of the CP43 subcomplex and assembly of the OEC, are thought to be similar to those of de novo PSII assembly.Citation20)
Numerous factors facilitating PSII assembly and repair have been identified. These factors have been summarized in various reviewsCitation20–22) and will therefore not be described in detail here. With regard to the proper association of extrinsic subunits, the C-terminal sequence of the D1 precursor must be processed by the luminal CtpA protease.Citation29) Psb27 prevents premature binding of the extrinsic subunits, thereby enabling proper processing of D1 and assembly of the Mn4CaO5 cluster to take place.Citation30,35,36) Psb27 also stabilizes the pre-assembled CP43 complex and assists in its integration into the RC47 complex.Citation28) The removal of the C-terminal extension of pD1 has been proposed to lead to reduced binding affinity between CP43 and Psb27 and to allow the binding of extrinsic PsbO and PsbV.Citation27) Association of extrinsic proteins may require additional factors or may induce structural changes in membrane-intrinsic proteins for OEC assembly. Further studies are required to address these issues.
3. Additional extrinsic proteins: CyanoP and CyanoQ
As already mentioned, cyanobacteria have ancestral homologs of eukaryotic PsbP and PsbQ, CyanoP, and CyanoQ. Although CyanoQ is not observed in currently available crystal structures of PSII from thermophilic cyanobacteria, CyanoQ has been clearly demonstrated to bind to PSII tightlyCitation37) and to optimize oxygen evolution.Citation38) A recent study using chemical cross-linking followed by immunodetection and tandem mass spectrometric analysis suggests that PsbQ in the model cyanobacterium Synechocystis sp. PCC 6803 is closely associated with PsbO and CP47 proteins.Citation39) The results of that study further imply that two molecules of CyanoQ may interact at the interface of the PSII dimers, indicating that CyanoQ, together with PsbO, is important for PSII dimer stability.
In contrast to CyanoQ, the molecular function of CyanoP in PSII has been enigmatic because of its low abundance in isolated PSII protein fractions.Citation7) A Synechocystis strain lacking CyanoP was found to exhibit slower photoautotrophic growth and lower PSII activity under Ca2+ and/or Cl−-depleted conditions,Citation7,40) but these phenotypes have not been clearly observed in other studies.Citation41,42) Comparative experiments using insertional and/or partial-deletion knockout mutants carrying different antibiotic resistance cassettes suggested that CyanoP plays a constrictive role to stabilize the donor side of PSII.Citation43) However, a recent study using surface plasmon resonance spectroscopy has indicated that the CyanoP binding site is in the PSII center, which is occupied by the PsbO subunit in mature PSII complexes.Citation44) It is therefore more likely that CyanoP functions in the PSII assembly process, possibly in the association of CP47 and CP43. The above facts suggest that mature cyanobacterial PSII has four extrinsic proteins: PsbO, U, V, and CyanoQ. Returning to the role of CyanoP in PSII functionality is minor in cyanobacteria, this protein has evolved into various functionally important proteins, such as PsbP, PsbP-like proteins (PPLs), and PsbP-domain proteins (PPDs), that support various protein complexes in eukaryotic thylakoid membranes.Citation8,9,45,46)
II. PSII extrinsic proteins in higher plants and green algae
1. Molecular evolution of PsbP and PsbQ in PSII
Higher plant and green algal chloroplasts contain multiple homologs of PsbP and PsbQ. In model plant species Arabidopsis and rice, two PsbP-like proteins (PPL1, 2), seven (possibly eight) PsbP-domain proteins (PPD1–7), and three PsbQ-like proteins (PQL1–3) have been identified.Citation9,45) Genetic studies using Arabidopsis mutants have demonstrated that PPL1 is involved in the repair of PSII following photodamage, while PPL2 and PQL1–3 proteins are required for the function of the chloroplast NAD(P)H dehydrogenase complex (NDH) involved in photosystem I (PSI) cyclic electron transfer.Citation47–49) New nomenclature has been proposed for the PsbP and PsbQ homologs in the NDH complex, namely, PnsL1 (PPL2), PnsL2 (PQL1), and PnsL3 (PQL2), where “PnsL” stands for Photosynthetic NDH subcomplex Lumen.Citation50) PQL3 is required for NDH activity,Citation49) but its molecular function in this complex stills needs to be clarified.
Indeed, PPD1 has recently been reported to be essential for PSI assembly.Citation51) Deletion of PPD1 leads to the specific loss of the stable PSI complex and prevents the resulting mutant from growing photoautotrophically. Biochemical and molecular biological analyses revealed that PPD1 interacts directly, and specifically, with PsaA and PsaB and assists their proper folding and integration into thylakoid membranes.Citation52) Molecular functions of other PPD proteins have also been investigated; a homolog of the PPD2 protein is suggested to be involved in singlet-oxygen signaling in Chlamydomonas,Citation53) while PPD5 has been linked to plant development via strigolactone synthesis.Citation54) These facts suggest that the acquisition of PsbP and PsbQ as PSII extrinsic subunits is the result of intensive gene duplication and functional diversification.Citation8,9,55)
The crucial event in the evolution of the green plant-type PSII complex may have been the development of the PsbP protein as a principal extrinsic subunit of PSII. Genetic studies using knockdown or knockout plants have suggested that PsbP, but not PsbQ, is essential for the oxygen-evolving activity of PSII in vivo.Citation56–58) Consistent with this idea, in vitro reconstitution studies combined with Fourier transform infrared (FTIR) measurement have given prominence to PsbP, but not PsbQ, as being essential for the conformational change around the OEC that optimize Ca2+ and Cl− cofactor availability in the active Mn4CaO5 cluster in PSII.Citation59) The N-terminal sequence of PsbP, which is not visible in the crystal structure (1.6 Å: PDB ID 1V2BCitation14)), has a critical role in the specific and functional interaction of PsbP with PSII.Citation60,61) Given PsbQ can restore the ability of the N-terminal truncated form of PsbP to induce the proper conformational changes and activate oxygen evolution in the OEC, PsbQ is inferred to have an auxiliary role to support PsbP binding and function in higher plants.Citation60,62) It should be noted that PsbQ in green algae would have a different function when compared to this protein in higher plants, as the primary sequence of green-algal PsbQ is more closely related to PsbQ′ in red algae and diatoms and can be categorized to PsbQ′ (Fig. ).Citation9,49)
2. Localization in the PSII complex
PsbO has been widely believed to bind directly to the PSII core, followed by PsbP binding to PsbO, and then PsbQ binding to PsbP or PsbO. However, recent studies using chemical cross-linking combined with mass spectrometry suggest the multiple and direct interaction of PsbP and PsbQ with membrane-intrinsic PSII subunits. Using the zero-length cross-linker 1-ethyl-3-(3-diethylaminopropyl) carbodiimide (EDC), Ido et al.Citation63,64) revealed the existence of direct interactions between PsbP and PsbE and between PsbP and PsbR (Fig. ). Intriguingly, PsbP and PsbQ were further linked to the CP26 and CP43 light-harvesting proteins. In addition, the cross-linked sites between PsbP:1A and PsbE:57E, PsbP:27K and PsbR:22D, and PsbP:174K and CP26:96E were identified by tandem mass spectrometry. The binding topology of PsbP with the PSII core has also been analyzed by site-directed mutagenesis, which indicated that the region including PsbP:48R, :143K, and :160 K is important for electrostatic interaction with the PSII core.Citation65) These observations require the current organizational models of higher plant PSII extrinsic subunits to be overhauled.
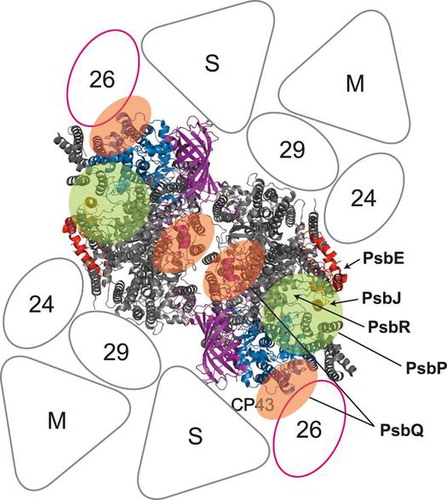
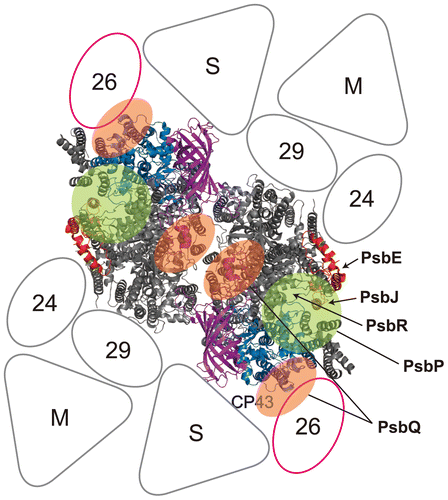
Fig. 3. Proposed location of the extrinsic proteins in the photosystem II (PSII)-light-harvesting complex II (LHCII) supercomplex of higher plants.
Notes: A model of the PSII-LHCII supercomplex (the C2S2M2 complex), viewed from the top onto the luminal surface and utilizing the high resolution structure of the cyanobacterial PSII core,Citation3) is based upon comparisons with the literature.Citation92,93) Putative PsbP- and PsbQ-binding sites, proposed by Ido et al.Citation64) and Mummadisetti et al.Citation66) are indicated by light green and orange overlays, respectively. It should be noted that two different binding sites for PsbQ have been proposed (see text for details). PsbV and PsbU, known to be absent in plant PSII, were excluded from the model. CP43 and PsbE, which interact with PsbP, are shown in light blue and red, respectively. PsbJ and PsbO, required for the stable binding of PsbP and PsbQ, are shown in orange and purple, respectively. PsbE, PsbJ, and putative location of PsbR are indicated by arrows. Positioning of the strongly (S) or moderately (M) bound LHC trimers, as well as the minor LHC antennae CP24 (24), CP26 (26), and CP29 (29), are schematically depicted. CP26 is outlined in magenta to indicate its interaction with PsbP and PsbQ.Citation64)
A very recent study using the cross-linker bis-sulfosuccinimidyl suberate (BS3), which has a spacer length of 11.4 Å, has provided additional information.Citation66) Although cross-linking by BS3 failed to detect an interaction between PSII extrinsic and intrinsic proteins, it indicated that the N-terminal 15-amino-acid residue domain of PsbP is in close proximity (≤11.4 Å) to the C-terminal region of PsbP. In addition, amino acid residues in the structurally unresolved loop domain of PsbP including PsbP:93Y and :96K were likely to be near PsbQ:1E. Cross-linked domains within PsbQ were also identified, indicating that two PsbQ molecules may interact in higher plants in a manner similar to that observed for CyanoQ. A direct interaction between PsbP and PsbQ has already been identified in Chlamydomonas,Citation67) but the cross-linked site is completely different, indicating that green algal PsbQ (PsbQ′) binds in a different manner. Modification by OH produced by synchrotron radiolysis of water has been used to further define buried surface regions of PsbP and PsbQ,Citation66) yielding results that are mostly consistent with previous observations using chemical modification or site-directed mutagenesis.Citation65,68,69)
Integration of all of the above biochemical data into a single model of PSII extrinsic protein binding is currently difficult. One of the major bottlenecks is the interaction of PsbP and PsbQ with the minor antenna protein CP26, which has been clearly revealed by both mass spectrometry and immunoblotting.Citation64) Assuming that PsbP and PsbQ are located near the interface between CP43 and CP26, the N-terminal sequence of PsbP should extend toward PsbE; however, this model is apparently unsupported by BS3 crosslinking experiments.Citation66) At the same time, PsbQ dimer formation at the PSII dimer interface is also restricted. To reconcile this discrepancy, a PsbQ–PsbQ interaction between the PSII dimers in the PSII supercomplex has been proposed.Citation66) Another possibility is that a secondary binding site at which PsbP and PsbQ can interact may exist in the PSII-LHCII supercomplex.Citation64) In fact, binding of a second copy of PsbO in higher plant PSII has been proposed on the basis of continuous biochemical studies.Citation70,71) High-resolution structural analysis, presumably by crystallography or 3D cryo-transmission electron microscopy, is obviously required to reveal the detailed extrinsic luminal relationships between PsbP, PsbQ, CP26, PsbR, PsbO, extrinsic loops of CP43 and CP47, and the whole PSII-LHCII supercomplex.
3. Assembly of the PSII complex and extrinsic proteins
While de novo assembly and repair of PSII generally occurs in a similar fashion in cyanobacteria and chloroplasts, different auxiliary assembly factors have arisen during evolution.Citation20) Several factors have been implicated in the assembly of PSII extrinsic proteins in Arabidopsis. CYP38, a cyclophilin-type immunophilin located in the thylakoid lumen, is proposed to be involved in the folding of D1 and CP43 and the subsequent assembly of the OEC.Citation72,73) Unlike typical cyclophilins, CYP38 does not possess peptidyl-prolyl cis-/trans-isomerase enzymatic activity, and may thus have a previously uncharacterized function.Citation74) The interaction of CYP38 with the E-loop of CP47 through its cyclophilin domain has been detected by a yeast two-hybrid assay. This finding supports a role for CYP38 in PSII assembly, but may require its proposed function to be reconsidered. In addition, LTO1 has been identified as a luminal thiol oxidoreductase that catalyzes the formation of intramolecular disulfide bonds in PsbO.Citation75) Like cyanobacteria, Arabidopsis has homologs of CtpA and Psb27 in the thylakoid lumen, but their role in chloroplasts seems to be different than in cyanobacteria. Although AtCtpA is essential for pD1 C-terminal processing and OEC functional assembly, the extrinsic PsbO is able to bind to the pD1-containing PSII assembly, which indicates that PSII extrinsic protein assembly is controlled differently between species.Citation73) pD1 processing is also facilitated by the Psb27 homolog (At1g05385) LPA19,Citation76) while another Psb27 homolog, Psb27-H1 (At1g03600), is involved in efficient repair of photodamaged PSIICitation77) and adaptation to fluctuating light.Citation78) Similar to observations of PsbP homologs,Citation9) these results suggest that the Psb27 homologs have developed distinct functions in the PSII life cycle during the course of evolution.
With respect to PSII assembly, a “catalytic” function has been proposed for PsbP proteins in addition to their structural roles as OEC subunits protecting the Mn4CaO5 cluster in PSII.Citation9) This view is based on the observation that complete elimination of PsbP in an Arabidopsis mutant impairs the photoautotrophy that causes a seedling-lethal phenotype,Citation58) while a minimum amount of PsbP enables photoautotrophic growth and accumulation of the PSII reaction center.Citation56,57) These results suggest that PsbP still retains its original function as an assembly factor, as has been reported for other PsbP homologs, namely, CyanoP, PPD1, and PPL1.Citation46) The mechanism by which PsbP exerts a catalytic function during PSII assembly remains elusive.
PsbP and PsbQ also appear to play an important role in defining the architecture of PSII-light-harvesting complex (LHC) II supercomplexes in higher plants.Citation8) In one study, PsbP knockdown by RNA interference caused a severe decrease in levels of PSII-LHCII supercomplexes whereas amounts of unattached LHCII trimers and minor LHCs were significantly increased.Citation79) Similarly in another study, the abundance of PSII-LHCII supercomplexes decreased in mutants lacking PsbQ and/or PsbR.Citation58) In fact, depletion of PsbQ and/or PsbR affects short-term regulatory mechanisms such as state transitions and non-photochemical quenching.Citation58) These observations are relevant to the study that showed that PsbP, together with PsbQ and PsbR, can act as a structural link to stabilize the association of a minor antenna protein, CP26, to the inner, membrane-facing domains of CP43.Citation64) A small number of factors implicated in PSII dimer and/or PSII-LHCII supercomplex formation such as the immunophilin FKBP20-2Citation80) and the protease Deg1,Citation81) have been reported in the thylakoid lumen. Interactions and functional relationships among these thylakoid luminal factors are still unclear, and thus require further study.
III. PSII extrinsic proteins in other algae
1. Red algae
The functions of extrinsic proteins (PsbO, PsbQ′, PsbV, and PsbU) from a red alga, Cyanidium caldarium, and their effect upon the overall OEC structure, were investigated using FTIR spectroscopy. The conformational changes caused by depletion of all the extrinsic proteins were mostly recovered by PsbV binding with the support of other extrinsic proteins.Citation82) The recovery of the OEC conformations correlated well with oxygen-evolving activity, suggesting that PsbV function in red algae resembles that of PsbP in higher plants. The manner in which PsbV binds in red algae has been suggested to be somewhat different from that was shown in cyanobacteria.Citation83) In opposition to this idea, however, red algal PsbV has been shown to be functional in cyanobacterial PSII, and its binding site is thus unlikely to be totally different between the two types of organisms. These facts support a model proposed by Ido et al.Citation64) to explain the binding of higher plant PSII extrinsic proteins, in which PsbP occupies a position roughly similar to that occupied by PsbV in the cyanobacterial crystal structure.
Although PsbV is a low-potential cytochrome (Cyt c550), the functionality of its heme during water oxidation in cyanobacteria has not yet been proven.Citation84) Unlike all other red algal extrinsic proteins, which are nuclear-encoded,Citation85,86) PsbV is still encoded by the chloroplast genome.Citation87) Several PsbP homologs have been found in red algae, but they have not been detected in purified PSII preparations. These facts may indicate that green plant-type PsbP, which is crucial for the elimination of unnecessary heme from the PSII donor side, has not developed in red algae. Alternatively, PsbV may have different redox properties in vivo, as indicated for cyanobacterial PsbV,Citation88) that may contribute to PSII stability;Citation84) however, further study is obviously required to address this point. As purification, crystallization, and preliminary X-ray diffraction analysis of red algal PSII were carried some time ago,Citation89) elucidation of the exact structural organization of red algal PSII is expected in the near future.
2. Diatoms
In addition to the four red algal-type extrinsic proteins, diatom PSII contains a fifth extrinsic protein, Psb31. Psb31 is speculated to have originated via a secondary endosymbiosis event.Citation13) Reconstitution experiments using proteins from a centric diatom, Chaetoceros gracilis, have suggested that Psb31 binds directly to PSII intrinsic proteins.Citation12) Interestingly, PSII reconstituted with Psb31 alone can partially restore oxygen-evolving activity in the absence of PsbO, suggesting that Psb31 has a novel and specific function in diatom PSII. The recent development of a genetic transformation system for diatomsCitation90,91) will facilitate the in vivo investigation of physiological functions of diatom-specific proteins.
Analysis of the crystal structure of Psb31 has revealed a four-helix bundled structure showing partial structural similarity with PsbQ family proteins.Citation13) Thus, two copies of PsbQ-like proteins with different binding and functional properties seem to be present as extrinsic subunits in diatom PSII. In this respect, it is tempting to speculate that eukaryotic PSII may have more than one binding site for each type of extrinsic protein, as has been already mentioned for PsbOCitation70,71) and PsbP/QCitation64) in higher plant PSII. Crystallization and preliminary X-ray diffraction analysis of the diatom PSII complex has not yet been reported.
IV. Conclusions and future perspectives
As discussed in this review, much progress has been made toward the elucidation of the localization and function of PSII extrinsic proteins. In particular, chemical cross-linking combined with mass spectrometry has provided new insights into the location of weakly bound extrinsic subunits, such as CyanoQ in cyanobacteriaCitation39) and PsbP/PsbQ in higher plants.Citation63,64,66) These findings are mostly consistent with observations from genetic studies using knockout or knockdown mutants of each component. The current data obviously necessitate a substantial reconsideration of historical concepts regarding the binding, function, and evolution of the extrinsic subunits of PSII.
At the same time, many questions remain. My colleagues and I have shown that PsbP and PsbQ in higher plant PSII are capable of multiple interactions with PSII intrinsic subunits as well as other light-harvesting proteins present within the larger, stable PSII supercomplex form.Citation64) This interactive capability may indicate that PsbP and PsbQ not only replace the functions of PsbU and PsbV structurally, but may also have evolved the ability to stabilize the overall PSII-LHCII supercomplex. Nevertheless, substantial discrepancies within the data and between reports still remain to be resolved. In addition, the possibility of multiple copies of PSII extrinsic proteins needs to be examined. Furthermore, studies using FTIR have detected a conformational change in the OEC upon the binding of extrinsic proteins in higher plantsCitation59,62,63,65) and red algae,Citation82) but the exact region in PSII showing this change is unknown. An additional FTIR study using a cyanobacterial system, where structural information is available at atomic resolution, would answer this question. Experimental evidence for the proposed roles of the extrinsic components in water or proton channels in PSII should also be evaluated in detail. In terms of the dynamic life cycle of PSII, molecular functions of PsbP and its homologs in chloroplasts are not perfectly understood. Finally, the physiological function of each PSII extrinsic component in other algal systems also requires clarification. Answering the above questions will reveal how the structural variations of PSII extrinsic proteins confer functional differences, presumably through fine tuning of light-induced charge separation and OEC reactivity, and how they may input into the processes of PSII-complex assembly and repair.
Acknowledgments
I would like to express my sincere gratitude to Dr Fumihiko Sato, professor, Kyoto University, for his continuous guidance and encouragement throughout the study. I thank Dr Tsuyoshi Endo, associate professor, Kyoto University; Dr Takumi Noguchi, professor, Nagoya University; Dr Jon Nield, senior lecturer, Queen Mary, University of London; and Dr Yoichiro Fukao, associate professor, Nara Institute of Science and Technology, for their collaboration and kind support. I also thank all my collaborators in this research and the current and previous members of the Laboratory of Molecular and Cellular Biology of Totipotency, Graduate School of Biostudies, Kyoto University. The contributions of Dr Kunio Ido and Dr Seiko Ishihara are especially appreciated. I am also grateful for the financial support offered by the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Japan Society for the Promotion of Science, and the Japan Science and Technology Agency.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
Notes
This review was written in response to the author’s receipt of the Japan Society for Bioscience, Biotechnology, and Agrochemistry Award for the Encouragement of Young Scientists in 2011.
References
- Hankamer B, Barber J, Boekema EJ. Structure and membrane organization of photosystem II in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:641–671.10.1146/annurev.arplant.48.1.641
- Satoh K. Protein-pigments and the photosystem II reaction center: a glimpse into the history of research and reminiscences. Photosynth. Res. 2008;98:33–42.10.1007/s11120-008-9348-4
- Umena Y, Kawakami K, Shen J-R, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55–60.10.1038/nature09913
- Vinyard DJ, Ananyev GM, Dismukes CG. Photosystem II: the reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 2013;82:577–606.10.1146/annurev-biochem-070511-100425
- Bricker TM, Roose JL, Fagerlund RD, Frankel LK, Eaton-Rye JJ. The extrinsic proteins of photosystem II. Biochim Biophys Acta. 2012;1817:121–142.10.1016/j.bbabio.2011.07.006
- Roose JL, Wegener KM, Pakrasi HB. The extrinsic proteins of photosystem II. Photosynth. Res. 2007;92:369–387.10.1007/s11120-006-9117-1
- Thornton LE, Ohkawa H, Roose JL, Kashino Y, Keren N, Pakrasi HB. Homologs of plant PsbP and PsbQ proteins are necessary for regulation of photosystem II activity in the cyanobacterium Synechocystis 6803. Plant Cell. 2004;16:2164–2175.10.1105/tpc.104.023515
- Ifuku K, Ido K, Sato F. Molecular functions of PsbP and PsbQ proteins in the photosystem II supercomplex. J. Photochem. Photobiol., B. 2011;104:158–164.10.1016/j.jphotobiol.2011.02.006
- Ifuku K. The PsbP and PsbQ family proteins in the photosynthetic machinery of chloroplasts. Plant Physiol. Biochem. 2014;81:108–114.
- Enami I, Kikuchi S, Fukuda T, Ohta H, Shen JR. Binding and functional properties of four extrinsic proteins of photosystem II from a red alga, Cyanidium caldarium, as studied by release−reconstitution experiments. Biochemistry. 1998;37:2787–2793.10.1021/bi9724624
- Nagao R, Ishii A, Tada O, Suzuki T, Dohmae N, Okumura A, Iwai M, Takahashi T, Kashino Y, Enami I. Isolation and characterization of oxygen-evolving thylakoid membranes and photosystem II particles from a marine diatom Chaetoceros gracilis. Biochim. Biophys. Acta. 2007;1767:1353–1362.10.1016/j.bbabio.2007.10.007
- Nagao R, Moriguchi A, Tomo T, Niikura A, Nakajima S, Suzuki T, Okumura A, Iwai M, Shen J-R, Ikeuchi M, Enami I. Binding and functional properties of five extrinsic proteins in oxygen-evolving photosystem II from a marine centric diatom, Chaetoceros gracilis. J. Biol. Chem. 2010;285:29191–29199.10.1074/jbc.M110.146092
- Nagao R, Suga M, Niikura A, Okumura A, Koua FH, Suzuki T, Tomo T, Enami I, Shen J-R. Crystal structure of Psb31, a novel extrinsic protein of photosystem II from a marine centric diatom and implications for its binding and function. Biochemistry. 2013;52:6646–6652.10.1021/bi400770d
- Ifuku K, Nakatsu T, Kato H, Sato F. Crystal structure of the PsbP protein of photosystem II from Nicotiana tabacum. EMBO Rep. 2004;5:362–367.10.1038/sj.embor.7400113
- Calderone V, Trabucco M, Vujičić A, Battistutta R, Giacometti GM, Andreucci F, Barbato R, Zanotti G. Crystal structure of the PsbQ protein of photosystem II from higher plants. EMBO Rep. 2003;4:900–905.10.1038/sj.embor.embor923
- Balsera M, Arellano JB, Revuelta JL, de las Rivas J, Hermoso JA. The 1.49 Å resolution crystal structure of PsbQ from photosystem II of Spinacia oleracea reveals a PPII structure in the N-terminal region. J. Mol. Biol. 2005;350:1051–1060.10.1016/j.jmb.2005.05.044
- Ifuku K, Ishihara S, Shimamoto R, Ido K, Sato F. Structure, function, and evolution of the PsbP protein family in higher plants. Photosynth. Res. 2008;98:427–437.10.1007/s11120-008-9359-1
- Linke K, Ho FM. Water in photosystem II: structural, functional and mechanistic considerations. Biochim. Biophys. Acta. 2014;1837:14–32.10.1016/j.bbabio.2013.08.003
- Vogt L, Vinyard DJ, Khan S, Brudvig GW. Oxygen-evolving complex of photosystem II: an analysis of second-shell residues and hydrogen-bonding networks. Curr. Opin. Chem. Biol. 2015;25:152–158.10.1016/j.cbpa.2014.12.040
- Nickelsen J, Rengstl B. Photosystem II assembly: from cyanobacteria to plants. Annu. Rev. Plant Biol. 2013;64:609–635.10.1146/annurev-arplant-050312-120124
- Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 2010;106:1–16.10.1093/aob/mcq059
- Komenda J, Sobotka R, Nixon PJ. Assembling and maintaining the photosystem II complex in chloroplasts and cyanobacteria. Curr. Opin. Plant Biol. 2012;15:245–251.10.1016/j.pbi.2012.01.017
- Rengstl B, Oster U, Stengel A, Nickelsen J. An intermediate membrane subfraction in cyanobacteria is involved in an assembly network for photosystem II biogenesis. J. Biol. Chem. 2011;286:21944–21951.10.1074/jbc.M111.237867
- Stengel A, Gügel IL, Hilger D, Rengstl B, Jung H, Nickelsen J. Initial steps of photosystem II de novo assembly and preloading with manganese take place in biogenesis centers in Synechocystis. Plant Cell. 2012;24:660–675.10.1105/tpc.111.093914
- Komenda J, Reisinger V, Müller BC, Dobáková M, Granvogl B, Eichacker LA. Accumulation of the D2 protein is a key regulatory step for assembly of the photosystem II reaction center complex in Synechocystis PCC 6803. J. Biol. Chem. 2004;279:48620–48629.10.1074/jbc.M405725200
- Boehm M, Yu J, Reisinger V, Beckova M, Eichacker LA, Schlodder E, Komenda J, Nixon PJ. Subunit composition of CP43-less photosystem II complexes of Synechocystis sp. PCC 6803: implications for the assembly and repair of photosystem II. Philos. Trans. R. Soc., B. 2012;367:3444–3454.10.1098/rstb.2012.0066
- Liu H, Chen J, Huang RY-C, Weisz D, Gross ML, Pakrasi HB. Mass spectrometry-based foot printing reveals structural dynamics of loop E of the chlorophyll-binding protein CP43 during photosystem II assembly in the cyanobacterium Synechocystis 6803. J. Biol. Chem. 2013;288:14212–14220.10.1074/jbc.M113.467613
- Komenda J, Knoppová J, Kopečná J, Sobotka R, Halada P, Yu J, Nickelsen J, Boehm M, Nixon PJ. The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2012;158:476–486.10.1104/pp.111.184184
- Roose JL, Pakrasi HB. Evidence that D1 processing is required for manganese binding and extrinsic protein assembly into photosystem II. J. Biol. Chem. 2004;279:45417–45422.10.1074/jbc.M408458200
- Roose JL, Pakrasi HB. The Psb27 protein facilitates manganese cluster assembly in photosystem II. J. Biol. Chem. 2008;283:4044–4050.10.1074/jbc.M708960200
- Kato Y, Sakamoto W. Protein quality control in chloroplasts: a current model of D1 protein degradation in the photosystem II repair cycle. J. Biochem. 2009;146:463–469.10.1093/jb/mvp073
- Grasse N, Mamedov F, Becker K, Styring S, Rögner M, Nowaczyk MM. Role of novel dimeric photosystem II (PSII)-Psb27 protein complex in PSII repair. J. Biol. Chem. 2011;286:29548–29555.10.1074/jbc.M111.238394
- Silva P, Thompson E, Bailey S, Kruse O, Mullineaux CW, Robinson C, Mann NH, Nixon PJ. FtsH is involved in the early stages of repair of photosystem II in Synechocystis sp PCC 6803. Plant Cell. 2003;15:2152–2164.10.1105/tpc.012609
- Nixon PJ, Barker M, Boehm M, de Vries R, Komenda J. FtsH-mediated repair of the photosystem II complex in response to light stress. J. Exp. Bot. 2005;56:357–363.
- Nowaczyk MM, Hebeler R, Schlodder E, Meyer HE, Warscheid B, Rögner M. Psb27, a cyanobacterial lipoprotein, is involved in the repair cycle of photosystem II. Plant Cell. 2006;18:3121–3131.10.1105/tpc.106.042671
- Liu H, Roose JL, Cameron JC, Pakrasi HB. A genetically tagged Psb27 protein allows purification of two consecutive photosystem II (PSII) assembly intermediates in Synechocystis 6803, a cyanobacterium. J. Biol. Chem. 2011;286:24865–24871.10.1074/jbc.M111.246231
- Kashino Y, Lauber WM, Carroll JA, Wang Q, Whitmarsh J, Satoh K, Pakrasi HB. Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry. 2002;41:8004–8012.10.1021/bi026012+
- Roose JL, Kashino Y, Pakrasi HB. The PsbQ protein defines cyanobacterial photosystem II complexes with highest activity and stability. Proc. Natl. Acad. Sci. 2007;104:2548–2553.10.1073/pnas.0609337104
- Liu H, Zhang H, Weisz DA, Vidavsky I, Gross ML, Pakrasi HB. MS-based cross-linking analysis reveals the location of the PsbQ protein in cyanobacterial photosystem II. Proc. Natl. Acad. Sci. 2014;111:4638–4643.10.1073/pnas.1323063111
- Aoi M, Kashino Y, Ifuku K. Function and association of CyanoP in photosystem II of Synechocystis sp. PCC 6803. Res. Chem. Intermed. 2014;40:3209–3217.10.1007/s11164-014-1827-y
- Ishikawa Y, Schröder WP, Funk C. Functional analysis of the PsbP-like protein (sll1418) in Synechocystis sp. PCC 6803. Photosynth. Res. 2005;84:257–262.10.1007/s11120-005-0477-8
- Summerfield TC, Winter RT, Eaton-Rye JJ. Investigation of a requirement for the PsbP-like protein in Synechocystis sp. PCC 6803. Photosynth. Res. 2005;84:263–268.10.1007/s11120-004-6431-3
- Sveshnikov D, Funk C, Schröder WP. The PsbP-like protein (sll1418) of Synechocystis sp. PCC 6803 stabilises the donor side of photosystem II. Photosynth. Res. 2007;93:101–109.
- Cormann KU, Bartsch M, Rögner M, Nowaczyk MM. Localization of the CyanoP binding site on photosystem II by surface plasmon resonance spectroscopy. Front. Plant Sci. 2014;5:595.
- Ifuku K, Ishihara S, Sato F. Molecular functions of oxygen-evolving complex family proteins in photosynthetic electron flow. J. Integr. Plant Biol. 2010;52:723–734.10.1111/jipb.2010.52.issue-8
- Bricker TM, Roose JL, Zhang P, Frankel LK. The PsbP family of proteins. Photosynth. Res. 2013;116:235–250.10.1007/s11120-013-9820-7
- Suorsa M, Sirpiö S, Paakkarinen V, Kumari N, Holmström M, Aro E-M. Two proteins homologous to PsbQ are novel subunits of the chloroplast NAD(P)H dehydrogenase. Plant Cell Physiol. 2010;51:877–883.10.1093/pcp/pcq070
- Ishihara S, Takabayashi A, Ido K, Endo T, Ifuku K, Sato F. Distinct functions for the two PsbP-like proteins PPL1 and PPL2 in the chloroplast thylakoid lumen of Arabidopsis. Plant Physiol. 2007;145:668–679.10.1104/pp.107.105866
- Yabuta S, Ifuku K, Takabayashi A, Ishihara S, Ido K, Ishikawa N, Endo T, Sato F. Three PsbQ-like proteins are required for the function of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell Physiol. 2010;51:866–876.10.1093/pcp/pcq060
- Ifuku K, Endo T, Shikanai T, Aro EM. Structure of the chloroplast NADH dehydrogenase-like complex: nomenclature for nuclear-encoded subunits. Plant Cell Physiol. 2011;52:1560–1568.10.1093/pcp/pcr098
- Liu J, Yang H, Lu Q, Wen X, Chen F, Peng L, Zhang L, Lu C. PsbP-domain protein1, a nuclear-encoded thylakoid lumenal protein, is essential for photosystem I assembly in Arabidopsis. Plant Cell. 2012;24:4992–5006.10.1105/tpc.112.106542
- Roose JL, Frankel LK, Bricker TM. The PsbP-domain protein 1 functions in the assembly of lumenal domains in photosystem I. J. Biol. Chem. 2014;289:23776–23785.
- Brzezowski P, Wilson KE, Gray GR. The PSBP2 protein of Chlamydomonas reinhardtii is required for singlet oxygen-dependent signaling. Planta. 2012;236:1289–1303.10.1007/s00425-012-1683-1
- Roose JL, Frankel LK, Bricker TM. Developmental defects in mutants of the PsbP domain protein 5 in Arabidopsis thaliana. PLoS One. 2011;6:e28624.10.1371/journal.pone.0028624
- Sato N. Phylogenomic and structural modeling analyses of the PsbP superfamily reveal multiple small segment additions in the evolution of photosystem II-associated PsbP protein in green plants. Mol. Phylogenet. Evol. 2010;56:176–186.10.1016/j.ympev.2009.11.021
- Ifuku K, Yamamoto Y, Ono T-A, Ishihara S, Sato F. PsbP protein, but not PsbQ protein, is essential for the regulation and stabilization of photosystem II in higher plants. Plant Physiol. 2005;139:1175–1184.10.1104/pp.105.068643
- Yi X, Hargett SR, Liu H, Frankel LK, Bricker TM. The PsbP protein is required for photosystem II complex assembly/stability and photoautotrophy in Arabidopsis thaliana. J. Biol. Chem. 2007;282:24833–24841.10.1074/jbc.M705011200
- Allahverdiyeva Y, Suorsa M, Rossi F, Pavesi A, Kater MM, Antonacci A, Tadini L, Pribil M, Schneider A, Wanner G, Leister D, Aro E-M, Barbato R, Pesaresi P. Arabidopsis plants lacking PsbQ and PsbR subunits of the oxygen-evolving complex show altered PSII super-complex organization and short-term adaptive mechanisms. Plant J. 2013;75:671–684.10.1111/tpj.2013.75.issue-4
- Tomita M, Ifuku K, Sato F, Noguchi T. FTIR evidence that the PsbP extrinsic protein induces protein conformational changes around the oxygen-evolving Mn cluster in photosystem II. Biochemistry. 2009;48:6318–6325.10.1021/bi9006308
- Ifuku K, Sato F. A truncated mutant of the extrinsic 23-kDa protein that absolutely requires the extrinsic 17-kDa protein for Ca2+ retention in photosystem II. Plant Cell Physiol. 2002;43:1244–1249.10.1093/pcp/pcf136
- Ifuku K, Nakatsu T, Shimamoto R, Yamamoto Y, Ishihara S, Kato H, Sato F. Structure and function of the PsbP protein of photosystem II from higher plants. Photosynth. Res. 2005;84:251–255.10.1007/s11120-004-7160-3
- Kakiuchi S, Uno C, Ido K, Nishimura T, Noguchi T, Ifuku K, Sato F. The PsbQ protein stabilizes the functional binding of the PsbP protein to photosystem II in higher plants. Biochim. Biophys. Acta. 2012;1817:1346–1351.10.1016/j.bbabio.2012.01.009
- Ido K, Kakiuchi S, Uno C, Nishimura T, Fukao Y, Noguchi T, Sato F, Ifuku K. The conserved His-144 in the PsbP protein is important for the interaction between the PsbP N-terminus and the Cyt b559 subunit of photosystem II. J. Biol. Chem. 2012;287:26377–26387.10.1074/jbc.M112.385286
- Ido K, Nield J, Fukao Y, Nishimura T, Sato F, Ifuku K. Cross-linking evidence for multiple interactions of the PsbP and PsbQ proteins in a higher plant photosystem II supercomplex. J. Biol. Chem. 2014;289:20150–20157.10.1074/jbc.M114.574822
- Nishimura T, Uno C, Ido K, Nagao R, Noguchi T, Sato F, Ifuku K. Identification of the basic amino acid residues on the PsbP protein involved in the electrostatic interaction with photosystem II. Biochim. Biophys. Acta. 2014;1837:1447–1453.10.1016/j.bbabio.2013.12.012
- Mummadisetti MP, Frankel LK, Bellamy HD, Sallans L, Goettert JS, Brylinski M, Limbach PA, Bricker TM. Use of protein cross-linking and radiolytic footprinting to elucidate PsbP and PsbQ interactions within higher plant Photosystem II. Proc. Natl. Acad. Sci. 2014;111:16178–16183.10.1073/pnas.1415165111
- Nagao R, Suzuki T, Okumura A, Niikura A, Iwai M, Dohmae N, Tomo T, Shen J-R, Ikeuchi M, Enami I. Topological analysis of the extrinsic PsbO, PsbP and PsbQ proteins in a green algal PSII complex by cross-linking with a water-soluble carbodiimide. Plant Cell Physiol. 2010;51:718–727.10.1093/pcp/pcq042
- Tohri A, Dohmae N, Suzuki T, Ohta H, Inoue Y, Enami I. Identification of domains on the extrinsic 23 kDa protein possibly involved in electrostatic interaction with the extrinsic 33 kDa protein in spinach photosystem II. Eur. J. Biochem. 2004;271:962–971.10.1111/ejb.2004.271.issue-5
- Meades GD, McLachlan A, Sallans L, Limbach PA, Frankel LK, Bricker TM. Association of the 17-kDa extrinsic protein with photosystem II in higher plants. Biochemistry. 2005;44:15216–15221.10.1021/bi051704u
- Popelkova H, Yocum CF. PsbO, the manganese-stabilizing protein: analysis of the structure-function relations that provide insights into its role in photosystem II. J. Photochem. Photobiol. B. 2011;104:179–190.
- Roose JL, Yocum CF, Popelkova H. Binding stoichiometry and affinity of the manganese-stabilizing protein affects redox reactions on the oxidizing side of photosystem II. Biochemistry. 2011;50:5988–5998.10.1021/bi2008068
- Sirpiö S, Khrouchtchova A, Allahverdiyeva Y, Hansson M, Fristedt R, Vener AV, Scheller HV, Jensen PE, Haldrup A, Aro E-M. AtCYP38 ensures early biogenesis, correct assembly and sustenance of photosystem II. Plant J. 2008;55:639–651.10.1111/tpj.2008.55.issue-4
- Fu A, He Z, Cho HS, Lima A, Buchanan BB, Luan S. A chloroplast cyclophilin functions in the assembly and maintenance of photosystem II in Arabidopsis thaliana. Proc. Natl. Acad. Sci. 2007;104:15947–15952.10.1073/pnas.0707851104
- Vasudevan D, Fu A, Luan S, Swaminathan K. Crystal structure of Arabidopsis cyclophilin38 reveals a previously uncharacterized immunophilin fold and a possible autoinhibitory mechanism. Plant Cell. 2012;24:2666–2674.10.1105/tpc.111.093781
- Karamoko M, Cline S, Redding K, Ruiz N, Hamel PP. Lumen thiol oxidoreductase1, a disulfide bond-forming catalyst, is required for the assembly of photosystem II in Arabidopsis. Plant Cell. 2011;23:4462–4475.10.1105/tpc.111.089680
- Wei L, Guo J, Ouyang M, Sun X, Ma J, Chi W, Lu C, Zhang L. LPA19, a Psb27 homolog in Arabidopsis thaliana, facilitates D1 protein precursor processing during PSII biogenesis. J. Biol. Chem. 2010;285:21391–21398.10.1074/jbc.M110.105064
- Chen H, Zhang D, Guo J, Wu H, Jin M, Lu Q, Lu C, Zhang L. A Psb27 homologue in Arabidopsis thaliana is required for efficient repair of photodamaged photosystem II. Plant Mol. Biol. 2006;61:567–575.10.1007/s11103-006-0031-x
- Hou X, Fu A, Garcia VJ, Buchanan BB, Luan S. PSB27: a thylakoid protein enabling Arabidopsis to adapt to changing light intensity. Proc. Natl. Acad. Sci 2015;112:1613–1618.
- Ido K, Ifuku K, Yamamoto Y, Ishihara S, Murakami A, Takabe K, Miyake C, Sato F. Knockdown of the PsbP protein does not prevent assembly of the dimeric PSII core complex but impairs accumulation of photosystem II supercomplexes in tobacco. Biochim. Biophys. Acta. 2009;1787:873–881.10.1016/j.bbabio.2009.03.004
- Lima A, Lima S, Wong JH, Phillips RS, Buchanan BB, Luan S. A redox-active FKBP-type immunophilin functions in accumulation of the photosystem II supercomplex in Arabidopsis thaliana. Proc. Natl. Acad. Sci. 2006;103:12631–12636.10.1073/pnas.0605452103
- Sun X, Ouyang M, Guo J, Ma J, Lu C, Adam Z, Zhang L. The thylakoid protease Deg1 is involved in photosystem-II assembly in Arabidopsis thaliana. Plant J. 2010;62:240–249.10.1111/j.1365-313X.2010.04140.x
- Uno C, Nagao R, Suzuki H, Tomo T, Noguchi T. Structural coupling of extrinsic proteins with the oxygen-evolving center in red algal photosystem II as revealed by light-induced FTIR difference spectroscopy. Biochemistry. 2013;52:5705–5707.10.1021/bi4009787
- Enami I, Yoshihara S, Tohri A, Okumura A, Ohta H, Shen JR. Cross-reconstitution of various extrinsic proteins and photosystem II complexes from cyanobacteria, red algae and higher plants. Plant Cell Physiol. 2000;41:1354–1364.10.1093/pcp/pcd069
- Roncel M, Kirilovsky D, Guerrero F, Serrano A, Ortega JM. Photosynthetic cytochrome c550. Biochim. Biophys. Acta. 2012;1817:1152–1163.10.1016/j.bbabio.2012.01.008
- Enami I, Iwai M, Akiyama A, Suzuki T, Okumura A, Katoh T, Tada O, Ohta H, Shen J-R. Comparison of binding and functional properties of two extrinsic components, Cyt c550 and a 12 kDa protein, in cyanobacterial PSII with those in red algal PSII. Plant Cell Physiol. 2003;44:820–827.10.1093/pcp/pcg106
- Ohta H, Suzuki T, Ueno M, Okumura A, Yoshihara S, Shen J-R, Enami I. Extrinsic proteins of photosystem II: an intermediate member of PsbQ protein family in red algal PS II. Eur. J. Biochem. 2003;270:4156–4163.10.1046/j.1432-1033.2003.03810.x
- Glöckner G, Rosenthal A, Valentin K. The structure and gene repertoire of an ancient red algal plastid genome. J. Mol. Evol. 2000;51:382–390.
- Guerrero F, Sedoud A, Kirilovsky D, Rutherford AW, Ortega JM, Roncel M. A high redox potential form of cytochrome c550 in photosystem II from Thermosynechococcus elongatus. J. Biol. Chem. 2011;286:5985–5994.10.1074/jbc.M110.170126
- Adachi H, Umena Y, Enami I, Henmi T, Kamiya N, Shen J-R. Towards structural elucidation of eukaryotic photosystem II: Purification, crystallization and preliminary X-ray diffraction analysis of photosystem II from a red alga. Biochim. Biophys. Acta. 2009;1787:121–128.10.1016/j.bbabio.2008.11.004
- Miyahara M, Aoi M, Inoue-Kashino N, Kashino Y, Ifuku K. Highly efficient transformation of the diatom Phaeodactylum tricornutum by multi-pulse electroporation. Biosci. Biotechnol. Biochem. 2013;77:874–876.
- Ifuku K, Yan D, Miyahara M, Inoue-Kashino N, Yamamoto YY, Kashino Y. A stable and efficient nuclear transformation system for the diatom Chaetoceros gracilis. Photosynth. Res. 2015;123:203–211.10.1007/s11120-014-0048-y
- Caffarri S, Kouřil R, Kereïche S, Boekema EJ, Croce R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 2009;28:3052–3063.10.1038/emboj.2009.232
- Pagliano C, Nield J, Marsano F, Pape T, Barera S, Saracco G, Barber J. Proteomic characterization and three-dimensional electron microscopy study of PSII-LHCII supercomplexes from higher plants. Biochim. Biophys. Acta. 2014;1837:1454–1462.10.1016/j.bbabio.2013.11.004
