Abstract
The bitter acids in hops (Humulus lupulus L.) and beer, such as α-, β-, and iso-α-acids, are known to affect beer quality and display various physiological effects. However, these compounds readily oxidize, and the effect of the oxides on the properties of beer or their potential health benefits are not well understood. In this study, we developed a simple preparative method for the bitter acid oxide fraction derived from hops and designated the constituents as matured hop bitter acids (MHBA). HPLC-PDA-ESI/HRMS and MS2 revealed that MHBA are primarily composed of α-acid-derived oxides, which possess a common β-tricarbonyl moiety in their structures similar to α-, β-, and iso-α-acids. We also developed a quantitative analytical method of whole MHBA by HPLC, which showed high precision and reproducibility. Using our newly developed method, the concentration of whole MHBA in several commercial beers was evaluated. Our results will promote the study of bitter acid oxides.
Graphical abstract
Preparative and analytical methods of hop bitter acid oxides formed through the oxidation of α- and β-acids have been developed.
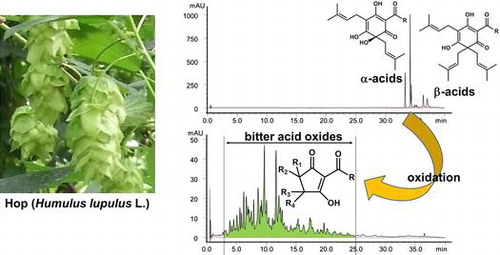
Hops (Humulus lupulus L.) are widely used in the brewing industry to add characteristic bitterness and aroma to beer. The lupulin glands from female inflorescences of hops are known to accumulate essential oils, prenylflavonoids, and bitter acids.Citation1) The bitter acids in hops are phloroglucinol derivatives usually classified as α-acids and β-acids. Both groups comprise three main congeners [normal-(n-), co-, and ad-homologs], which differ in their acyl side chains [α-acids, cohumulone (1a), n-humulone (1b), and adhumulone (1c); β-acids, colupulone (2a), n-lupulone (2b), and adlupulone (2c)] (Fig. ).Citation2,3) During the wort boiling process, α-acids thermally isomerize into iso-α-acids via an acyloin-type ring contraction, resulting in the generation of two epimeric isomers: cis-iso-α-acids (3a–c) and trans-iso-α-acids (4a–c) (Fig. ).Citation4) Iso-α-acids are mainly responsible for the typical bitter taste of beer,Citation2,5) as well as the stability of beer foam,Citation6) and are known to exhibit antibacterial properties.Citation7)
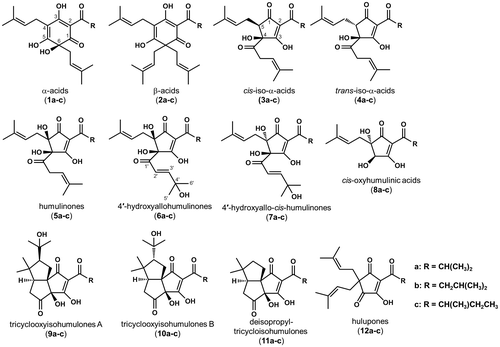
Fig. 1. Chemical structures of compounds 1–12. α-Acids, cohumulone (1a), n-humulone (1b), and adhumulone (1c); β-acids, colupulone (2a), n-lupulone (2b), and adlupulone (2c); cis-iso-α-acids, cis-isocohumulone (3a), cis-iso-n-humulone (3b), and cis-isoadhumulone (3c); trans-iso-α-acids, trans-isocohumulone (4a), trans-iso-n-humulone (4b), and trans-isoadhumulone (4c); humulinones, cohumulinone (5a), n-humulinone (5b), and adhumulinone (5c); 4′-hydroxyallohumulinones, 4′-hydroxyallocohumulinone (6a), 4′-hydroxyallo-n-humulinone (6b), and 4′-hydroxyalloadhumulinone (6c); 4′-hydroxyallo-cis-humulinones, 4′-hydroxyallo-cis-cohumulinone (7a), 4′-hydroxyallo-cis-n-humulinone (7b), and 4′-hydroxyallo-cis-adhumulinone (7c); cis-oxyhumulinic acids, cis-oxycohumulinic acid (8a), cis-oxy-n-humulinic acid (8b), and cis-oxyadhumulinic acid (8c); tricyclooxyisohumulones A, tricyclooxyisocohumulone A (9a), tricyclooxyiso-n-humulone A (9b), and tricyclooxyisoadhumulone A (9c); tricyclooxyisohumulones B, tricyclooxyisocohumulone B (10a), tricyclooxyiso-n-humulone B (10b), and tricyclooxyisoadhumulone B (10c); deisopropyltricycloisohumulones, deisopropyltricycloisocohumulone (11a), deisopropyltricycloiso-n-humulone (11b), and deisopropyltricycloisoadhumulone (11c); and hulupones, cohulupone (12a), n-hulupone (12b), and adhulupone (12c).
In addition, hop bitter acids have also received considerable attention because of their potential health benefits.Citation8) The α- and β-acids have been reported to possess various biological activities, such as anti-cancer,Citation9–11) anti-inflammatory,Citation12–14) and anti-oxidation activities.Citation15–17) An isomerized hop extract, which primarily consists of iso-α-acids, was shown to prevent diet-induced obesity by the modulation of lipid metabolism in the liver and inhibition of intestinal lipid absorption.Citation18) Thus, utilization of hop bitter acids for health promotion may be expected. However, development of functional foods or supplements containing α-, β-, and/or iso-α-acids as bioactive ingredients is technically challenging due to the instability of these compounds. It is well known that α- and β-acids in hops readily oxidize,Citation19,20) and iso-α-acids in beer are also degraded by oxidation and proton-catalyzed cyclization.Citation21–24)
The bitterness quality of beer brewed with stored hops, which contain oxidation products derived from α- and β-acids, has been studied, but the conclusion is disputed.Citation5,25–30) Moreover, there are very few published studies on the physiological effects of these oxidation products.Citation31) Until recently, most of the constituents of the oxidation products in hops were unidentified. Our studies confirmed that α-acids are oxidized into humulinones (5a–c), 4′-hydroxyallohumulinones (6a–c), tricyclooxyisohumulones A (9a–c) and B (10a–c), and deisopropyltricycloisohumulones (11a–c) and that β-acids are oxidized into hulupones (12a–c) (Fig. ).Citation32,33) We also revealed that 4′-hydroxyallohumulinones (6a–c) isomerize into 4′-hydroxyallo-cis-humulinones (7a–c) and degrade into cis-oxyhumulinic acids (8a–c) during the wort boiling process (Fig. ).Citation34) Nonetheless, the chemical properties of a large number of other α- and/or β-acid-derived oxidation products remain unknown. In order to evaluate the effects of bitter acid oxides on beer properties as well as their potential health benefits, the chemical structures of the main constituents of bitter acid oxides must be determined. Furthermore, it is essential to develop methods to selectively prepare and analyze the bitter acid oxide fraction, which exclude other hop constituents such as polyphenols, lipids, waxes, and polysaccharides. In addition, understanding chemical properties common to the constituents in whole bitter acid oxides is also helpful in studying the effects of these bitter acid oxides.
Here, we developed a simple liquid–liquid extraction method to obtain the total bitter acid oxide fraction from a water extract of stored hops and designated the constituents of this fraction as matured hop bitter acids (MHBA). The chemical properties of MHBA were then thoroughly investigated. A quantitative analytical methodology for whole MHBA was developed and validated, and the concentrations of whole MHBA in several commercial beers were investigated using this technique.
Materials and methods
Chemicals and materials
The following chemicals were obtained commercially: ethylenediaminetetraacetic acid (EDTA), caffeine, H3PO4, MeCN, EtOH, and CH2Cl2 (Wako Pure Chemicals, Osaka, Japan). Deionized water for chromatography was purified by means of a Milli-Q Gradient A10 system (Millipore, Billerica, MA). An isomerized hop extract and hop pellets were purchased from Hopsteiner (Mainburg, Germany).
Preparation of reference standard compounds
cis-Iso-α-acids (3a–c), trans-iso-α-acids (4a–c), humulinones (5a–c), and hulupones (12a–c) were prepared according to a protocol reported previously.Citation32) Tricyclooxyiso-n-humulones A (9b) and B (10b) and deisopropyltricycloiso-n-humulone (11b) were isolated from the autoxidation products of n-humulone.Citation33) 4′-Hydroxyallohumulinones (6a–c), 4′-hydroxyallo-cis-cohumulinone (7a), 4′-hydroxyallo-cis-n-humulinone (7b), cis-oxycohumulinic acid (8a), and cis-oxy-n-humulinic acid (8b) were isolated in our laboratory.Citation34)
Preparation of a MHBA fraction from stored hops
Hop pellets (600 g) were stored at 60 °C for 120 h to oxidize α- and β-acids and then extracted with pre-warmed H2O (50 °C, 6 L) for 1 h. During extraction, the temperature of the extract was maintained at 50 °C. The extract was filtered to remove the debris of hops and then treated with activated charcoal and polyvinylpolypyrrolidone (PVPP) for 2 h. After treatment, the mixture was filtered to separate the extract from activated charcoal and PVPP. The filtrate was heated to 90 °C for 4 h and cooled to room temperature before lyophilizing to yield a pale brown powder as a water extract of stored hops (126 g). A portion (63 g) of this extract was dissolved in H2O (3 L), and total bitter acid oxides were extracted with CH2Cl2 (6 L) after acidifying the solution with 1 N HCl (300 mL). The CH2Cl2 layer was dried over anhydrous Na2SO4 and evaporated to yield a pale brown, amorphous solid (12.6 g) as a MHBA fraction.
Molecular formula analysis of the MHBA components
The MHBA fraction was dissolved in EtOH and analyzed using HPLC-PDA-ESI/HRMS to determine the molecular formula of the MHBA components. The HPLC-PDA-HRMS system consisted of an LTQ Orbitrap XL mass spectrometer (ThermoScientific, San Jose, CA) with an Agilent 1200 series binary pump (G1312B), a degasser (G1379B), an autosampler (G1367C), a column compartment (G1316B), and a PDA detector (G1315C) (Agilent, Palo Alto, CA). The LC conditions were as follows: column: 100 × 2.1 mm id, 3 μm, L-column 2 ODS (Chemicals Evaluation and Research Institute, Tokyo, Japan); solvent: 5 mM HCOONH4 (pH 8.5) (solvent A) and MeCN (solvent B), a linear gradient from 10 to 36% B in 0 → 39 min, 36 to 80% B in 39 → 44 min, and 80% B for 44 → 52 min; flow rate: 0.25 mL/min; and column temperature: 40 °C. The PDA recorded spectra from 190 to 500 nm. The HRMS was operated in negative ionization mode with an ESI source using the following conditions: sheath gas at 50 (arbitrary units), aux gas at 20 (arbitrary units), sweep gas at 0 (arbitrary units), spray voltage at −2.0 kV, capillary temperature at 300 °C, capillary voltage at −20 V, and tube lens at −70 V. The mass range was from m/z 100 to 800 with a resolution of 30,000.
Precursor ion scan analysis of the MHBA components
The MHBA fraction was dissolved in EtOH and analyzed using HPLC-PDA-ESI/MS/MS to investigate the common partial structure of the MHBA components. The HPLC-PDA-MS/MS system consisted of a 4000 Q-Trap mass spectrometer (AB Sciex, Tokyo, Japan) connected to a Shimadzu Prominence UFLC system (Shimadzu, Kyoto, Japan). The LC conditions were the same as described for the HPLC-PDA-ESI/HRMS method. The 4000 Q-Trap mass spectrometer was operated in negative ionization mode with an ESI source for the precursor ion scans of m/z 111 (co-homolog) and m/z 125 (n- and ad-homologs). Nitrogen was used as the turbo gas at 600 °C. Ion source gases 1 and 2 were set to 50 and 70 psi, respectively, N2 curtain gas was set to 30 psi, and the collision cell gas was set to 7 psi. The ion spray voltage was set to −4500 V, entrance potential (EP) was set to −10 V, and collision cell exit potential ramp was set to −41–−7 V. A declustering potential ramp (−115 to −45 V) and collision energy (CE) ramp (−50 to −20 V) were used. Q1 and Q3 resolutions were set to unit. The product ion scan of reference standards was performed with the same parameters as described above, except for CE which was set to −50 V. Analyst software version 1.6.1 (AB Sciex, Tokyo, Japan) was used for the experiments.
Acid-base partition experiments of the MHBA components
The water extract of stored hops (62.5 mg) was dissolved in H2O (10 mL) and partitioned with CH2Cl2 (20 mL) after acidifying the solution with 1 N HCl (1.0 mL). A portion of the CH2Cl2 layer (10 mL) was partitioned with 0.1 M NH4HCO3 buffer (pH 8.0) (10 mL), and both the aqueous layer and CH2Cl2 layer were analyzed by HPLC.
Quantitative HPLC-UV analytical method for whole MHBA
The following extraction experiments were conducted independently four times. The water extract of stored hops (600 mg) was dissolved in H2O (250 mL), and the MHBA fraction was extracted with CH2Cl2 (500 mL) after acidifying the solution with 1 N HCl (25 mL). The CH2Cl2 layer was dried over anhydrous Na2SO4 and evaporated to yield a pale brown, amorphous solid.
The obtained respective solid was dissolved in EtOH (0.500 mg/mL) and analyzed by a Prominence UFLC system (Shimadzu, Kyoto, Japan). The analyses were performed using the following conditionsCitation32,33): column: 100 × 2.1 mm id, 3 μm, Alltima C18 column (Systech, Tokyo, Japan); solvent: H2O/H3PO4 (85%), 1000/0.2, (v/v) containing EDTA (0.02% w/v) (solvent A) and MeCN (solvent B), a linear gradient from 10 to 52% B in 0 → 26.7 min, 52% B for 26.7 → 30 min, 52 to 75% B in 30 → 32.7 min, 75 to 85% B in 32.7 → 36.7 min, and 85% B for 36.7 → 37.7 min; flow rate: 0.6 mL/min; detector: 270 nm; and column temperature: 40 °C. The injection volume was 3.0 μL.
Baseline drift generated from mobile phase gradient was eliminated by subtracting the chromatogram signals of blank (H2O) analysis using a baseline correction function of LCsolution software version 1.25 (Shimadzu, Kyoto, Japan). Peaks detected in the region immediately after elution of caffeine and immediately before elution of trans-isocohumulone (4a) were integrated. The integrated area was quantified as whole MHBA using the calibration curve of tricyclooxyiso-n-humulone (9b).
Analytical method validation for whole MHBA in a model beverage
A bottled carbonated beverage, containing the water extract of stored hops with a final concentration of whole MHBA being around 120 mg/L, was prepared. To evaluate intra- and inter-day precision and reproducibility of quantitative analysis of whole MHBA, the beverage was analyzed (n = 6) for six consecutive days. The beverage was degassed by sonication for 15 min, and a portion (10 mL) was partitioned with CH2Cl2 (20 mL) after acidifying the solution with 1 N HCl (1.0 mL). The two-layered solution was then centrifuged at 6000 g for 10 min at 20 °C, and a portion (10 mL) of the CH2Cl2 layer was collected, dried under N2 gas, and redissolved in EtOH (1.0 mL). After filtration, this solution was subjected to quantitative HPLC analysis. To evaluate the extraction efficiency of whole MHBA in the analytical procedure, the residual aqueous solution was extracted with CH2Cl2 (20 mL) for a second time and the resulting extract was analyzed using the same protocol.
Quantitative analysis of whole MHBA in commercial beers
Commercially available beers were purchased in a Japanese market. The purchased beers were a selection of both Japanese (A–E) and foreign brands (F–K).
Each beer was degassed by sonication for 15 min, and a portion (10 mL) was partitioned with CH2Cl2 (20 mL) after acidifying the solution with 1 N HCl (1.0 mL). The two-layered solution was then centrifuged at 6000 g for 10 min at 20 °C, and a portion (10 mL) of the CH2Cl2 layer was collected, dried under N2 gas, and redissolved in EtOH (1.0 mL). After filtration, this solution was subjected to quantitative HPLC analysis. Iso-α-acid content (sum of 3a–c and 4a–c) in the beer was measured simultaneously using ICS-I3 as a calibration standard (American Society of Brewing Chemists, Saint Paul, MN).Citation32)
Results and discussion
Preparation of a water extract of stored hops and a MHBA fraction
In this study, we designated total bitter acid oxides derived from α-, β-, and/or iso-α-acids, including structurally unknown constituents, as MHBA, which showed more hydrophilic properties than iso-α-acids in HPLC analysis (Fig. ). The MHBA fraction could be prepared from a water extract of stored hops as described below in detail.
Fig. 2. HPLC chromatograms of the (A) EtOH extract of fresh hops, (B) isomerized hop extract, and water extract of hops stored at 60 °C for 120 h (C) before and (D) after heating the extract. The chromatograms of (C) and (D) represent the components extractable in CH2Cl2 under acidic conditions. Structures of the compounds are given in Fig. .
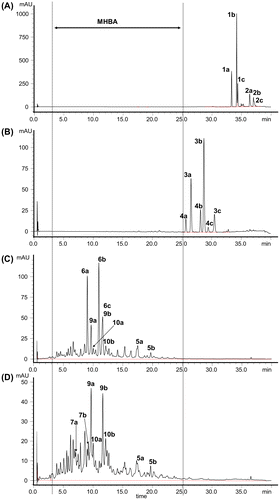
First, fresh hops were oxidized to transform α- and β-acids into their respective oxidation products, which were then extracted into water. The poorly water-soluble components such as xanthohumol, lipids, and waxes could be excluded in this extraction step, and an extract, containing the oxidation compounds such as 4′-hydroxyallohumulinones (6a–c) and tricyclooxyisohumulones A (9a–c) and B (10a–c), was obtained (Fig. C). Next, an aqueous solution of the extract was heated. The heating process induced chemical reactions that occur during the wort boiling process, such as transformation of unstable 4′-hydroxyallohumulinones (6a–c) into 4′-hydroxyallo-cis-humulinones (7a–c) and cis-oxyhumulinic acids (8a–c),Citation34) as well as potentially other reactions, resulting in the stabilization of the composition of the extract (Fig. D). Finally, MHBA could be selectively extracted with CH2Cl2 from an aqueous solution of the extract after acidification. CH2Cl2 was selected as the extraction solvent because the oxidation compounds, which constitute MHBA, were shown to be efficiently extracted with CH2Cl2 in our previous study.Citation34) Indeed, CH2Cl2 was found to be suitable for the extraction of bitter acid oxides. However, isooctane, which is widely used to extract bitter acids in beer,Citation35,36) was unsuitable for the extraction of hydrophilic oxides (Fig. S1 in Supplemental material).
The solid content yield of the water extract from stored hops was about 21% (w/w), and the MHBA fraction accounted for about 20% of the extract. The chemical properties of MHBA as a whole were further investigated.
Chemical properties of MHBA
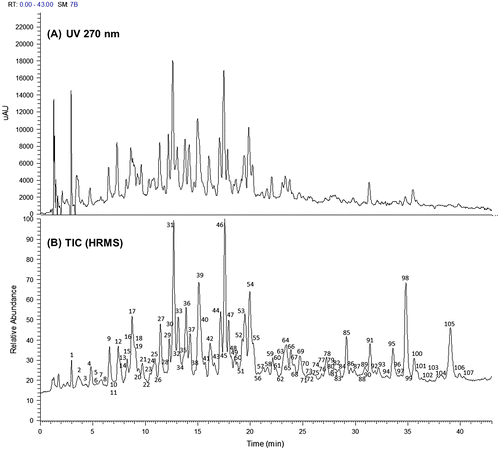
First, we analyzed the MHBA fraction using HPLC-PDA-ESI/HRMS. The chromatogram at 270 nm and total ion chromatogram (TIC) are shown in Fig. . Except for some peaks [i.e., peak numbers 85, 95, 98 (cohulupone (12a)), and 105 (n-hulupone (12b) and adhulupone (12c)) in Fig. (B)] which were detected with high intensity by the MS detector in comparison with the UV detector probably because of their especially high efficiency of ionization, the chromatogram at 270 nm (Fig. (A)) and TIC (Fig. (B)) showed almost the same profile. This result suggested that the MHBA fraction was composed mostly of substances (i) possessing UV absorption around 270 nm and (ii) capable of easily forming negative ions in MS. The molecular formulas of the constituents of each peak in Fig. (B) indicated by the HRMS data are summarized in Table S1 (in Supplemental material). Humulinones (5a–c), 4′-hydroxyallohumulinones (6a–c), 4′-hydroxyallo-cis-cohumulinone (7a), 4′-hydroxyallo-cis-n-humulinone (7b), cis-oxycohumulinic acid (8a), cis-oxy-n-humulinic acid (8b), tricyclooxyiso-n-humulones A (9b) and B (10b), deisopropyltricycloiso-n-humulone (11b), and hulupones (12a–c) were identified in MHBA from direct comparison to the reference standards. It is known that iso-α-acid derivatives are eluted in the order of co-congener, ad-congener, and n-congener in ODS HPLC using an alkaline mobile phase.Citation4,37) Based on the elution pattern described above and observed m/z, 4′-hydroxyallo-cis-adhumulinone (7c), cis-oxyadhumulinic acid (8c), tricyclooxyisocohumulones A (9a) and B (10a), tricyclooxyisoadhumulones A (9c) and B (10c), deisopropyltricycloisocohumulone (11a), and deisopropyltricycloisoadhumulone (11c) were tentatively identified in MHBA (Table S1). All other structurally unidentified components in MHBA, as far as we investigated, gave a molecular formula corresponding to the oxidation products of α- and β-acids and related compounds (94% of the components seem to be α-acid oxides and 6% of the components seem to be β-acid oxides) (Table S1). For example, oxygen adducts, water adducts and dehydration products of oxidized α-acids, and compounds produced by oxidative elimination of an acetone (C3H6O)Citation33,38,39) and a methylhexenoyl group (C6H8O)Citation34,39,40) were detected (Table S1). Thus, MHBA appear to be composed of oxidation products derived primarily from α-acids.
Fig. 3. HPLC-PDA-HRMS analysis of the MHBA fraction. (A) Chromatogram of UV at 270 nm, and (B) TIC of HRMS analysis. Observed m/z and indicated molecular formula of the constituents of each peak shown in TIC and identified compounds are summarized in Table S1 (in Supplemental material).
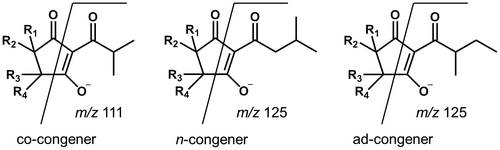
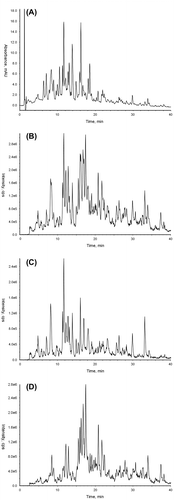
All the structurally elucidated compounds in MHBA possess a β-tricarbonyl moiety in their structures similar to α-, β-, and iso-α-acids (Fig. ). In the product ion scan of the reference standards using HPLC-ESI/MS/MS, common fragment ions (m/z 111 for co-congener and m/z 125 for n- and ad-congeners) originating from the β-tricarbonyl moiety including the acyl side chains were detected (Fig. and Table ). To check whether all the components in MHBA (including unidentified ones) possess the β-tricarbonyl moiety, precursor ion scans of m/z 111 and m/z 125 were conducted. The results shown in Fig. clearly demonstrate that the MHBA components give fragment ions of m/z 111 and m/z 125 by MS/MS analysis, suggesting that all the components in MHBA possess a β-tricarbonyl moiety. The behavior of the MHBA components on CH2Cl2/H2O partition (i.e., extractable in CH2Cl2 under acidic but not alkaline conditions) also supported the presence of the β-tricarbonyl moiety, which possesses acidic properties (Fig. S2 in Supplemental material).
Table 1. Fragment ions of reference compounds produced by MS/MS analysis.
Fig. 5. HPLC-PDA-MS/MS analysis of the MHBA fraction. (A) Chromatogram of UV at 270 nm, (B) TIC of precursor ion scans of m/z 111 and 125, (C) chromatogram of precursor ion scan of m/z 111, and (D) chromatogram of precursor ion scan of m/z 125.
Taken together, these findings suggest MHBA are mainly composed of α-acid-derived oxides, which possess a common β-tricarbonyl moiety.
Development of a quantitative analytical method for HPLC analysis of whole MHBA
Because the UV absorption characteristics of the MHBA components were attributed to the common chromophore (β-tricarbonyl moiety), the difference in the molar absorbance coefficient among the components at 270 nm was assumed to be small.Citation41) Therefore, we quantified the total MHBA components using the calibration curve prepared from tricyclooxyiso-n-humulone A (9b), which is an abundant (Fig. ) and stable constituent of MHBA.Citation34)
Fig. 6. HPLC chromatograms of (A) caffeine, (B) ICS-I3 standard, and (C) the MHBA fraction.
Note: ICS-I3 contains mainly trans-isocohumulone (4a), trans-iso-n-humulone (4b), and trans-isoadhumulone (4c). Integrated peak area of whole MHBA is shown in color.
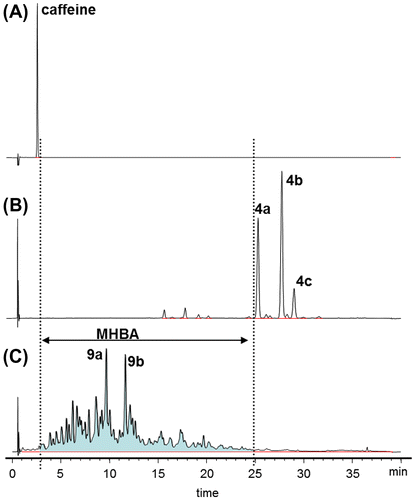
In the HPLC analysis, we define the MHBA elution region as shown in Fig. [i.e., from just after the elution of caffeine to just before that of trans-isocohumulone (4a)]. The caffeine peak was used as an indicator to distinguish the peaks belonging to MHBA from the peaks around the solvent shock, which were not retained by the ODS column. Because the MHBA region was quite broad (i.e., from tR ~3 min to tR ~25 min), a correction for baseline drift generated from the solvent gradient was required in order to integrate the total peak area precisely. The baseline drift could be eliminated by subtracting the chromatogram signals of blank (H2O) analysis using the analytical software function (Fig. S3 in Supplemental material).
The MHBA fraction was prepared independently four times and the whole MHBA content was subsequently determined by HPLC analysis. The quantitative value of MHBA accounted for 95.8% of the solid content of the fraction with few errors [%RSD, 2.05 (n = 4)] (Table ). The almost complete consistency of the solid content and quantitative value obtained by HPLC analysis demonstrated that this analytical method was suitable for the quantitative analysis of whole MHBA.
Table 2. MHBA concentration determined by HPLC analysis of the MHBA fraction.
Evaluation of the intra- and inter-day analytical precision of whole MHBA
A model carbonated beverage containing a water extract of stored hops was prepared to evaluate the precision of our analytical procedure for the analysis of whole MHBA in a beverage. MHBA in the beverage were extracted with CH2Cl2 under acidic conditions and then analyzed by HPLC. To evaluate the extraction efficiency, the residual aqueous solution was extracted with CH2Cl2 for a second time. Subsequent analysis showed that the second extract contained very few MHBA components (Fig. S4 in Supplemental material). Indeed, the entire MHBA peak area of the second extract was less than 4% of that of the first extract. Thus, whole MHBA was found to be almost completely extracted in the analytical procedure. The intra- and inter-day precision of the analyses were found to be below 1.55% RSD and 1.10% RSD, respectively (Table ). These results established that this analytical method had a high degree of precision and reproducibility. Efficient extraction of whole MHBA together with the high level of precision obtained using an external standard method indicates that no internal standard is required for the analysis. Further studies to verify the precision of analysis between laboratories are needed to confirm the versatility of our novel analytical method.
Table 3. The intra- and inter-day precision of HPLC analysis of MHBA in a model beverage.
Quantitative analysis of whole MHBA in commercial beers
Using the developed analytical method, we investigated the amount of MHBA in several commercial beers. The MHBA concentration along with the determined iso-α-acid concentration in the beers is summarized in Table . The major brands of beer made in Japan (brands A–E) contained 19–38 mg/L MHBA and 16–27 mg/L iso-α-acids. These were all lager type beers. However, beer made in foreign countries (brands F–K) contained 20–210 mg/L MHBA and 3.3–64 mg/L iso-α-acids. Among them, India Pale Ale (brands G, H, and I) had the highest content of MHBA and iso-α-acids. These results seem reasonable, given that India Pale Ale is brewed with large amounts of hops. Lambic (brands J and K) also showed high concentrations of MHBA, whereas the iso-α-acid concentration was the lowest among the beers analyzed in this study. Because Lambic is manufactured over a long period of time for aging, iso-α-acids are presumably subject to degradation during this periodCitation21–23) and may indeed be transformed into MHBA components.
Table 4. Concentrations of MHBA and iso-α-acids in commercial beers.
Unlike the model carbonated beverage, beers contain extracts of malt and other raw materials. The constituents derived from these materials (i.e., other than hops) may co-elute at the MHBA region in the HPLC analysis and the calculated MHBA concentration in beers may be overestimated. Nonetheless, whole MHBA content is certainly reflected in the quantified value because whole MHBA could be extracted by our method but not the method using isooctane as extracting solvent (Fig. S1 in Supplemental material). Bittering components, such as iso-α-acids, in beer are usually measured by extracting them with isooctane, and the bitterness unit (BU) is thus calculated using the isooctane extract.Citation35,36) It is empirically known that beers with the same BU or iso-α-acid concentration often differ in their bitterness quality.Citation5,25) In these cases, MHBA, which could not be measured by previous techniques, may influence the bitterness quality of the beer. Further studies are needed to clarify the effects of MHBA on beer properties.
In conclusion, it was found that MHBA could be selectively prepared from a water extract of stored hops simply by extraction with CH2Cl2 under acidic conditions. Detailed analyses of the chemical properties of MHBA show that they are (i) principally composed of the oxidation products of α-acids and (ii) possess a common β-tricarbonyl moiety. A quantitative analytical HPLC method for whole MHBA was developed and validated, which enabled the quantification of whole MHBA in a carbonated beverage. All of these findings are helpful for evaluating the effects of MHBA on the properties of beer. Moreover, this technique will be invaluable for examining the bioactivities related to the health benefits of ingesting MHBA. Studies on the health benefits brought about by consuming MHBA are currently in progress.
Supplemental material
The supplementary material for this paper is available online at http://dx.doi.org/10.1080/09168451.2015.1042832.
150409_Supplemental_material_FigureS1234.pdf
Download PDF (388.2 KB)150409_Supplemental_material_TableS1.pdf
Download PDF (386.2 KB)Acknowledgements
We thank members of our research groups for valuable discussions, especially Yumie Morimoto-Kobayashi, Fumitoshi Manabe, Yuji Kaneko, and Yasuji Kawachi. We also gratefully acknowledge the technical assistance of Hiromi Ozaki and Makiko Yamada.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Clark SM, Vaitheeswaran V, Ambrose SJ, Purves RW, Page JE. Transcriptome analysis of bitter acid biosynthesis and precursor pathways in hop (Humulus lupulus). BMC Plant Biol. 2013;13:12.10.1186/1471-2229-13-12
- Almaguer C, Schoenberger C, Gastl M, Arendt EK, Becker T. Humulus lupulus—a story that begs to be told. A review. J. Inst. Brew. 2014;120:289–314.
- Steenackers B, De Cooman L, De Vos D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: a review. Food Chem. 2015;172:742–756.10.1016/j.foodchem.2014.09.139
- Urban J, Dahlberg CJ, Carroll BJ, Kaminsky W. Absolute configuration of beer′s bitter compounds. Angew. Chem., Int. Ed. 2013;52:1553–1555.10.1002/anie.201208450
- Kowaka M, Kokubo E. Composition of bitter substances of hops and characteristics of beer bitterness. J. Am. Soc. Brew. Chem. 1977;35:16–21.
- Bishop LR, Whitear AL, Inman WR. A scientific basis for beer foam formation and cling. J. Inst. Brew. 1974;80:68–80.10.1002/jib.1974.80.issue-1
- Teuber M, Schmalreck AF. Membrane leakage in Bacillus subtilis 168 induced by the hop constituents lupulone, humulone, isohumulone, and humulinic acid. Arch. Mikrobiol. 1973;94:159–171.10.1007/BF00416690
- Van Cleemput M, Cattoor K, De Bosscher K, Haegeman G, De Keukeleire D, Heyerick A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009;72:1220–1230.10.1021/np800740m
- Shimamura M, Hazato T, Ashino H, Yamamoto Y, Iwasaki E, Tobe H, Yamamoto K, Yamamoto S. Inhibition of angiogenesis by humulone, a bitter acid from beer hop. Biochem. Biophys. Res. Commun. 2001;289:220–224.10.1006/bbrc.2001.5934
- Chen W-J, Lin J-K. Mechanisms of cancer chemoprevention by hop bitter acids (beer aroma) through induction of apoptosis mediated by fas and caspase cascades. J. Agric. Food Chem. 2004;52:55–64.10.1021/jf034737u
- Lamy V, Roussi S, Chaabi M, Gossé F, Lobstein A, Raul F. Lupulone, a hop bitter acid, activates different death pathways involving apoptotic TRAIL-receptors, in human colon tumor cells and in their derived metastatic cells. Apoptosis. 2008;13:1232–1242.10.1007/s10495-008-0250-5
- Yasukawa K, Takeuchi M, Takido M. Humulon, a bitter in the hop, inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology. 1995;52:156–158.10.1159/000227448
- Yamamoto K, Wang J, Yamamoto S, Tobe H. Suppression of cyclooxygenase-2 gene transcription by humulon of beer hop extract studied with reference to glucocorticoid. FEBS Lett. 2000;465:103–106.
- Hougee S, Faber J, Sanders A, Berg WB, Garssen J, Smit HF, Hoijer MA. Selective inhibition of COX-2 by a standardized CO2 extract of Humulus lupulus in vitro and its activity in a mouse model of zymosan-induced arthritis. Planta Med. 2006;72:228–233.10.1055/s-2005-916212
- Tagashira M, Watanabe M, Uemitsu N. Antioxidative activity of hop bitter acids and their analogues. Biosci. Biotechnol. Biochem. 1995;59:740–742.
- Tobe H, Kubota M, Yamaguchi M, Kocha T, Aoyagi T. Apoptosis to HL-60 by humulone. Biosci. Biotechnol. Biochem. 1997;61:1027–1029.10.1271/bbb.61.1027
- Liu Y, Gu X-h, Tang J, Liu K. Antioxidant activities of hops (Humulus lupulus) and their products. J. Am. Soc. Brew. Chem. 2007;65:116–121.
- Yajima H, Noguchi T, Ikeshima E, Shiraki M, Kanaya T, Tsuboyama-Kasaoka N, Ezaki O, Oikawa S, Kondo K. Prevention of diet-induced obesity by dietary isomerized hop extract containing isohumulones, in rodents. Int. J. Obes. (London). 2005;29:991–997.
- Burton JS, Stevens R. Evaluation of hops. XI. The hard resin and presence of hulupinic acid. J. Inst. Brew. 1965;71:51–56.10.1002/jib.1965.71.issue-1
- Laws DRJ. Hop resins and beer flavour V. The significance of oxidized hop resins in brewing. J. Inst. Brew. 1968;74:178–182.10.1002/jib.1968.74.issue-2
- Intelmann D, Hofmann T. On the autoxidation of bitter-tasting iso-α-acids in beer. J. Agric. Food Chem. 2010;58:5059–5067.10.1021/jf100083e
- Intelmann D, Kummerloewe G, Haseleu G, Desmer N, Schulze K, Froehlich R, Frank O, Luy B, Hofmann T. Structures of storage-induced transformation products of the beer's bitter principles, revealed by sophisticated NMR spectroscopic and LC-MS techniques. Chem. Eur. J. 2009;15:13047–13058.10.1002/chem.v15:47
- Intelmann D, Demmer O, Desmer N, Hofmann T. 18O stable isotope labeling, quantitative model experiments, and molecular dynamics simulation studies on the trans-specific degradation of the bitter tasting iso-α-acids of beer. J. Agric. Food Chem. 2009;57:11014–11023.10.1021/jf903000c
- Rakete S, Berger R, Böhme S, Glomb MA. Oxidation of isohumulones induces the formation of carboxylic acids by hydrolytic cleavage. J. Agric. Food Chem. 2014;62:7541–7549.10.1021/jf501826h
- Ono M, Kakudo Y, Yamamoto R, Nagami K, Kumada J. Simultaneous analysis of hop bittering components by high-performance liquid chromatography. II. Evaluation of hop deterioration. J. Am. Soc. Brew. Chem. 1987;45:61–69.
- Foster RT II, Weber K, Jangaard NO. The effect of hop acids transformation on kettle utilization and finished beer flavor and aroma. Tech. Q.—Master Brew. Assoc. Am. 1981;18:109–115.
- Aitken RA, Bruce A, Harris JO, Seaton JC. The bitterness of hop-derived materials in beer. J. Inst. Brew. 1970;76:29–36.10.1002/jib.1970.76.issue-1
- Whitear AL. Changes in resin composition and brewing behaviour of hops during storage. J. Inst. Brew. 1966;72:177–183.10.1002/jib.1966.72.issue-2
- Howard GA, Martin PA. Bittering power of stored hops. J. Inst. Brew. 1964;70:424–439.10.1002/jib.1964.70.issue-5
- Almaguer C, Gastl M, Arendt EK, Becker T. Contributions of hop hard resins to beer quality. BrewingSci.—Monatsschr. Brauwiss. 2012;65:118–129.
- Sasaoka N, Sakamoto M, Kanemori S, Kan M, Tsukano C, Takemoto Y, Kakizuka A. Long-term oral administration of hop flower extracts mitigates alzheimer phenotypes in mice. PLoS One. 2014;9:e87185/1–e87185/12, 12 pp.
- Taniguchi Y, Matsukura Y, Ozaki H, Nishimura K, Shindo K. Identification and quantification of the oxidation products derived from α-acids and β-acids during storage of hops (Humulus lupulus L.). J. Agric. Food Chem. 2013;61:3121–3130.10.1021/jf3047187
- Taniguchi Y, Taniguchi H, Matsukura Y, Kawachi Y, Shindo K. Structural elucidation of humulone autoxidation products and analysis of their occurrence in stored hops. J. Nat. Prod. 2014;77:1252–1261.10.1021/np4008427
- Taniguchi Y, Taniguchi H, Yamada M, Matsukura Y, Koizumi H, Furihata K, Shindo K. Analysis of the components of hard resin in hops (Humulus lupulus L.) and structural elucidation of their transformation products formed during the brewing process. J. Agric. Food Chem. 2014;62:11602–11612.10.1021/jf504394h
- Howard GA. Institute of brewing analysis committee estimation of the bitterness of beer. J. Inst. Brew. 1968;74:249–251.
- Bitterness units of beer and wort. J. Am. Soc. Brew. Chem. 1996;54:186–187.
- Vanhoenacker G, De Keukeleire D, Sandra P. Analysis of iso-α-acids and reduced iso-α-acids in beer by direct injection and liquid chromatography with ultraviolet absorbance detection or with mass spectrometry. J. Chromatogr. A. 2004;1035:53–61.10.1016/j.chroma.2004.02.038
- Haseleu G, Intelmann D, Hofmann T. Structure determination and sensory evaluation of novel bitter compounds formed from β-acids of hop (Humulus lupulus L.) upon wort boiling. Food Chem. 2009;116:71–81.10.1016/j.foodchem.2009.02.008
- Intelmann D, Haseleu G, Dunkel A, Lagemann A, Stephan A, Hofmann T. Comprehensive sensomics analysis of hop-derived bitter compounds during storage of beer. J. Agric. Food Chem. 2011;59:1939–1953.10.1021/jf104392y
- Haseleu G, Lagemann A, Stephan A, Intelmann D, Dunkel A, Hofmann T. Quantitative sensomics profiling of hop-derived bitter compounds throughout a full-scale beer manufacturing process. J. Agric. Food Chem. 2010;58:7930–7939.10.1021/jf101326v
- Verzele M, Steenbeke G, Verhagen LC, Strating J. Improved analysis by liquid chromatography of iso-alpha acids. J. High Resolut. Chromatogr. 1990;13:826–831.10.1002/(ISSN)1521-4168