Abstract
Acetic acid has been shown to promote glycogen replenishment in skeletal muscle during exercise training. In this study, we investigated the effects of acetic acid on endurance capacity and muscle oxidative metabolism in the exercise training using in vivo mice model. In exercised mice, acetic acid induced a significant increase in endurance capacity accompanying a reduction in visceral adipose depots. Serum levels of non-esterified fatty acid and urea nitrogen were significantly lower in acetic acid-fed mice in the exercised mice. Importantly, in the mice, acetic acid significantly increased the muscle expression of key enzymes involved in fatty acid oxidation and glycolytic-to-oxidative fiber-type transformation. Taken together, these findings suggest that acetic acid improves endurance exercise capacity by promoting muscle oxidative properties, in part through the AMPK-mediated fatty acid oxidation and provide an important basis for the application of acetic acid as a major component of novel ergogenic aids.
Graphical abstract
A proposed signaling pathway regulated by acetic acid administration in skeletal muscle of exercise-trained mice.
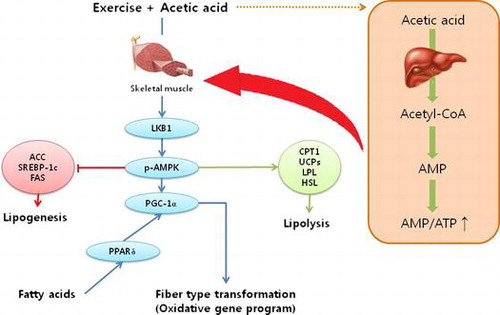
One of the major determinants of endurance capacity is increased fat oxidation, which leads to the sparing of glycogen consumption in muscle and the liver during exercise.Citation1,2) β-oxidation in muscular mitochondria followed by aerobic respiration is required to generate adequate ATP for muscular energy during exerciseCitation3); thus, regulation of energy metabolism by increasing fat oxidation and decreasing carbohydrate consumption enhances endurance capacity during prolonged exercise. AMP-activated protein kinase (AMPK) is a key regulator in mediating the acute and prolonged effects of exercise on fatty acid metabolism in skeletal muscle.Citation4,5) Importantly, AMPK is activated by depletion of ATP and increased AMP, such as diet restriction/hypoglycemia, exercise, and muscular contraction. Activated AMPK is known to stimulate an increase in muscle glucose transportCitation6) and fatty acid oxidationCitation7) and to inhibit ATP-consuming process to restore the energy balance.Citation8,9)
Mouse skeletal muscle fibers are classified into four types based on their contractile and metabolic properties: type I (oxidative); type IIa (mixed oxidative-glycolytic); type IIx (glycolytic); and type IIb (glycolytic) myofibers. These are characterized based on their energy source during exercise or physical activity: type I fibers preferentially metabolize fatty acids as an energy substrate and express the slow isoforms of contractile proteins, whereas type II fibers preferentially use carbohydrates and express the fast isoforms of contractile proteins.Citation10,11) Exercise training stimulates a remodeling program in skeletal muscle; increased expression of genes involved in the oxidative slow-twitch contractile apparatus, mitochondrial respiration, and fatty acid oxidation.Citation12–14) In addition, skeletal muscles rich in oxidative fibers are resistant to muscle wastingCitation15) and may contribute to enhanced endurance performance.
Acetic acid, a major organic acid in vinegar, has been shown to promote fatty acid oxidation and muscle glycogen repletion during exercise in rodents.Citation16,17) Recently, it is reported that administration of acetic acid increased energy expenditure and suppressed body fat mass,Citation18) implying that acetic acid could enhance endurance capacity by reprogramming muscle fiber type and energy utilization in muscle during exercise. Therefore, we investigated the effects of acetic acid on endurance capacity and related physiological metabolism in mice, and this finding may provide an important basis for using acetic acid as a novel source for ergogenic aid.
Methods
Animal and diet
The care and treatment of experimental animals conformed to a protocol approved by the Institutional Animal Care and Use Committee of Korea University (Seoul, Korea). Sixty female C57BL/6 mice (5-week-old) were purchased from Samtako Co. Ltd (Osan, Korea) and were housed in individual cages in a windowless room with a 12 h light–dark cycle. During a 2-week adaptation period, all mice in the exercised group were subjected to running exercise 3 times (velocity of 10 m/min on 0° inclination for 15 min with shock grid OFF, followed by 10 min with shock grid ON) to acclimate to the treadmill. At the end of the adaptation period, all animals were subjected to an endurance test for the measurement of their baselines on the running time to exhaustion. To minimize individual variations on endurance capacity baseline, we selected 12 mice with the closest value to the mean baseline from the original 30 mice. Finally, selected mice were divided into two groups (Ex-control and Ex-acetic acid). Food grade acetic acid was dissolved in normal saline and administered orally to mice at 10 mL/kg body weight once daily for 8 weeks with vehicle. Body weight and food intake were recorded weekly. At the end of study, the mice were fasted for 4 h, ran for 30 min according to the endurance protocol, and then immediately euthanized by Avertin (2,2,2-Tribromoethanol, Sigma-Aldrich). Blood was collected by cardiac puncture and internal organs (visceral fat and skeletal muscle) were also weighed.
Exercise training and endurance protocol
During the experimental period, all animals were trained on a motorized treadmill (YS-03-2; Mirae-ST Corp., Daejeon, Korea) three times a week. Training was performed for 15 min (5 min at 10 m/min, then an increase of 1 m/min every minute for 10 min) on a 10° incline with a shock grid (0.97 mA, 1 Hz) to encourage the mice to run. Endurance capacity was determined every other week by placing animals on an individual treadmill at room temperature. The exercise regimen was started with shock grid ON and 10° inclination at 10 m/min for 5 min, speed was increased by 1 m/min up to 20 m/min (10 min with increase speed), and then held at 20 m/min until exhaustion. Based on previous studies that measured treadmill endurance capacity,Citation19–21) the mice were defined as exhausted if they were willing to sustain on the shock grid five times for more than 2 s or remain on the shock grid for five consecutive seconds. At the moment of exhaustion, the mouse was removed from the treadmill. The total running time until exhaustion was recorded and used as the index of endurance capacity.
Biochemical parameter analysis in serum
The serum was separated from whole blood samples by centrifugation at 2000 × g for 20 min at 4 °C. Serum levels of glucose, urea nitrogen, creatine kinase, triglyceride (TG), total cholesterol, and lactate were measured with an ARCO PC (Biotecnica Instruments SpA, Rome, Italy) using commercial kits as specified by the manufacturer.
Total RNA extraction and determination of mRNA level by quantitative PCR
Soleus muscle tissues were homogenized in 1 mL of Easyblue reagent, and then total RNA was isolated according to the manufacturer’s protocol (iNtRON Biotechnology, Sungnam, Kyunggi, Korea). RNA was quantified by spectroscopy (NanoDrop, Wilmington, NC, USA). Total RNA (2 μg) was reverse transcribed to cDNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster, CA, USA) using random and oligo (dT) primers in a 20 μL reaction according to the manufacturer’s protocol. The resulting cDNA was used in the qPCR along with specific primers for the following target genes: carnitine palmitoyltransferase 1β (CPT1β; NM_013200.1), uncoupling protein 2 (UCP2; NM_062227.2), UCP3 (NM_013167.2), hormone-sensitive lipase (HSL; NM_012859.1), acetyl-CoA carboxylase 2 (ACC2; NM_053922.1), AMP-activated protein kinase (AMPK; NM_023991.1), peroxisome proliferator-activated receptor δ (PPARδ; NM_013141.2), myosin heavy chain I (MHC I; NM_001135158.1), and MHC IIb (NM_019325.1). Each 20 μL reaction contained 100 ng of cDNA, 10× power SYBR Green Mastermix (mBiotech, Hanam, Korea), and forward and reverse primers. All reactions were carried out in an ABI 7500 thermocycler (Applied Biosystems) using the following thermal cycling program: 50 °C for 2 min, 95 °C for 10 min, and then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The results were normalized to β-actin (NM_031144.2) as an internal standard, and the relative quantity of each gene is presented in terms of 2–ΔΔCt, which was calculated using the ΔCt and ΔΔCt values.
Immunoblot analysis
Antibodies were obtained from the following sources: AMPKα and phospho-AMPKα Thr172 (Cell Signaling Technology, Danvers, MA, USA), LKB1, PGC-1α, and β-actin (Santa Cruz Biotechnology, Dallas, TX, USA), UCP (Abcam, Gyeonggi, Korea), MHC I, IIa and IIb (BA-F8, SC-71, and BF-F3, respectively, Developmental Studies Hybridoma Bank, Iowa city, IA). Conventional immunoblotting procedures were used to detect the target proteins. Soleus muscle tissues were collected to extract protein using RIPA buffer (Cell Signaling Technology). Lysates were then cleared by centrifugation at 15,000 × g for 20 min. Total protein concentration was determined by Bradford assay. Equal amounts of protein were separated on 12% SDS/PAGE and the proteins were transferred to polyvinylidene difluoride membranes. The membranes were then blocked for 30 min in a PBS solution containing 5% BSA and 0.1% Tween-20, and then probed with primary antibody overnight in 5% BSA and 0.1% Tween-20 in PBS. After washing, membranes were incubated for 1 h with horseradish peroxidase-linked secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) in PBS solution containing 5% nonfat milk powder and 0.1% Tween-20. Finally, after three 5 min washes in 0.1% PBS/Tween-20, proteins were visualized by ImageQuant LAS 4000 (General Electric, Pittsburgh, PA, USA). Band intensities were quantified with imageJ software (Rasband W S, ImageJ, US National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/).
Statistical analysis
Data were analyzed by one-way or two-way ANOVA using the SAS software for Windows release 9.2 (SAS Institute Inc., Cary, NC, USA) on the W64_VSHOME platform. Two-way ANOVA with repeated measures was performed to assess mean differences between groups for body weight, adipose depot, and serum analysis. One-way ANOVA with repeated measures was performed to assess mean differences between groups for gene expression and endurance capacity over time. The least squares means option using a Tukey–Kramer adjustment was used for multiple comparisons among the experimental groups. Data are shown as the mean ± SE. p values of <0.05 are reported as statistically significant.
Results
Acetic acid prevents diet-induced visceral fat accumulation
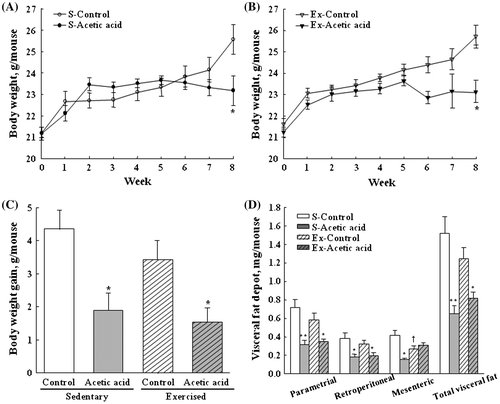
Acetic acid administration significantly reduced weight gain and visceral fat depot in both sedentary and exercised mice compared to the corresponding control groups (p = 0.0011 and p < 0.0001 for overall acetic acid effect). Mesenteric fat depot was significantly decreased in exercised control mice compared to that of sedentary mice, while no significant differences were observed in weight gain and total visceral fat between sedentary and exercised control mice (Fig. ). There were no significant differences in food intake among all treatment groups (data not shown).
Fig. 1. Effects of dietary acetic acid on body weight of sedentary and trained groups (A and B, respectively), body weight gain (C), and visceral fat depot (D) of exercise-trained mice.
Notes: Animals were subjected to the exercise training program for 8 weeks. Visceral fat depot includes epididymal, mesenteric, and retroperitoneal fat. Values represent mean ± SE (n = 6). *Significantly different from respective control (*p < 0.05, **p < 0.01). †S-control vs. Ex-Control (p < 0.05).
Acetic acid promotes endurance capacity by increasing fatty acid oxidation in muscle
The endurance capacity of mice was determined by the maximum running time on the treadmill. The maximum running time was significantly increased in acetic acid group compared to the control group at Week 6 and Week 8 (p < 0.05, Fig. ). Exercise training of control group induced an average 1.4-fold increase in maximum running time of mice at Week 8 compared to their baseline, while acetic acid administration with exercise induced an average 1.8-fold increase compared to their baseline. The serum levels of major metabolic substrates after treadmill running showed that acetic acid administration significantly decreased serum levels of glucose (p = 0.005 for overall acetic acid effect), non-esterified fatty acid (NEFA) (p = 0.028 for overall acetic acid effect), and urea nitrogen (p = 0.010 for overall acetic acid effect) (Table ).
Fig. 2. Effects of dietary acetic acid on endurance running time.
Notes: Endurance capacity was evaluated with treadmill exercise every 2 weeks. Values represent mean ± SE (n = 6). *Significantly different from Ex-Control (p < 0.05).
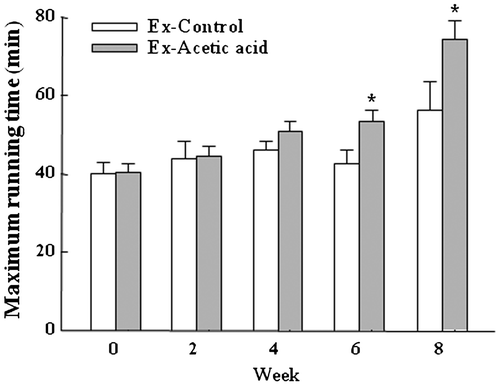
Table 1. Serum parameters.
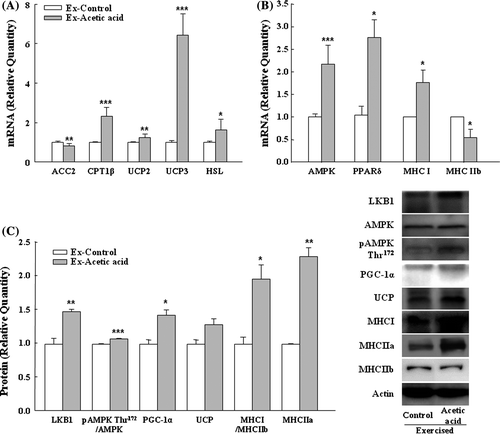
To further explore the impact of acetic acid on fatty acid oxidation in muscle, we analyzed the mRNA expressions of key regulators in muscle oxidative metabolism. Since the serum parameters showed no effect of acetic acid in sedentary group, we focused on the exercised group for further metabolic analyses. Acetic acid significantly up-regulated muscle mRNA expressions of CPT1β, UCP2, UCP3, and HSL, and down-regulated ACC2 mRNA compared to those of the control group (Fig. (A)). In addition, acetic acid administration increased the expressions of proteins involved in regulation of muscle oxidative properties such as LKB1, pAMPK, and PGC-1α (Fig. (C)).
Fig. 3. Effects of dietary acetic acid on selected gene expressions for fat oxidation (A, B), and protein expressions (C) from soleus muscle of exercise-trained mice.
Notes: ACC2, acetyl CoA carboxylase; CPT1β, carnitine palmitoyltransferase 1 beta; UCP2, uncoupling protein 3; UCP3, uncoupling protein 3; HSL, hormone-sensitive lipase; AMPK, AMP-activated protein kinase; pAMPK, phosphorylated AMPK; PPARδ, peroxisome proliferator-activated receptor δ; MHC I, myosin heavy chain I; MHCIIa, myosin heavy chain IIa; MHC IIb, myosin heavy chain IIb; LKB1, Liver kinase B1; PGC-1α, Peroxisome proliferator-activated receptor gamma coactivator-1α. Relative quantities of each gene were presented in terms of 2–ΔΔCt, calculated using the ΔCt and ΔΔCt values. Values represent means ± SE (n = 3). *Significantly different from Ex-Control (*p < 0.05, **p < 0.01, ***p < 0.001).
Acetic acid enhances exercise-mediated responses in the skeletal muscle
AMPK is known to be the main regulator of exercise-mediated responses in the skeletal muscle, and PPARδ, a key enzyme for such muscle fiber transformation, is one of the primary downstream targets of AMPK.Citation22–24) The gene expressions of AMPK and PPARδ were significantly up-regulated by acetic acid, resulting in increased MHCI (oxidative type I-isoform) and MHCIIa (mixed oxidative-glycolytic type IIa-isoform), and decreased MHCIIb (glycolytic type IIb-isoform) expressions in the soleus muscle of mice (Fig. (B)). The twitch speed of type IIa fiber is fast and it contains high glycogen content, but mitochondrial density and oxidative capacity are high. Thus, type IIa fiber is more sensitive to the mode of exercise. Consistent with the changes of MHCI and MHCIIb mRNA levels, their protein ratio was significantly increased by acetic acid treatment in exercised mice (Fig. (C)).
Discussion
There is strong evidence both in vitro and in vivo that acetic acid reduces body fat by increasing hepatic fatty acid oxidation and energy expenditure.Citation18,25,26) Consistently, our current study confirmed that supplementation with acetic acid prevented visceral fat accumulation without change in food intake in exercise-trained mice. Our results also showed that acetic acid administration during treadmill exercise of mice is associated with the enhanced muscle oxidative properties through AMPK-mediated signaling pathway.
It is reported that the beneficial effects of acetic acid on hepatic lipid metabolism are mediated by AMPK.Citation26,27) These effects include the both down-regulation of lipogenic genes and up-regulation of fat oxidative genes in the liver by AMPK signaling pathway. However, there is no evidence that the effects of acetic acid on fat oxidative properties in exercise-trained mice, especially in muscle. We showed that acetic acid suppressed the expression of the lipogenic gene ACC2 and increased the expression of several oxidative genes, including CPT1β, UCP2, UCP3, and HSL, through enhanced AMPK activity in skeletal muscle. In addition to oxidative mRNA expression, we also found the up-regulated protein expression involved in fat oxidation such as LKB1, pAMPK/AMPK, and PGC-1α, indicating that acetic acid controls fuel utilization by enhancing fatty acid oxidation in muscle during exercise. In fact, it was suggested that LKB1-AMPK-PGC-1 signaling is critical to the beneficial effects of training.Citation28) AMPK can be activated by increased AMP-to-ATP ratios and by phosphorylation through upstream AMPK kinases such as LKB1.Citation29,30) Accordingly, LKB1 phosphorylates and thus activates AMPK at site Thr172 of the α-subunit.Citation31,32) Moreover, AMPK plays major role in the regulation of PGC-1α expression.Citation33) Suwa et al.Citation34) reported that the 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR)-induced activation of AMPK in muscle caused up-regulated expression of PGC-1α, and Terada et al.Citation35) reported the increase in mRNA expression of PGC-1α after endurance training.Citation35)
Meanwhile, phosphorylation/inhibition of muscle ACC2 mediated by activation of AMPK is the principal pathway regulating fatty acid oxidation in muscle during exercise.Citation36,37) Phosphorylation of ACC2 suppresses the malonyl-CoA-mediated inhibition of CPT-1, which is a rate-limiting enzyme in mitochondrial fatty acid beta-oxidation.Citation36) The importance of ACC2 in the regulation of muscle lipid metabolism is shown by the increased rates of fatty acid oxidation, energy expenditure, and reduced malonyl-CoA levels in mice followed by disruption of the ACC2 gene.Citation38) Taken together, the present findings strongly suggest that acetic acid could promote exercise-based adaptations in muscle oxidative metabolism.
Acetic acid has been shown to affect hepatic and muscular glycogen metabolism by facilitating glycogen replenishment in rodents with or without exercise.Citation17,39,40) This effect of acetic acid suggests the preferential use of fat (over glucose) as an energy source in the liver and muscle during exercise, resulting in improved endurance performance. Therefore, we measured serum levels of major metabolic substrates after exercise to test the effect of acetic acid on fuel utilization. Serum levels of NEFA, which is one of the major fuels available when glycogen and glucose are used for oxidative metabolism during exercise,Citation41) were significantly lower in the acetic acid group. Consistent with the reduced NEFA levels, animals that were fed acetic acid exhibited reduced serum urea nitrogen levels. When the body cannot obtain energy from carbohydrate and fat sources, proteins are catabolized to a greater degree. Accordingly, urea nitrogen concentration was dramatically increased following sustained exercise, indicating that urea nitrogen is a very sensitive index of endurance ability.Citation42,43) Therefore, our results, along with those of previous studies on glycogen replenishment, suggest that acetic acid could stimulate fatty acid oxidation and reduce carbohydrate and protein catabolism following exercise. However, further studies are required to confirm the effects of acetic acid on endurance performance with respiratory measurements.
Exercise-based adaptations in muscle are linked to increased expression of genes involved in fatty acid oxidation and oxidative fiber-type transformation,Citation13,44,45) and these adaptations may contribute to improved endurance performance.Citation46,47)
In the present study, acetic acid supplementation along with training enhanced exercise-based adaptations in muscle, as reflected by the increased expression of genes involved in energy homeostasis such as LKB1, AMPK, PPARδ, and PGC-1α. Indeed, PPARδ, the primary downstream target of AMPK, is a key regulator of lipid and carbohydrate metabolism in muscle,Citation48) and targeted activation of PPARδ in muscle leads to increased oxidative muscle fibers and enhanced running endurance.Citation11,49) Narkar et al.Citation10) showed that simultaneous treatment with AMPK and PPARδ agonists in mice induced the expression of muscle oxidative genes in a synergistic fashion, suggesting that the interaction between AMPK and PPARδ contributes to skeletal muscle transcriptome reprogramming following exercise. In addition, the MHC isoforms I, IIa, and IIb have been widely used as the primary biomarkers to determine fiber-type composition, and it has been shown that the expression of MHCI and IIb is up-regulated and down-regulated, respectively, in muscle after endurance exercise training.Citation50) Interestingly, muscle-specific deletion of PPARδ decreased MHCI mRNA levels and increased MHCIIb mRNA levels in muscle.Citation51) In addition, increased expression of PGC-1α in muscle caused the fast-to-slow fiber-type transformation accompanied suggesting that PGC-1α solely converts all fibers, but other signals also influence the fiber phenotype such as calcineurin-Mef2 pathway.Citation52) Indeed, PGC-1α is downstream of the calcium/calcineurin/CaM kinase pathway. Although the exact mechanism of PGC-1α on the fiber-type transformation has not been fully elucidated, it is quite obvious that the PGC-1α is one of key factors influencing the fiber-type transformation. Our current results, showing that acetic acid promotes exercise-based alterations in MHC isoform expression through up-regulated mRNA expression of AMPK, PPARδ, and protein expression of pAMPK/AMPK and PGC-1α in muscle, is consistent with these previous findings. There is strong possibility for acetic acid along with exercise having a combinatorial effect on the biological response during exercise.
The present study demonstrated that acetic acid administration promotes the expression of genes and proteins that enhance muscle oxidative properties following sustained exercise training. In addition, the reduced serum levels of NEFA and urea nitrogen indicate increased fatty acid oxidation during exercise in mice given acetic acid. These observations provide an important basis for the application of acetic acid as a major component of novel ergogenic aids and warrant further clinical evaluation.
Author Contributions
JH Pan and JH Kim designed the study and drafted the manuscript. YJ Kim, D-H Shin, S Kim, and M Shin reviewed the manuscript. HM Kim and ES Lee participated in a part of treadmill experiment and animal care. SH Kim and JH Lee consulted about the design of the study and troubleshooting of western blot. All authors reviewed and approved the final manuscript.
Acknowledgments
We thank “Carcinogenesis laboratory” members of Kyungbook National University for technical advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jung K, Kim IH, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J. Ethnopharmacol. 2004;93:75–81.10.1016/j.jep.2004.03.022
- Mancini D, Benaminovitz A, Cordisco ME, Karmally W, Weinberg A. Slowed glycogen utilization enhances exercise endurance in patients with heart failure. J. Am. Coll. Cardiol. 1999;34:1807–1812.10.1016/S0735-1097(99)00413-1
- Fushiki T, Matsumoto K, Inoue K, Kawada T, Sugimoto E. Swimming endurance capacity of mice is increased by chronic consumption of medium-chain triglycerides. J. Nutr. 1995;125:531–539.
- Aschenbach WG, Sakamoto K, Goodyear LJ. 5′ adenosine monophosphate-activated protein kinase, metabolism and exercise. Sports Med. 2004;34:91–103.10.2165/00007256-200434020-00003
- Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem. Sci. 2004;29:18–24.
- Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2001;280:E677–84.
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am. J. Physiol. 1996;270:E299–304.
- Bergeron R, Russell RR 3rd, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am. J. Physiol. 1999;276:E938–44.
- Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell. 2001;7:1085–1094.
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou YH, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPAR delta agonists are exercise mimetics. Cell. 2008;134:405–415.
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPAR delta. PLoS Biol. 2004;2:1532–1539.
- Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19:1498–1500.
- Schmitt B, Fluck M, Decombaz J, Kreis R, Boesch C, Wittwer M, Graber F, Vogt M, Howald H, Hoppeler H. Transcriptional adaptations of lipid metabolism in tibialis anterior muscle of endurance-trained athletes. Physiol. Genomics. 2003;15:148–157.
- Yoshioka M, Tanaka H, Shono N, Snyder EE, Shindo M, St-Amand J. Serial analysis of gene expression in the skeletal muscle of endurance athletes compared to sedentary men. FASEB J. 2003;17:1812–1819.
- Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, Hesselink MK. Skeletal muscle wasting and contractile performance in septic rats. Muscle Nerve. 2005;31:339–348.10.1002/(ISSN)1097-4598
- Fushimi T, Tayama K, Fukaya M, Kitakoshi K, Nakai N, Tsukamoto Y, Sato Y. The efficacy of acetic acid for glycogen repletion in rat skeletal muscle after exercise. Int. J. Sports Med. 2002;23:218–222.
- Nakao C, Yamada E, Fukaya M, Tayama K, Tsukamoto Y, Sato Y. Effect of acetate on glycogen replenishment in liver and skeletal muscles after exhaustive swimming in rats. Scand. J. Med. Sci. Sports. 2001;11:33–37.10.1034/j.1600-0838.2001.011001033.x
- Hattori M, Kondo T, Kishi M, Yamagami K. A single oral administration of acetic acid increased energy expenditure in C57BL/6J mice. Biosci. Biotechnol., Biochem. 2010;74:2158–2159.10.1271/bbb.100486
- Fueger PT, Shearer J, Krueger TM, Posey KA, Bracy DP, Heikkinen S, Laakso M, Rottman JN, Wasserman DH. Hexokinase II protein content is a determinant of exercise endurance capacity in the mouse. J. Physiol. London. 2005;566:533–541.
- Koch LG, Meredith TA, Fraker TD, Metting PJ, Britton SL. Heritability of treadmill running endurance in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;275:R1455–R1460.
- Lightfoot JT, Turner MJ, Knab AK, Jedlicka AE, Oshimura T, Marzec J, Gladwell W, Leamy LJ, Kleeberger SR. Quantitative trait loci associated with maximal exercise endurance in mice. J. Appl. Physiol. 2007;103:105–110.10.1152/japplphysiol.01328.2006
- Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell. Mol. Life Sci. 2010;67:3407–3423.10.1007/s00018-010-0454-z
- Matsakas A, Narkar VA. Endurance exercise mimetics in skeletal muscle. Curr. Sports Med. Rep. 2010;9:227–232.10.1249/JSR.0b013e3181e93938
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415.
- Kim JY, Ok EYJ, Kim YJ, Choi KS, Kwon O. Oxidation of fatty acid may be enhanced by a combination of pomegranate fruit phytochemicals and acetic acid in HepG2 cells. Nutr. Res. Pract. 2013;7:153–159.10.4162/nrp.2013.7.3.153
- Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food. Chem. 2009;57:5982–5986.10.1021/jf900470c
- Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem. Biophys. Res. Commun. 2006;344:597–604.10.1016/j.bbrc.2006.03.176
- Sriwijitkamol A, Ivy JL, Christ-Roberts C, DeFronzo RA, Mandarino LJ, Musi N. LKB1-AMPK signaling in muscle from obese insulin-resistant Zucker rats and effects of training. Am. J. Physiol. Endocrinol. Metab. 2006; 290: E925–E932.
- Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur. J. Biochem. 1997;246:259–273.10.1111/ejb.1997.246.issue-2
- Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP activated protein kinase. Trends Biochem. Sci. 1999;24:22–25.
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335.10.1073/pnas.0308061100
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LGD, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008.
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90.
- Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 2003;95:960–968.10.1152/japplphysiol.00349.2003
- Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem. Biophys. Res. Commun. 2002;296:350–354.10.1016/S0006-291X(02)00881-1
- Jorgensen SB, Richter EA, Wojtaszewski JFP. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. J. Physiol. London. 2006;574:17–31.
- Ruderman NB, Park H, Kaushik VK, Dean D, Constant S, Prentki M, Saha AK. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol. Scand. 2003;178:435–442.
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KAH, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616.10.1126/science.1056843
- Fushimi T, Sato Y. Effect of acetic acid feeding on the circadian changes in glycogen and metabolites of glucose and lipid in liver and skeletal muscle of rats. Br. J. Nutr. 2005;94:714–719.10.1079/BJN20051545
- Fushimi T, Tayama K, Fukaya M, Kitakoshi K, Nakai N, Tsukamoto Y, Sato Y. Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. J. Nutr. 2001;131:1973–1977.
- Fernandez C, Hansson O, Nevsten P, Holm C, Klint C. Hormone-sensitive lipase is necessary for normal mobilization of lipids during submaximal exercise. Am. J. Physiol. Endocrinol. Metab. 2008;295:E179–E186.
- Kim JH, Park HG, Pan JH, Kim SH, Yoon HG, Bae GS, Lee H, Eom SH, Kim YJ. Dietary conjugated linoleic acid increases endurance capacity of mice during treadmill exercise. J. Med. Food. 2010;13:1057–1060.10.1089/jmf.2009.1358
- Wang L, Zhang HL, Lu R, Zhou YJ, Ma R, Lv JQ, Li XL, Chen LJ, Yao Z. The decapeptide CMS001 enhances swimming endurance in mice. Peptides. 2008;29:1176–1182.10.1016/j.peptides.2008.03.004
- Mahoney DJ, Tarnopolsky MA. Understanding skeletal muscle adaptation to exercise training in humans: contributions from microarray studies. Phys. Med. Rehabil. Clin. N. Am. 2005;16:859–873.10.1016/j.pmr.2005.08.018
- Siu PM, Donley DA, Bryner RW, Alway SE. Myogenin and oxidative enzyme gene expression levels are elevated in rat soleus muscles after endurance training. J. Appl. Physiol. 2004;97:277–285.
- Aagaard P, Andersen JL, Bennekou M, Larsson B, Olesen JL, Crameri R, Magnusson SP, Kjaer M. Effects of resistance training on endurance capacity and muscle fiber composition in young top-level cyclists. Scand. J. Med. Sci. Sports. 2011;21:E298–E307.10.1111/j.1600-0838.2010.01283.x
- Diaz-Herrera P, Torres A, Morcuende JA, Garcia-Castellano JM, Calbet JAL, Sarrat R. Effect of endurance running on cardiac and skeletal muscle in rats-Histol. Histopathol. 2001;16:29–35.
- Kramer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A. Role of AMP kinase and PPAR delta in the regulation of lipid and glucose metabolism in human skeletal muscle. J. Biol. Chem. 2007;282:19313–19320.10.1074/jbc.M702329200
- Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17:2299–2301.
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Coenen-Schimke JM, Rys P, Nair KS. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J. Appl. Physiol. 2005;99:95–102.10.1152/japplphysiol.00129.2005
- Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1α expression is controlled in skeletal muscles by PPARβ, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006;4:407–414.10.1016/j.cmet.2006.10.003
- Lin J, Wu H, Tarr PT, Zhang CY, Wu ZD, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801.10.1038/nature00904
