Abstract
Here, we investigated the protective effect of cacao polyphenol extract (CPE) on carbon tetrachloride (CCl4)-induced hepato-renal oxidative stress in rats. Rats were administered CPE for 7 days and then received intraperitoneal injection of CCl4. Two hours after injection, we found that CCl4 treatment significantly increased biochemical injury markers, lipid peroxides (phosphatidylcholine hydroperoxide (PCOOH) and malondialdehyde (MDA)) and decreased glutathione peroxidase activity in kidney rather than liver, suggesting that kidney is more vulnerable to oxidative stress under the present experimental conditions. CPE supplementation significantly reduced these changes, indicating that this compound has antioxidant properties against CCl4-induced oxidative stress. An inhibitory effect of CPE on CCl4-induced CYP2E1 mRNA degradation may provide an explanation for CPE antioxidant property. Together, these results provide quantitative evidence of the in vivo antioxidant properties of CPE, especially in terms of PCOOH and MDA levels in the kidneys of CCl4-treated rats.
Graphical abstract
Rats were supplemented with CPE prior to CCl4 treatment. CCl4 significantly increased PCOOH and MDA in kidney rather than liver, and CPE significantly ameliorated these damages.
Medical and nutritional experts have recently given serious attention to the antioxidant properties of food constituents due to the ability of oxidative stress-mediated peroxidation of biological molecules (e.g. lipids) to induce a variety of pathological conditions such as atherogenesis, aging, and liver diseases.Citation1) Although many in vitro studies have investigated the antioxidant properties of food constituents, there is still insufficient data for the biological functions of dietary antioxidants in vivo, with the exception of a few major antioxidants (e.g. tocopherols, ascorbic acid, certain carotenoids, and catechin).Citation2–4) Since the bioavailability of food constituents is limited by their digestibility and metabolic fate, oral administration trials are favored to evaluate their biological activities in vivo.
For such administration trials, researchers frequently use a carbon tetrachloride (CCl4) experimental model, which mimics oxidative injury in many physiological situations,Citation5) and allows the evaluation of the preventive efficacy of dietary antioxidants against CCl4-induced oxidative stress using a colorimetric thiobarbituric acid reactive substance (TBARS) assay. However, this assay has methodological problems, including the generation of artifactual TBARS depending on the assay conditions.Citation6,7) Thus, performing CCl4 experiments with a TBARS assay does not always provide clear information on the biological functions of dietary antioxidants in vivo.
Target organs in the CCl4 model are another important issue. While most studies focused on CCl4-induced hepatotoxicity, CCl4 inhalation or ingestion is also known to cause renal damage in humans.Citation8) However, there is still insufficient data about the renal effects of antioxidants present in food using the CCl4 model.
Among commonly consumed antioxidative foods, cacao has recently received attention due to its significant polyphenol content, especially procyanidin.Citation9) However, there has been only one report concerning the antioxidant properties of cacao polyphenol against liver damage in CCl4-treated rats,Citation10) and there is still insufficient data for kidney.
Therefore, we supplemented the diets of CCl4-treated rats with cacao polyphenol extract (CPE) to assess its effects on liver and kidney oxidative status using reliable chromatographic methods for lipid peroxidation products. Levels of phosphatidylcholine hydroperoxide (PCOOH), a primary oxidation product of phosphatidylcholine, were determined by liquid chromatography (LC) with chemiluminescence (CL) detection. Meanwhile, levels of malondialdehyde (MDA), a secondary lipid oxidation product, were measured by LC with fluorescence (FL) detection, but not with a classic colorimetric TBARS assay. The oxidative/antioxidative profile, which included measurements of protein carbonyl, 8-hydroxydeoxyguanosine (8-OHdG), glutathione peroxidase (GPx) activity, α-tocopherol, and CYP2E1 gene expression was also evaluated. Although the antioxidant potential of CPE in vivo requires further exploration, here we provide quantitative evidence of the antioxidant properties of CPE, and in particular, its effect on PCOOH and MDA levels in the kidneys of CCl4-treated rats.
Materials and methods
Chemicals
CPE was obtained from Barry Callebaut Belgium NV (Lebeke–Wieze, Belgium) and was composed of 60.5% polyphenol (53.1% procyanidin and 7.4% other phenolics) with the remaining 39.0% being composed of 12.0% carbohydrate, 9.5% protein, 8.0% alkaloid, 5.5% fiber, 3.0% ash, and 1.0% fat (wt/wt). All other reagents used were of analytical grade.
Animal experiments
Male F344 rats (12 weeks of age) were obtained from CLEA (Tokyo, Japan), and housed in cages kept at 23 °C with a 12 h light:dark cycle. After rats were acclimated with CE-2 rodent chow (CLEA) and water for 1 week prior to initiating the study, rats were divided into four groups of five rats each.
CPE powder was mixed with water at a ratio of 1:3 (g powder/mL water). CCl4 was suspended in canola oil at a ratio of 1:1 by volume. Each rat received either CPE (500 mg/kg body weight) or vehicle water by oral gavage once a day for seven successive days. On day 7, the rats were fasted for 24 h, and then given intraperitoneal (i.p.) injections of CCl4 (1200 mg/kg body weight) or vehicle canola oil. Two hours after the injection, the rats were anesthetized with isoflurane. Rats were then sacrificed, and blood was collected and centrifuged at 1000 × g for 10 min at 4 °C to prepare plasma. Livers and kidneys were removed and weighed. These protocols were reviewed by the Committee on the Ethics of Animal Experiments of Tohoku University (2013-Noudou-060), and this experiment was carried out in accordance with the Animal Experiment Guidelines of Tohoku University.
Biochemical parameters
Plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), uric acid (UA), total lipids (TL), triglyceride (TG), cholesterol (Cho), and phospholipid (PL) levels were measured at the Nagahama Life-science Laboratory of Oriental East (Shiga, Japan).
Liver and kidney proteins were estimated by the Lowry method.Citation11) TL was extracted from the tissues by the Folch methodCitation12) with 0.002% butylated hydroxytoluene (BHT), while TG and Cho were measured with commercial kits (Wako, Osaka, Japan). PL was determined by a modified Bartlett method.Citation13)
Analysis of lipid peroxidation products
For determination of PCOOH, liver and kidney TL were loaded onto Sep-Pak aminopropyl cartridges pre-equilibrated with methanol and chloroform/2-propanol (2:1), and the PL fraction including PCOOH was eluted with methanol. PL was concentrated to dryness, resuspended in methanol, and subjected to LC-CL.Citation14) For LC-CL, a Finepak SIL NH2–5 column (4.6 × 250 mm; Japan Spectroscopic, Tokyo, Japan) was used. The eluent was 2-propanol/methanol/water (135:45:20, v/v/v), and the flow rate was 1 mL/min. A CLD-100 detector (Tohoku Electronic Industries, Miyagi, Japan) was used for post-column CL detection. A mixture of luminol and cytochrome c in 50 mM borate buffer (pH 10.0) was prepared as a hydroperoxide-specific post-column CL reagent, with a flow rate of 1.2 mL/min.
MDA was evaluated by the LC method.Citation15) Briefly, sodium dodecyl sulfate, TBA, and BHT were added to the liver and kidney homogenates and mixed. After the mixture was heated in boiling water, TBA-MDA adducts were extracted with a butanol/pyridine solution and the extract was subjected to LC-FL using a COSMOSIL 5C18-MS-II column (4.6 × 250 mm; Nacalai Tesque, Kyoto, Japan). The eluent was water/methanol (70:30), and the flow rate was 1 mL/min. TBA-MDA adducts were detected with a RF-10AXL fluorescence detector (Shimadzu, Kyoto, Japan) at excitation and emission wavelengths of 515 and 553 nm, respectively.
Other oxidative/antioxidative markers
Protein carbonyl was measured using the Carbonyl Protein Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), which is based on the derivatization of carbonyl with 2,4-dinitrophenylhydrazine. 8-OHdG was evaluated by an immunohistochemical method using anti-8-OHdG antibody (Nikken Foods, Shizuoka, Japan). GPx activity was assessed with a commercially available kit (BioVision, Milpitas, CA, USA), while α-tocopherol levels were determined by LC-FL.Citation16) Previous studies reported that hepato-renal α-tocopherol was positively correlated with TG.Citation17,18) Hence, in this study, hepato-renal α-tocopherol levels were expressed as the ratio of α-tocopherol to TG.
Gene expression analysis
CYP2E1 gene expression was evaluated by real-time quantitative reverse transcription-PCR (RT-PCR). Total RNA was extracted from livers and kidneys using a commercial kit (Qiagen, Valencia, CA, USA), and cDNA was synthesized using a PrimeScript RT reagent kit (Takara, Tokyo, Japan). PCR amplification was performed with a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, New South Wales, Australia) using SYBR Premix Ex Taq™ II (Takara) and gene-specific primers for CYP2E1 and GAPDH (Supplementary Table 1). PCR conditions were 95 °C for 30 s for 1 cycle and 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. The GAPDH content in each sample was used to normalize the results.
Statistical analysis
Data were analyzed with two-way analysis of variance (ANOVA) with CCl4 treatment (Non-CCl4 or CCl4) and CPE supplementation (water or CPE) as independent factors. In case of singificant interaction, Tukey post hoc tests were performed. ANOVA and Tukey post hoc tests were performed using Ekuseru-Toukei 2010 (Social Survey Research Information, Tokyo, Japan). Data were expressed as the mean ± SEM. Differences were considered significant at p < 0.05.
Results and discussion
Animal experimental design
Scientists at the National Institute of Environmental Health Sciences (NIEHS) have investigated in detail the time- and dose-dependent effects of CCl4 i.p. injection on a series of oxidative stress markers in rat plasma.Citation19,20,21) The largest induction of plasma stress markers (e.g. lipid peroxidation products) was seen 2 h after injection of 1200 mg/kg CCl4, after which the levels of lipid peroxidation products tended to decrease with time. Given that there are no available data concerning the oxidative status in liver and kidney following CCl4 injection, we performed a preliminary study using these reported conditions and found that CCl4 injection increased lipid peroxidation (e.g. PCOOH formation) in rat plasma and tissues, especially in the kidney. Based on these findings and those reported by NIEHS, here we used CCl4 treatment (1200 mg/kg, 2 h) as a model of oxidative stress in rat liver and kidney injury, and estimated the preventive efficiency of the dietary antioxidant CPE.
As mentioned in the Introduction, Kilicgun and AltinerCitation10) evaluated the antioxidant potential of cacao (cacao solids) using a CCl4 model, and found that cacao could decrease CCl4-induced lipid and protein oxidation in liver. However, given the crude nature of cacao solids, describing the contribution of separate cacao constituents (e.g. polyphenol) to oxidative stress is difficult. In the present study, we used CPE, a highly concentrated cacao polyphenol (e.g. procyanidin), to clarify whether cacao polyphenols are indeed bioactive. The CPE dose was set at 500 mg (equivalent to 303 mg polyphenol)/kg body weight for 7 days, based on earlier, similar animal studies.Citation22–24) We first confirmed that under these CPE supplementation conditions, no toxicity was observed in rats (our preliminary data). The rats were then divided into 4 groups (Water + Non-CCl4, Water + CCl4, CPE + Non-CCl4, and CPE + CCl4). Rats were supplemented with or without CPE (500 mg/kg body weight) for 7 days, and then given an i.p. injection of CCl4 (1200 mg/kg body weight) or vehicle canola oil. A comparison of the Water + Non-CCl4 and Water + CCl4 groups showed evidence of CCl4-induced injuries and oxidative stress in the liver and kidney. Moreover, amelioration of CCl4-induced injuries and oxidative stress in liver and kidney occurred in the CPE + CCl4 group compared with the Water + CCl4 group.
CCl4-induced tissue injury and its amelioration by CPE
Leakage of liver enzymes into the bloodstream indicates hepatic damage.Citation25) In this study, i.p. injection of CCl4 in rats induced higher concentrations of AST, ALT, and LDH in plasma (Table ), suggesting that liver injury had occurred. Other hallmarks of liver damage (e.g. lowered levels of TL, TG, Cho, and PL in plasma) were also seen in the CCl4 group, probably due to decreased secretion of lipoproteins induced by CCl4.Citation26) In addition to liver injury, renal damage was confirmed by increased UA in the plasma of the Water + CCl4 group, as was observed in an earlier report.Citation27) Because some pathological changes, including high level of TG and low levels of PL and protein,Citation25,28) were seen in the kidneys rather than the livers of the CCl4 group (Table ), renal damage appears to be more prominent than liver damage under these experimental conditions.
Table 1. Body weights and plasma parameters of rat administered CPE (500 mg/kg body weight) for 7 days and injected i.p. with CCl4 (1200 mg/kg body weight) 2 h before sacrifice.
Table 2. Hepato-renal parameters and antioxidants in rat administered CPE (500 mg/kg body weight) for 7 days and injected i.p. with CCl4 (1200 mg/kg body weight) 2 h before sacrifice.
CPE administration to rats significantly decreased CCl4-induced elevation of plasma AST, ALT, and LDH, although CPE did not affect CCl4-induced reduction of plasma TL, TG, and PL (Table ). Additionally, CPE moderated the increase in plasma UA caused by CCl4 (Table ), and attenuated the pathological changes in renal PL and protein induced by CCl4 (Table ). Overall, these results demonstrate the hepato-renal protective properties of CPE against CCl4-induced damage. To the best of our knowledge, this is the first report to show a protective effect of cacao polyphenol on kidney injury caused by CCl4. To determine whether the protective actions of CPE are due to antioxidant properties, we next measured the hepato-renal oxidative status.
CCl4-induced oxidative stress and its amelioration by CPE
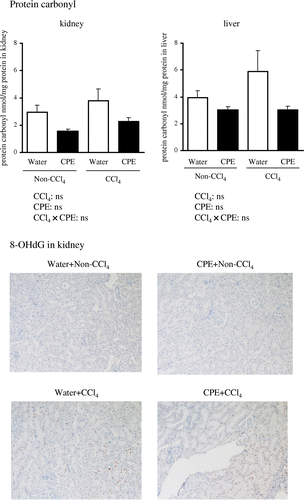
The classic colorimetric TBARS assay has been criticized for a lack of specificity because TBA can react with several compounds other than MDA to produce a red color, which can interfere with MDA measurements.Citation29) Therefore, we used LC-CL and LC-FL methods to determine levels of PCOOH and MDA, respectively, with high sensitivity and selectivity.Citation15,30) As shown in Fig. , renal levels of PCOOH and MDA were significantly increased in the Water + CCl4 group compared with the Water + Non-CCl4 group, while there were no significant changes in the liver. These results suggest that CCl4-induced renal injury is associated with lipid peroxidation. In contrast, there were no significant differences in the content of protein carbonyls (an indicator of oxidative protein damage) (Fig. ), or in 8-OHdG (a marker of oxidative DNA damage) levels between the Water + CCl4 and Water + Non-CCl4 groups in the kidney (Fig. ) and liver (data not shown). Thus, an increase in lipid peroxidation, rather than in oxidized protein and DNA, may be responsible for CCl4-induced tissue injury.
Fig. 1. Hepato-renal PCOOH and MDA levels in rats administered CPE (500 mg/kg body weight) for 7 days and given i.p. injection of CCl4 (1200 mg/kg body weight) 2 h before sacrifice.
Notes: PCOOH and MDA levels were measured with LC techniques as described in Materials and Methods. Data points represent the mean ± SEM. Asterisks denote statistical differences (p < 0.05, two-way ANOVA). Different letters denote statistical difference (p < 0.05, Tukey test).
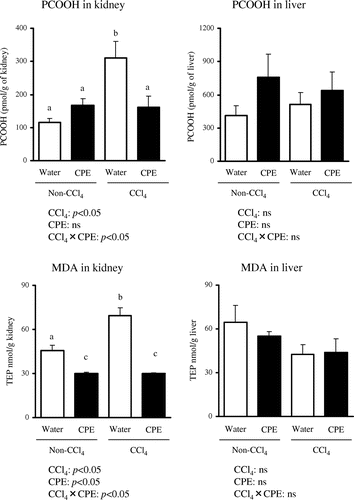
Fig. 2. Hepato-renal protein carbonyl content and renal immunohistochemical staining for 8-OHdG in rats administered CPE (500 mg/kg body weight) for 7 days and i.p. injection of CCl4 (1200 mg/kg body weight) 2 h before sacrifice.
Notes: Protein carbonyl levels were measured using a commercial kit (Cayman Chemical). 8-OHdG was determined by immunoassay. Data points represent the mean ± SEM.
We next measured GPx activity and α-tocopherol concentration in the liver and kidney. As shown in Table , the liver tissue had an approximately twofold higher GPx activity than kidneys in the Water + Non-CCl4 group, suggesting that the antioxidative capacity of the liver is greater than the kidney. Additionally, hepatic GPx activity was higher than that in kidneys from the Water + CCl4 group. This result may be related to the low oxidative stress levels in the liver even after CCl4 exposure. In accordance with these phenomena, hepatic α-tocopherol levels per TG were not reduced, while there was a significant decrease in renal α-tocopherol levels per TG after CCl4 injection. These results of GPx activity and α-tocopherol per TG are consistent with those of Ping et al.,Citation31) who reported that kidney is more vulnerable than liver to oxidative stress caused by i.p. injection of iron oxide. However, when hepato-renal α-tocopherol concentrations were calculated relative to tissue weight, the levels were significantly increased in Water + CCl4 group. Further work is therefore needed to evaluate hepato-renal α-tocopherol profile in CCl4 model.
As oxidative stress was observed mainly in the kidney, we evaluated whether the protective effects of CPE on CCl4-induced damage (Tables and ) were due to the antioxidant properties of CPE. The accumulation of PCOOH and MDA in the kidney was significantly suppressed in the CPE + CCl4 group compared with the Water + CCl4 group (Fig. ). Furthermore, CPE significantly restored hepato-renal GPx activities as well as the renal α-tocopherol concentration (Table ). Thus, the protective effect of CPE on CCl4-induced renal injury may be due to its antioxidant action. Meanwhile, questions have been raised about the antioxidant action mechanism (e.g. radical scavenging) of CPE.Citation32) Baba et al.Citation33) reported that the oral absorption rate of cacao polyphenol, calculated from urinary excretion data in rat study, is about 30–50%, and metabolized forms are mainly present in the body. Previous research reported that cacao polyphenol metabolites showed low antioxidant activity compared to intact cacao polyphenol, as they had lost the moiety responsible for radical scavenging activity.Citation9) Hence, to study the possible mechanism of CPE antioxidant properties against CCl4-induced oxidative stress, we measured the gene expression of CYP2E1, which in a CCl4 model is known to be responsible for generating free radicalsCitation34) that are thought to degrade CYP2E1 mRNA.Citation35) As shown in Table , CPE was effective in preventing CCl4-induced CYP2E1 mRNA degradation, which is in agreement with the previous report showing that cacao polyphenol metabolites acted as potent inhibitors of CYP2E1 activity.Citation36) The inhibitory effects of CPE on CYP2E1 activity may provide an explanation for CPE antioxidant properties against CCl4-induced oxidative stress beyond its capacity to scavenge CCl4-induced radicals directly, although further work is required to establish the causal relationship between CPE and PCOOH, MDA, GPx, and CYP2E1 activity.
Table 3. Gene expression of CYP2E1 of rat administered CPE (500 mg/kg body weight) for 7 days and injected i.p. with CCl4 (1200 mg/kg body weight) 2 h before sacrifice.
In conclusion, the present study was designed to yield quantitative evidence of the antioxidant properties of CPE against CCl4-induced oxidative stress. The lipid peroxidation products of PCOOH and MDA produced by CCl4 were more notable in the kidney than in the liver, and PCOOH and MDA accumulation in the kidney could be prevented by CPE treatment, which might be due to the reduction of CCl4-induced radicals by the suppression of CYP2E1 activity. Further study is needed to clarify the mechanism of the antioxidant activities of CPE.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1039481.
Author contribution
K.N., F.K., and Teruo.M. designed research; K.S. and Taiki.M. performed research; T.Y and M.K. discussed data; K.S. wrote the paper.
Additional information
Funding
References
- Dryden GW Jr, Deaciuc I, Arteel G, McClain CJ. Clinical implications of oxidative stress and antioxidant therapy. Curr. Gastroenterol. Rep. 2005;7:308–316.10.1007/s11894-005-0024-y
- Huang HY, Appel LJ, Croft KD, Miller ER III, Mori TA, Puddey IB. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: results of a randomized controlled trial. Am. J. Clin. Nutr. 2002;76:549–555.
- Nakagawa K, Kiko T, Hatade K, Sookwong P, Arai H, Miyazawa T. Antioxidant effect of lutein towards phospholipid hydroperoxidation in human erythrocytes. Br. J. Nutr. 2009;102:1280–1284.10.1017/S0007114509990316
- Nakagawa K, Ninomiya M, Okubo T, Aoi N, Juneja LR, Kim M, Yamanaka K, Miyazawa T. Tea catechin supplementation increases antioxidant capacity and prevents phospholipid hydroperoxidation in plasma of humans. J. Agric. Food Chem. 1999;47:3967–3973.10.1021/jf981195l
- McGregor D, Lang M. Carbon tetrachloride: genetic effects and other modes of action. Mutat. Res. 1996;366:181–195.
- Rauchová H, Vokurková M, Koudelová J. Hypoxia-induced lipid peroxidation in the brain during postnatal ontogenesis. Physiol. Res. 2012;61:89–101.
- Garcia YJ, Rodríguez-Malaver AJ, Peñaloza N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J. Neurosci. Methods. 2005;144:127–135.10.1016/j.jneumeth.2004.10.018
- Ruprah M, Mant TG, Flanagan RJ. Acute carbon tetrachloride poisoning in 19 patients: implications for diagnosis and treatment. Lancet. 1985;325:1027–1029.10.1016/S0140-6736(85)91624-1
- Jalil AM, Ismail A. Polyphenols in cocoa and cocoa products: is there a link between antioxidant properties and health? Molecules. 2008;13:2190–2219.10.3390/molecules13092190
- Kilicgun H, Altiner D. The antioxidant activity of cocoa. Phcog. Mag. 2009;5:298–300.10.4103/0973-1296.58148
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275.
- Folch J, Lees M, Sloane S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509.
- Barenholz Y, Amselem S. Liposome technology: liposome preparation and related techniques. In: Gregoriadis G, editor. Liposome Technology, Vol. 1. Boca Raton, FL: CRC Press; 1993. p. 527–616.
- Miyazawa T, Suzuki T, Fujimoto K, Yasuda K. Chemiluminescent simultaneous determination of phosphatidylcholine hydroperoxide and phosphatidylethanolamine hydroperoxide in the liver and brain of the rat. J. Lipid Res. 1992;33:1051–1059.
- Khoschsorur G, Winklhofer-Roob B, Rabl H, Auer T, Peng Z, Schaur R. Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromatographia. 2000;52:181–184.10.1007/BF02490453
- Schäffer MW, Roy SS, Mukherjee S, Nohr D, Wolter M, Biesalski HK, Ong DE, Das SK. Qualitative and quantitative analysis of retinol, retinyl esters, tocopherols and selected carotenoids out of various internal organs from different species by HPLC. Anal. Methods. 2010;2:1320–1332.10.1039/c0ay00288g
- Nagita A, Ando M. Assessment of hepatic vitamin E status in adult patients with liver disease. Hepatology. 1997;26:392–397.10.1002/(ISSN)1527-3350
- Galli F, Varga Z, Balla J, Ferraro B, Canestrari F, Floridi A, Kakuk G, Buoncristiani U. Vitamin E, lipid profile, and peroxidation in hemodialysis patients. Kidney Int. Suppl. 2001;59:148–154.10.1046/j.1523-1755.2001.07846.x
- Kadiiska MB, Gladen BC, Baird DD, Dikalova AE, Sohal RS, Hatch GE, Jones DP, Mason RP, Barrett JC. Biomarkers of oxidative stress study: are plasma antioxidants markers of CCl4 poisoning? Free Radical Biol. Med. 2000;28:838–845.10.1016/S0891-5849(00)00198-2
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, FitzGerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ II, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radical Biol. Med. 2005;38:698–710.10.1016/j.freeradbiomed.2004.09.017
- Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, Fitzgerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ II, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP. Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radical Biol. Med. 2005;38:711–718.
- Kalpana P, Alka M. Hepatoprotective activity of Mentha arvensis Linn. leaves against CCl4 induced liver damage in rats. Asian Pac. J. Trop. Dis. 2012;2:223–226.
- Gnanaprakash K, Madhusudhana C, Ramkanth S, Alagusundaram M, Tiruvengadarajan V, Angala P, Mohamed S. Aqueous extract of Flacourtia indica prevents carbon tetrachloride induced hepatotoxicity in rat. Int. J. Biol. life Sci. 2010;6:51–55.
- Singhal KG, Gupta GD. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pac. J. Trop. Med. 2012;5:677–685.10.1016/S1995-7645(12)60106-0
- Al-Yahya M, Mothana R, Al-Said M, Al-Dosari M, Al-Musayeib N, Al-Sohaibani M, Parvez MK, Rafatullah S. Attenuation of CCl4-induced oxidative stress and hepatonephrotoxicity by saudi sidr honey in rats. Evid. Based Complement. Alternat. Med. Forthcoming.
- Boll M, Weber LW, Becker E, Stampfl A. Hepatocyte damage induced by carbon tetrachloride: inhibited lipoprotein secretion and changed lipoprotein composition. Z Naturforsch. C. 2001;56:283–290.
- Manno M, Rezzadore M, Grossi M, Sbrana C. Potentiation of occupational carbon tetrachloride toxicity by ethanol abuse. Hum. Exp. Toxicol. 1996;15:294–300.10.1177/096032719601500404
- Kamalakkannan N, Rukkumani R, Viswanathan P, Rajasekharan K, Menon VP. Effect of curcumin and its analogue on lipids in carbon tetrachloride–induced hepatotoxicity: a comparative study. Pharm. Biol. 2005;43:460–466.10.1080/13880200590963880
- Knight JA, Pieper RK, McClellan L. Specificity of the thiobarbituric acid reaction: its use in studies of lipid peroxidation. Clin. Chem. 1988;34:2433–2438.
- Miyazawa T, Suzuki T, Fujimoto K, Kaneda T. Phospholipid hydroperoxide accumulation in liver of rats intoxicated with carbon tetrachloride and its inhibition by dietary alpha-tocopherol. J. Biochem. 1990;107:689–693.
- Ping M, Qing L, Jiaoe C, Yaping G, Juan D, Shumao D, Zhuge X, Xu Y. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012;7:4809–4818.
- Bors W, Michel C, Stettmaier K. Antioxidant effects of flavonoids. BioFactors. 1997;6:399–402.10.1002/biof.v6:4
- Baba S, Osakabe N, Natsume M, Muto Y, Takizawa T, Terao J. Absorption and urinary excretion of (−)-epicatechin after administration of different levels of cocoa powder or (−)-epicatechin in rats. J. Agric. Food Chem. 2001;49:6050–6056.10.1021/jf010965h
- Knight JA, Cheung AK, Pieper RK, Servilla K. Increased urinary lipoperoxide levels in renal transplant patients. Ann. Clin. Lab. Sci. 1989;19:238–241.
- Knockaert L, Berson A, Ribault C, Prost PE, Fautrel A, Pajaud J, Lepage S, Lucas-Clerc C, Bégué JM, Fromenty B, Robin MA. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver. Lab. Invest. 2012;92:396–410.10.1038/labinvest.2011.193
- Cheng J, Chen C, Kristopher KW, Manna SK, Scerba M, Friedman FK, Luecke H, Idle JR, Gonzalez FJ. Identification of 2-piperidone as a biomarker of CYP2E1 activity through metabolomic phenotyping. Toxicol. Sci. 2013;135:37–47.10.1093/toxsci/kft143
