Abstract
Bacillus subtilis subsp. natto produces poly-γ-glutamic acid under the control of quorum sensing. We identified ComXnatto pheromone as the quorum-sensing pheromone with an amino acid sequence of Lys-Trp-Pro-Pro-Ile-Glu and the tryptophan residue posttranslationally modified by a farnesyl group. ComXnatto pheromone is unique in the sense that the 5th tryptophan residue from the C-terminal is farnesylated.
Quorum sensing is the system involved in the activation of specific gene expressions dependent on cell density.Citation1) Bacillus subtilis secrets ComX pheromone that stimulates natural genetic competence controlled by quorum sensing.Citation2,3) ComX pheromone is a posttranslationally modified oligopeptide synthesized from ComX. The modification is catalyzed by an isoprenyl diphosphate synthase-like protein, ComQ, and is essential for the function of the pheromone.Citation4) Previous studies have demonstrated that striking polymorphism is exhibited in the amino acid sequence of the ComX pheromone variants in 6 strains, but each variant possesses a tryptophan residue at either the 3rd or 4th residue from the C-terminal.Citation3–6) Our studies revealed the precise nature of the ComX modification as follows: the tryptophan residue is modified with either a geranyl group or a farnesyl group at its γ-position, resulting in the formation of a tricyclic structure, including a newly formed proline-like five-membered ring.Citation7–10) In contrast, many details of processing remain to be clarified.
B. subtilis subsp. natto is closely related to B. subtilis laboratory strains and known as a starter strain for a traditional Japanese fermented food made from soybeans, called natto in Japanese. Molecular genetic analysis of B. subtilis subsp. natto strain BEST195 demonstrated that gene homologs, ComQ and ComX, are also present in its genome cluster.Citation11–13) The most obvious phenotypical distinction of B. subtilis subsp. natto from the laboratory strains is biofilm formation, which mainly involves the highly sticky polymer poly-γ-glutamic acid (γ-PGA). γ-PGA biosynthetic gene cluster involves the ComQXnatto gene.Citation12) This result suggests that the precursor ComXnatto is modified by ComQnatto, and resulting ComXnatto pheromone activates the gene expression for γ-PGA biosynthesis. However, there is no tryptophan residue at either the 3rd or the 4th residue from the C-terminal, but one tryptophan residue at the 54th among 73 amino acid residues in ComXnatto. Furthermore, our recent studies have demonstrated that the tryptophan residue must be located between the 2nd and the 4th residues from the C-terminal in order to be modified by ComQ.Citation14–16)
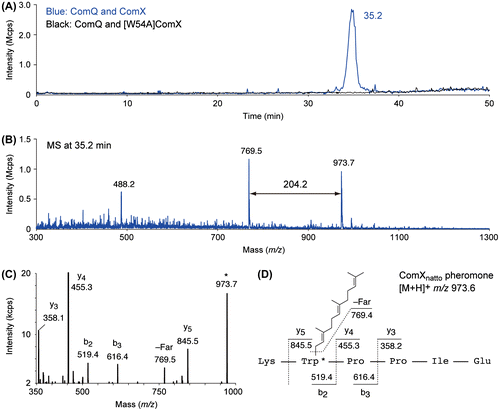
First, we prepared ComXnatto pheromone expression plasmid integrated with a ComQX gene cluster directly amplified from B. subtilis subsp. natto, and then the plasmid was introduced to Escherichia coli. We also prepared alanine-substituted mutant of the 54th residue tryptophan in ComXnatto ([W54A] ComXnatto) based on the constructed plasmid. Next, we analyzed these fermentation liquids of the transformants by LC-MS and compared resulting chromatograms at various values of m/z. As expected, only the sample prepared from the fermentation liquid ComQnatto and ComXnatto overexpressing transformant contained the characteristic compounds with a molecular ion at m/z 973.7 (Fig. (A)). Additionally, the MS at 35.2 min showed a fragment ion at m/z 769.5, 204.2 Da less than the value of the molecular ion (Fig. (B)). The difference in ions corresponds to farnesylation (C15H24 addition). In LC-MS analysis, no other modified peptides derived from ComXnatto were observed at all. Subsequently, MS/MS analysis was carried out (Fig. (C)), and the MS/MS of the compound at m/z 973.7 ([C54H81N8O9]+) was consistent with that of the six-residue-modified peptide that possessed the amino acid sequence, Lys-Trp-Pro-Pro-Ile-Glu, and the 2nd tryptophan residue-modified with a farnesyl group (Fig. (D)). The amino acid sequence of the compound matched that from the 53rd to the 58th residues in ComXnatto. It is notable that ComQnatto can catalyze farnesylation of the tryptophan residue up to at least the 5th position from the C-terminal, though it is not clear which biosynthetic step occurred first, farnesylation or truncation of amino acid residues. Based on the phylogenetic resemblance, the tryptophan residue of ComXnatto pheromone is probably similar to that of the ComXRO-C-2 pheromone.Citation8) Unfortunately, since ComXnatto pheromone is much more labile than the ComX pheromones previously isolated or synthesized, it was difficult to purify enough ComXnatto pheromone for NMR analysis.Citation7–9,17–20) We are now attempting to improve the chemical synthetic method for ComX pheromone,Citation17–20) therefore, the precise chemical structure of ComXnatto pheromone will be determined in the near future.
Fig. 1. LC-MS and MS/MS analyses of ComXnatto pheromone secreted by transformants.
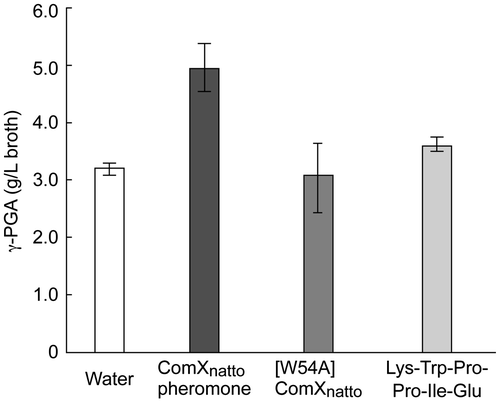
Subsequently, we investigated the effect of the modified ComXnatto peptide on γ-PGA production of B. subtilis subsp. natto in liquid culture. The addition of the modified ComXnatto peptide at nearly 10 nM increased the yield of γ-PGA more than 1.5 times than that of water, liquid culture broth of [W54A] ComXnatto, or a plain hexapeptide, Lys-Trp-Pro-Pro-Ile-Glu, at 1 μM (Fig. ). Unfortunately, since the modified ComXnatto peptide could not be detected in the liquid culture of B. subtilis subsp. natto by LC-MS, the concentration would be estimated to be less than 10 nM. These findings were consistent with previous molecular genetic researches.Citation3,6,12) It was thus confirmed that the modified ComXnatto peptide plays a crucial role in γ-PGA production as a quorum-sensing pheromone, ComXnatto pheromone, in B. subtilis subsp. natto.
Fig. 2. Effects of ComXnatto pheromone on γ-PGA production of B. subtilis subsp. natto.
In conclusion, it has been shown that ComXnatto pheromone stimulates γ-PGA production, which is a hexapeptide having an amino acid sequence of Lys-Trp-Pro-Pro-Ile-Glu, and the 2nd tryptophan residue is posttranslationally modified with a farnesyl group by ComQnatto. ComXnatto pheromone is unique in the sense that the 5th tryptophan residue from the C-terminal is modified with a farnesyl group, which corresponds to the 54th residue among 73 amino acid residues in ComXnatto. For industrial application, ComXnatto pheromone will attract attention as a promising additive for improving the production of γ-PGA by B. subtilis subsp. natto.
Additional information
Funding
References
- Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246.10.1016/j.cell.2006.04.001
- Hamoen LW, Venema G, Kuipers OP. Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology. 2003;149:9–17.10.1099/mic.0.26003-0
- Magnuson R, Solomon J, Grossman AD. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell. 1994;77:207–216.10.1016/0092-8674(94)90313-1
- Weinrauch Y, Msadek T, Kunst F, Dubnau D. Sequence and properties of ComQ, a new competence regulatory gene of Bacillus subtilis. J. Bacteriol. 1991;173:5685–5693.
- Bacon Schneider K, Palmer TM, Grossman AD. Characterization of comQ and comX, two genes required for production of ComX pheromone in Bacillus subtilis, J. Bacteriol. 2002;184:410–419.10.1128/JB.184.2.410-419.2002
- Ansaldi M, Marolt D, Stebe T, Mandic-Mulec I, Dubnau D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol. Microbiol. 2002;44:1561–1573.10.1046/j.1365-2958.2002.02977.x
- Okada M, Sato I, Cho SJ, Iwata H, Nishio T, Dubnau D, Sakagami Y. Structure of the Bacillus subtilis quorum-sensing peptide pheromone ComX. Nat. Chem. Biol. 2005;1:23–24.10.1038/nchembio709
- Okada M, Yamaguchi H, Sato I, Tsuji F, Dubnau D, Sakagami Y. Chemical structure of posttranslational modification with a farnesyl group on tryptophan. Biosci. Biotechnol. Biochem. 2008;72:914–918.10.1271/bbb.80006
- Okada M, Yamaguchi H, Sato I, Tsuji F, Qi J, Dubnau D, Sakagami Y. Acid labile ComX pheromone from Bacillus mojavensis RO-H-1. Biosci. Biotechnol. Biochem. 2007;71:1807–1810.10.1271/bbb.70245
- Okada M. Post-translational isoprenylation of tryptophan. Biosci. Biotechnol. Biochem. 2011;75:1413–1417.10.1271/bbb.110087
- Nishito Y, Osana Y, Hachiya T, Popendorf K, Toyoda A, Fujiyama A, Itaya M, Sakakibara Y. Whole genome assembly of a natto production strain Bacillus subtilis natto from very short read data. BMC Genomics. 2010;11:243–254.10.1186/1471-2164-11-243
- Tran L-SP, Nagai T, Itoh, Y. Divergent structure of the ComQXPA quorum sensing components: molecular basis of strain-specific communication mechanism in Bacillus subtilis. Mol. Microbiol. 2000;37:1159–1171.10.1046/j.1365-2958.2000.02069.x
- Tortosa P, Logsdon L, Kraigher B, Itoh Y, Mandic-Mulec I, Dubnau D. Specificity and genetic polymorphism of the Bacillus competence quorum-sensing system. J. Bacteriol. 2001;183:451–460.10.1128/JB.183.2.451-460.2001
- Tsuji F, Ishihara A, Kurata K, Nakagawa A, Okada M, Kitamura S, Kanamaru K, Masuda Y, Murakami K, Irie K, Sakagami Y. Geranyl modification on the tryptophan residue of ComXRO-E-2 pheromone by a cell-free system. FEBS Lett. 2012;586:174–179.10.1016/j.febslet.2011.12.012
- Tsuji F, Ishihara A, Nakagawa A, Okada M, Kitamura S, Kanamaru K, Masuda Y, Murakami K, Irie K, Sakagami Y. Lack of the consensus sequence necessary for tryptophan prenylation in the ComX pheromone precursor. Biosci. Biotechnol. Biochem. 2012;76:1492–1496.10.1271/bbb.120206
- Okada M, Ishihara A, Yamasaki R, Tsuji F, Hayashi S, Usami S, Sakagami Y. A region corresponding to second aspartate-rich motif in tryptophan isoprenylating enzyme, ComQ, serves as a substrate-binding site. Biosci. Biotechnol. Biochem. 2014;78:550–555.10.1080/09168451.2014.891932
- Okada M, Sato I, Cho SJ, Suzuki Y, Ojika M, Dubnau D, Sakagami Y. Towards structural determination of the ComX pheromone: synthetic studies on peptides containing geranyltryptophan. Biosci. Biotechnol. Biochem. 2004;68:2374–2387.10.1271/bbb.68.2374
- Okada M, Sato I, Cho SJ, Dubnau D, Sakagami Y. Chemical synthesis of ComX pheromone and related peptides containing isoprenoidal tryptophan residue. Tetrahedron. 2006;62:8907–8918.10.1016/j.tet.2006.06.074
- Okada M, Yamaguchi H, Sato I, Cho SJ, Dubnau D, Sakagami Y. Structure–activity relationship studies on quorum sensing ComXRO-E-2 pheromone. Bioorg. Med. Chem. Lett. 2007;17:1705–1707.10.1016/j.bmcl.2006.12.070
- Tsuji F, Kobayashi K, Okada M, Yamaguchi H, Ojika M, Sakagami Y. The geranyl-modified tryptophan residue is crucial for ComXRO-E-2 pheromone biological activity. Bioorg. Med. Chem. Lett. 2011;21:4041–4044.10.1016/j.bmcl.2011.04.123
