Abstract
Outer bran fraction of rice (OBFR) contains higher concentrations of crude fiber, γ-oryzanol, and phytic acid compared to whole rice bran (WRB). In this study, we examined the effects of feeding OBFR on lipid accumulation and fecal excretion in rats. Twenty-one male rats at seven-week-old were divided into a control group and two treatment groups. The control group was fed a control diet, and the treatment groups were fed OBFR- or WRB-containing diet for 21 days. There was no significant difference in growth performance. Feeding OBFR diet increased fecal number and weight accompanied by increased fecal lipid content, while it did not affect mRNA expressions encoding lipid metabolism-related protein in liver. In addition, feeding OBFR-diet decreased the abdominal fat tissue weight and improved plasma lipid profiles, while WRB-containing diet did not affect them. These results suggested that feeding OBFR-diet might prevent lipid accumulation via enhancing fecal lipid excretion in rats.
Graphical abstract
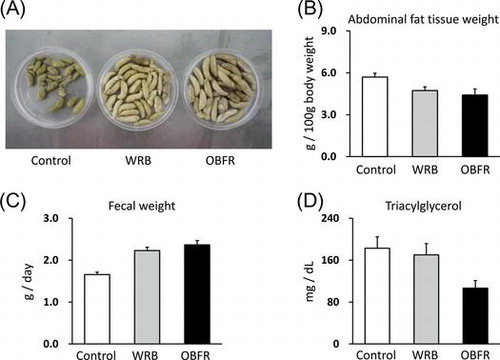
A, Excreted feces from a rat per day. B, Fecal weight from a rat per day. C, The weight of the abdominal fat tissue. D, Plasma triacylglycerol level. Values are expressed as mean ± SEM (n = 7). OBFR; outer bran fraction of rice, WRB; rice bran.
Introduction
Excessive energy is stored in white adipose tissue as triacylglycerols (TGs), and excess adipose tissue accumulation (i.e. obesity) is one of the main causes of metabolic dysfunction in human and even in companion animals. The reasons for obesity are various, e.g. excessive eating,Citation1) consumption of foods rich in fat,Citation2) or individual genetic factors.Citation3) In addition to these common reasons, recently the intestinal flora has been considered to be one of the contributing factors to obesity.Citation4)
It has been well defined that intake of dietary fiber, which is divided into insoluble (cellulose, hemicelluloses, and lignin) and soluble fiber (pectins and gums) depending on their solubility in water, reduces the risk of obesity,Citation5) because feeding dietary fiber decreases the digestibility of nutrients by disrupting nutrient absorption.Citation6,7)
Consequently, dietary fiber increased fecal excretion accompanied by increased excretion of energy, protein, and fat.Citation5,8) Interestingly, intake of insoluble fiber decreases energy digestibility, while soluble fiber increases it.Citation9)
Rice bran is a byproduct of rice milling process, and is well known to contain rich dietary fibers, because it consists of several cell layers, including pericarp, tegmen, and aleurone.Citation10) Rice bran also contains functional components (tocopherol, tocotrienol, feruic acid, γ-oryzanol, or phytic acid), e.g. they play a role as free-radical scavenging antioxidants, respectively.Citation11–14) Feruic acid and γ-oryzanol have roles to inhibit cholesterol absorption in intestine and excrete it into feces.Citation12,15) In addition, phytic acid inhibited activity of porcine pancreatic lipase,Citation16) and inhibition of pancreatic lipase resulted in reduced availability of dietary fat in the intestine.Citation17) Feeding rice bran remarkably improved bowel movement and fecal excretionCitation18) and decreased plasma TG and total cholesterol in rats.Citation19) Feeding rice bran oil also increased fecal weight and fecal bile acid excretion of rats.Citation20) In human, intake of rice bran significantly improve the bowel movement,Citation21) although the effects of rice bran on plasma lipid profiles were small.Citation22)
Recently, multibreak milling systems prevails in the rice industry. In the rice milling systems, multiple milling machines are used to remove bran from the rice kernels; therefore, more outer layer of rice bran is removed from initial break. Lloyd et al. compared the amount of functional components such as γ-oryzanol, tocopherol, and tocotrienol in rice bran collected from three milling breaks and found that these functional components were rich in the outer layer, i.e. rice bran collected after first milling break contains the highest levels of γ-oryzanol, while it was not detected in rice bran collected after third milling break.Citation23) The amount of tocopherol and tocotrienol was the highest in rice bran collected after second milling break.Citation23) In addition, the outer layer of rice bran, which mainly contains pericarp, was rich in lignin compared to the inner layer, while the amounts of pectic substance, hemicellulose, and α-cellulose were equal between the outer and inner layer.Citation24) Thus, since the outer layer of rice bran contains rich insoluble dietary fiber and functional components such as γ-oryzanol, tocopherol, and tocotrienol, the outer layer may be expected to exert equal or exceed health beneficial effects compared to whole rice bran (WRB). However, the health beneficial effects of the outer layer of rice bran have not been described.
In this study, we collected the outer bran fraction of rice (OBFR) from first and second break of commercial quadruple-break milling system and WRB from all breaks. We examined the effects of feeding OBFR or WRB on fecal excretion and lipid accumulation of rats. To evaluate the intestinal environment of rats fed either OBFR or WRB, cecal pH and fecal characteristics (number, weight, bile acid content, and lipid content) were examined in this study.
Materials and methods
Preparation of OBFR
OBFR and WRB were collected from a commercial rice mill plant (Shokkyo Co.,Ltd, Hiroshima, Japan). In this plant, quadruple break commercial milling systems (HCP-40A, Satake Corporation., Hiroshima, Japan) were used to polish rice. The OBFR was collected from first and second break, while WRB was collected from all breaks. In this study, 8.3% of OBFR and 9.7% of WRB were obtained from brown rice, respectively. Then, chemical composition (i.e. moisture, crude protein, crude fat, crude fiber, ash, and gross energy) of both OBFR and WRB was tested by the Japan Food Research Laboratories (Tokyo, Japan) and shown in Table .
Table 1. Chemical composition of WRB and OBFR used in this study.
Determination of γ-oryzanol content and phytic acid content
The γ-oryzanol content in WRB and OBFR was determined by Shimadzu HPLC model LC6A with Shim-Pack CLC-ODS column (6.0 × 150 mm) according to the method described by Chen and Bergman.Citation25) Phytic acid content in WRB and OBFR was determined according to the method described by Vaintraub and Lapteva.Citation26)
Experimental diet
Three experimental diets were formulated as follows: (1) control diet (AIN-93G); (2) diet-containing WRB, and (3) diet-containing OBFR. The crude protein (CP), crude fiber (CF), and gross energy (GE) were adjusted to the same levels as those in the control diet (17.89% CP, 5.04% CF, and 3.94 Mcal/kg GE). The ingredients and nutrient composition of the experimental diets are shown in Table .
Table 2. Composition of experimental diets.
Animals and treatment
This experiment was conducted in accordance with the guidelines of the Animal Care and Use Committee of Kagoshima University. Twenty-one male Sprague Dawley rats at 7 weeks of age were purchased from Japan SLC Co. Ltd (Shizuoka, Japan). The rats were individually housed in wire-bottomed aluminum cages (152 × 201 × 170 mm) and provided water and the control diet ad libitum for 6 days in a temperature-controlled room at 25 °C. On day 7, the rats were randomly divided into three groups and receive one of the three experimental diets. Body weight was measured once a week, and both feed and water intake was measured every day. Fecal number and weight were measured every day, and fecal samples were collected during last 3 days of the experiment. All rats were killed by cervical dislocation under ether anesthesia. The liver, heart, kidney, mesenteric fat tissue, retroperitoneal fat tissue, epididymal fat tissue, and cecum were collected and weighed. Total abdominal fat weight was the sum of mesenteric fat tissue weight, retroperitoneal fat tissue weight, and epididymal fat tissue weight. Cecal content, which removed from the cecum, was collected in plastic test tube and added 10 times the amount of double-distilled water to it. Then, the pH of suspension of the cecum content was measured using a pH meter (HM-30G, DKK-TOA Corporation, Tokyo, Japan). After removal of the cecal content, the cecum tissue was washed in PBS and wiped up, and then weighed.
Biochemical blood testing
The levels of TG, total cholesterol, and glucose in the plasma were measured by Fuji DRI-CHEM 3500 (Fujifilm, Tokyo, Japan), according to the manufacturer’s instructions.
Determination of dry weight, lipid, and bile acid content of feces and lipid content of liver
The moisture content of fecal samples was determined by air oven moisture content determination methods. The dried feces were powdered, and then total fat content was measured using the method described by Folch et al.Citation27) Fecal bile acids were measured using a total bile acids kit (Wako, Osaka, Japan). Total fat content in liver was also measured using the method described by Folch et al.Citation27)
Statistical analysis
Data are expressed as means ± SEM. Statistical comparisons were performed using Dunnett’s test. P values under 5% were considered to indicate statistical significance. All analyses were performed with the General Linear Model procedure of the Statistical Analysis System software package (SAS/STAT Version 0, Statistical Analysis Systems Institute Inc., Cary, NC, USA).
Results and discussion
Throughout a 21-day feeding period, the growth performance (i.e. body weight gain, feed intake, feed efficiency, and water intake) of rats fed either OBFR- or WRB-containing diet was not different with that of the control rats, respectively (Table ). On the other hand, feeding either OBFR- or WRB-containing diet remarkably increased fecal number, fecal wet weight, and fecal dry weight compared to those of the control rats, respectively (Table ). Since it has been well-known that feeding dietary fiber increased fecal excretion,Citation5) it was suggested that dietary fiber included in either OBFR or WRB might affect fecal excretion and/or bowel movement of rats. However, in this study, since the CF content was same level among three diets, the reason for this increased fecal excretion might be due to not the fiber amount but the fiber composition in the diet. Cummings et al.Citation28) reported that dietary fiber from various sources have different effects on fecal excretion of human, e.g. wheat bran increased fecal excretion compared to cabbage, carrots, apples and guar, despite equal loads of fiber in diet. In addition, Cummings et al.Citation28) also found that there was a positive correlation between lignin intake and fecal weight. In this study, although the source of fiber in the control diet was cellulose, either OBFR- or WRB-containing diet might include some fiber sources other than cellulose (e.g. lignin, hemicellulose, or pectic substance). Especially, since the outer layer of rice bran contains rich in insoluble fiber, such as lignin,Citation24) it was suggested that insoluble fiber included in either OBFR or WRB might be one of the causes of the increased fecal excretion in rats fed either OBFR- or WRB-containing diet.
Table 3. Effects of feeding OBFR on growth performance in rats.
Table 4. Effects of feeding OBFR on fecal excretion, fecal lipid content, fecal bile acid content, and lipid content in liver of rats.
Although feeding OBFR-containing diet did not affect the final body weight and the tissue weights of liver, heart, and kidney, it decreased total abdominal fat tissue weight (Table ). Especially, the mesenteric fat tissue was significantly decreased in rats fed OBFR-containing diet. Although there was no significant difference, feeding OBFR-containing diet tended to decrease the weights of the retroperitoneal and epididymal fat tissue, respectively. In addition, plasma levels of total cholesterol and TG in rats fed OBFR-containing diet were lower than those of the control rats (Table ). However, it did not affect mRNA expressions of genes related to lipid metabolism in liver (Supplemental Figure 1; see Biosci. Biotechnol. Biochem Web site). Feeding dietary fiber reduces the digestibility of nutrients by disrupting nutrient absorption,Citation6,7) and consequently it increases fecal excretion of energy, protein, and fat.Citation9) Indeed, the fecal lipid content of rats fed OBFR-containing diet was increased compared to that of the control rats (Table ). Therefore, the decreased abdominal fat tissue weight and plasma lipid levels might be due to dietary fiber involved in OBFR. Furthermore, feeding rice bran oil increased fecal bile acid excretionCitation20) and decreased either plasma TG or total cholesterol level.Citation29,30) Since γ-oryzanol has roles to inhibit cholesterol absorption in intestine and excrete it into feces,Citation15) it is considered that these lipid-lowering effects might be partially due to the functions of γ-oryzanol.Citation29,30) In addition, phytic acid inhibited activity of porcine pancreatic lipase,Citation16) and inhibition of pancreatic lipase resulted in reduced availability of dietary fat in the intestine.Citation17) Therefore, it was suggested that either γ-oryzanol or phytic acid might affect intestinal lipid absorption and fecal lipid excretion.
Table 5. Effects of feeding OBFR on body weight, tissue weights, and cecal pH in rats.
Table 6. Effects of feeding OBFR on plasma lipid and glucose profiles in rats.
The intestinal flora can ferment dietary fiber, and some species of enteric bacteria (e.g. Lactobacillus, Streptococcus, and Bifidobacterium) have an ability to produce short-chain fatty acids (SCFA). The production of SCFA resulted in a lower pH environment in the intestinal tract.Citation31) Tamura et al.Citation32) found that feeding rice bran oil increased the ratios of Lactobacillus in cecum of mice and there was positive correlation between the ratio of Lactobacillus and fecal bile acid content. In this study, feeding OBFR- and WRB-containing diet similarly decreased the cecal pH and increased bile acid excretion in rats. These results raising a possibility that feeding OBFR and/or WRB might increase the ratio of Lactobacillus, and consequently changed intestinal flora of rats. Furthermore, since SCFA, which is absorbed from intestinal tract, resulted in improvement in blood lipid levels,Citation33) feeding WRB- or OBFR-containing diet might improve lipid metabolism of rats partially via changes in intestinal flora.
However, in contrast to the OBFR-containing diet, feeding WRB-containing diet decreased neither abdominal fat tissue weight nor plasma lipid levels. One possible explanation for this difference between OBFR and WRB might be due to the different concentrations of γ-oryzanol and/or phytic acid. In this study, both γ-oryzanol and phytic acid concentrations in OBFR were 1.2 times higher than that in WRB, respectively (Table ). Therefore, it was suggested that the lipid-lowering effect might be higher in OBFR than WRB, and consequently only feeding OBFR-containing diet reduced lipid accumulation. However, since neither fecal nor liver lipid content in rats fed OBFR-containing diet was different from rats fed WRB-containing diet, this difference between OBFR and WRB could not be explained by only the roles of γ-oryzanol and phytic acid in the intestine. Recently, it was reported that rice bran extract has potential roles to accelerate energy consumption in human.Citation34) In addition, triterpene alcohols and sterols from rice bran inhibited postprandial release of glucose-dependent insulinotropic polypeptide (GIP), which promotes weight gain and insulin resistance, and prevent diet-induced obesity in mice.Citation35) Since γ-oryzanol is a mixture of ferulic acid esters of phytosterols and triterpene alcohols,Citation36) OBFR might contain higher amount of triterpene alcohols compared to WRB and increase energy expenditure via inhibition of GIP release. Moreover, rice bran contains functional components other than γ-oryzanol, phytic acid, and dietary fiber such as vitamins, essential minerals, protease inhibitors, and amylase inhibitors.Citation37) Therefore, these functional compounds might affect the lipid-lowering effect of OBFR and WRB, while the amount of α-tocopherol, which is the most biologically active form, was not different between OBFR and WRB (data not shown). Further studies are needed to gain more information about either the functional compounds in OBFR or the health benefit of OBFR.
In conclusion, feeding OBFR increased number and weight of feces accompanied by increased fecal excretion. And feeding OBFR has higher lipid lowering effect compared to that of WRB. These results suggested that OBFR could be a functional ingredient for human and other mammals such as companion animals.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1032883.
Supplemental Materials
Download MS Word (85.8 KB)Acknowledgments
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Loro AD Jr, Orleans CS. Binge eating in obesity: preliminary findings and guidelines for behavioral analysis and treatment. Addict. Behav. 1981;6:155–166.10.1016/0306-4603(81)90010-1
- Labayen I, Ruiz JR, Ortega FB, Huybrechts I. High fat diets are associated with higher abdominal adiposity regardless of physical activity in adolescents; the HELENA study. Clin. Nutr. 2014;33:859–866.10.1016/j.clnu.2013.10.008
- Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am. J. Med. Genet. A. 2007;143:3016–3034.10.1002/(ISSN)1552-4833
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031.10.1038/nature05414
- Spiller RC. Pharmacology of dietary fibre. Pharmacol. Ther. 1994;62:407–427.10.1016/0163-7258(94)90052-3
- Southgate DAT, Branch WJ, Hill MJ, Drasar BS, Walters RL, Davies PS, McLean Baird I, Metabolic responses to dietary supplements of bran. Metabolism. 1976;25:1129–1135.
- Baer DJ, Rumpler WV, Miles CW, Fahey GC Jr. Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J. Nutr. 1997;127:579–586.
- Southgate DAT, Durnin JVGA. Calorie conversion factors. An experimental reassessment of the factors used in the calculation of the energy value of human diets. Br. J. Nutr. 1970;24:517–535.10.1079/BJN19700050
- Renteria-Flores JA, Johnston LJ, Shurson GC, Gallaher DD. Effect of soluble and insoluble fiber on energy digestibility, nitrogen retention, and fiber digestibility of diets fed to gestating sows. J. Anim. Sci. 2008;86:2568–2575.10.2527/jas.2007-0375
- Shi J, Mazza G, Maguer ML, Functional foods: biochemical and processing aspects. Lancaster (FL): CRC Press; 1998. p. 71–72.
- Burton GW, Traber MG, Vitamin E. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu. Rev. Nutr. 1990;10:357–382.10.1146/annurev.nu.10.070190.002041
- Ou S, Kwok KC. Ferulic acid: pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004;84:1261–1269.10.1002/(ISSN)1097-0010
- Wang T, Hicks KB, Moreau R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J. Am. Oil Chem. Soc. 2002;79:1201–1206.10.1007/s11746-002-0628-x
- Graf E, Eaton JW. Antioxidant functions of phytic acid. Free Radical Biol. Med. 1990;8:61–69.10.1016/0891-5849(90)90146-A
- Rong N, Ausman LM, Nicolosi RJ. Oryzanol decreases cholesterol absorption and aortic fatty streaks in hamsters. Lipids. 1997;32:303–309.10.1007/s11745-997-0037-9
- Knuckles BE. Effect of phytate and other myo-inositol phosphate esters on lipase activity. J. Food Sci. 1988;53:250–252.10.1111/jfds.1988.53.issue-1
- Mukherjee M. Human digestive and metabolic lipases – a brief review. J. Mol. Catal. B: Enzym. 2003;22:369–376.10.1016/S1381-1177(03)00052-3
- Satchithanandam S, Klurfeld DM, Calvert RJ, Cassidy MM. Effects of dietary fibers on gastrointestinal mucin in rats. Nutr. Res. 1996;16:1163–1177.10.1016/0271-5317(96)00121-2
- Topping DL, Illman RJ, Roach PD, Trimble RP, Kambouris A, Nestel PJ. Modulation of the hypolipidemic effect of fish oils by dietary fiber in fats: studies with rice and wheat bran. J. Nutr. 1990;120:325–330.
- Chen CW, Cheng HHJ. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J. Nutr. 2006;136:1472–1476.
- Tomlin J, Read NW. Comparison of the effects of colonic function caused by feeding rice bran and wheat bran. Eur. J. Clin. Nutr. 1988;42:857–861.
- Kestin M, Moss R, Clifton PM, Nestel PJ. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 1990;52:661–666.
- Lloyd BJ, Siebenmorgen TJ, Beers KW. Effects of commercial processing on antioxidants in rice bran. Cereal Chem. 2000;77:551–555.
- Shibuya N, Nakane R, Yasui A, Tanaka K, Iwasaki T. Comparative studies on cell wall preparations from rice bran, germ, and endosperm. Cereal Chem. 1985;62:252–258.
- Chen MH, Bergman CJ. A rapid procedure for analysing rice bran tocopherol, tocotrienol and γ-oryzanol contents. J. Food Compos. Anal. 2005;18:139–151.10.1016/j.jfca.2003.09.004
- Vaintraub IA, Lapteva N. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Biochem. 1988;175:227–230.10.1016/0003-2697(88)90382-X
- Folch J, Lees M, Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509.
- Cummings JH, Branch W, Jenkins DJ, Southgate DA, Houston H, James WP. Colonic response to dietary fibre from carrot, cabbage, apple, bran, and guar gum. Lancet. 1978;311:5–9.10.1016/S0140-6736(78)90357-4
- Sugano M, Tsuji E. Rice bran oil and cholesterol metabolism. J. Nutr. 1997;127:521–524.
- Wilson TA, Nicolosi RJ, Woolfrey B, Kritchevsky D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypocholesterolemic hamsters. J. Nutr. Biochem. 2007;18:105–112.10.1016/j.jnutbio.2006.03.006
- Gibson GR, Roberfoid MB. Dietary modulation of the human colonie microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412.
- Tamura M, Hori S, Hoshi C, Nakagawa H. Effects of rice bran oil on the intestinal microbiota and metabolism of isoflavones in adult mice. Int. J. Mol. Sci. 2012;13:10336–10349.10.3390/ijms130810336
- Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779.10.1136/gut.22.9.763
- Fukuoka D, Hashizume K, Osaki N, Shimotoyodome A, International Patent WO2012008317 A1 (Jan 19, 2012).
- Fukuoka D, Okahara F, Hashizume K, Yanagawa K, Osaki N, Shimotoyodome A. Triterpene alcohols and sterols from rice bran lower postprandial glucose-dependent insulinotropic polypeptide release and prevent diet-induced obesity in mice. J. Appl. Physiol. 2014;117:1337–1348.10.1152/japplphysiol.00268.2014
- Itoh T, Tamura T, Matsumoto T. Sterol composition of 19 vegetable oils. J. Am. Oil Chem. Soc. 1973;50:122–125.10.1007/BF02633564
- Hamada JS. Characterization and functional properties of rice bran proteins modified by commercial exoproteases and endoproteases. J. Food Sci. 2000;65:305–310.10.1111/jfds.2000.65.issue-2
