Abstract
Soybean plants require high amounts of nitrogen, which are mainly obtained from biological nitrogen fixation. A field experiment was conducted by soybean (Glycine max) genotypes, growing two varieties (Shohag and BARI Soybean6) and two advanced lines (MTD10 and BGM02026) of soybean with or without Rhizobium sp. BARIRGm901 inoculation. Soybean plants of all genotypes inoculated with Rhizobium sp. BARIRGm901 produced greater nodule numbers, nodule weight, shoot and root biomass, and plant height than non-inoculated plants. Similarly, inoculated plants showed enhanced activity of nitrogenase (NA) enzyme, contributing to higher nitrogen fixation and assimilation, compared to non-inoculated soybean plants in both years. Plants inoculated with Rhizobium sp. BARIRGm901 also showed higher pod, stover, and seed yield than non-inoculated plants. Therefore, Rhizobium sp. BARIRGm901 established an effective symbiotic relationship with a range of soybean genotypes and thus increased the nodulation, growth, and yield of soybean grown in gray terrace soils in Bangladesh.
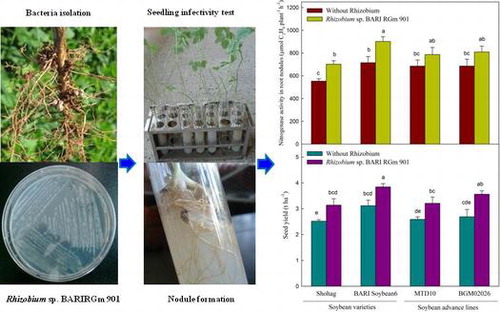
Graphical Abstract
Rhizobium sp. BARIRGm901 isolation, seedling infectivity test, nitrogenase enzyme assay, and seed yield in soybean genotypes in gray terrace soil.
Nitrogen is one of the most abundant elements on earth, but its availability often limits plant growth and crop production. In agriculture, it is converted to plant available forms by chemical fixation through industrial production and/or biological nitrogen fixation (BNF) involving micro-organisms. Soybean (Glycine max) plants require a large amount of nitrogen as their seeds contain high concentrations of protein (approximately 35–40%), and the total amount of nitrogen accumulating in the shoot is proportional to the seed yield.Citation1) Rhizobial bacteria play an important role in the nitrogen cycle of agro-ecosystems by infecting the roots of soybean plants and inducing the formation of nitrogen-fixing nodules. These nodules provide an appropriate environment for fixation of atmospheric nitrogen into ammonium by NA, a bacterial enzyme sensitive to high oxygen tensions.Citation2) Large amounts of nitrogen are fixed by the bacteria and transferred to the plants, reducing the need for nitrogenous fertilizer.Citation3) It is estimated that 150–200 million tons of mineral nitrogen are required annually for plant growth, of which almost 100 million tons of nitrogen are fixed via the industrial Haber–Bosch process,Citation4) and 175 million tons of atmospheric nitrogen are fixed biologically.Citation5) The ability of legumes to fix nitrogen reduces the need to supply crops with synthetic nitrogen fertilizers and, hence, the negative environmental impacts of fertilizer application.Citation6) The processes of symbiotic nitrogen fixation and transformation of molecular nitrogen into a form available to the plant are more efficient in varieties of soybean than in other plants.Citation7)
BNF is the most important process after photosynthesis. It requires adenosine triphosphate, produced by oxidative degradation of sugars and related molecules, as an energy source for reduction of atmospheric nitrogen.Citation8) The host soybean plant supplies the bacteria with carbohydrates in the form of sugars and related molecules, produced by photosynthesis, which are transferred to the nodules.Citation9) Thus, the rhizobial bacteria in the nodules receive energy and produce forms of nitrogen that can be assimilated by the host plant.Citation10)
The efficiency of BNF depends on the type and variety of crop, rhizobial strain, and the interactions between bacterial strains and plant varieties, as well as on management practices and environmental factors. Considerable genetic variation in nodule number and weight has been observed in leguminous crops, including soybean, and these traits are associated with variation in BNF ability. Recent studies showed differences in nitrogen fixation between soybean genotypes.Citation11)
Until 1980, Bradyrhizobium japonicum was considered to be the sole symbiont of soybean.Citation12) Since then, the isolation of rhizobia from locations around the world in combination with an increased availability of molecular techniques for analysis has enabled the identification and/or classification of new rhizobial symbionts of soybean. In addition, fast growing organisms that develop nitrogen-fixing nodules in soybean, including Rhizobium sp. NGR234,Citation13) Sinorhizobium fredii,Citation14) Sinorhizobium xinjiangensis,Citation15) and Mesorhizobium thianshanense,Citation16) were isolated. Soybean plants display a wide range of responses to rhizobial infection: some cultivars are fully incompatible with rhizobia, whereas others may restrict nodulation.Citation17) This high diversity means soybean cultivars may be grouped according to whether they are high or low nodulating.Citation18)
The BNF capability of soybean depends mostly on the mass of the root nodules and the specific activity of the NA enzyme produced by bacteria.Citation19–21) Bohrer and HungriaCitation21) observed that the nodulation capacity of soybean cultivars belonging to different maturity groups not only differed in nodulation response but also that cultivars could be grouped into those with high and low nodulation capacity. The Bangladesh Agricultural Research Institute (BARI) developed different soybean genotypes. Some of these are waiting for cultivation at the farmers’ level and have not yet been screened with respect to nodulation, nitrogen fixation, and yield characteristics in field conditions. There is scope to enhance the crop yield of soybean by improving nodulation, and thereby nitrogen fixation, by using effective nitrogen-fixing bacteria as inoculants by applying them to the seed or soil. This method provides the possibility of increasing production by exploiting better colonization of the roots and, hence, reducing the need of farmers to apply costly nitrogenous fertilizer. The use of high yielding soybean genotypes combined with effective rhizobia strains can enhance production. Therefore, an experiment was conducted to study the nodulation capacity of isolated Rhizobium sp. BARIRGm901 on newly released soybean genotypes and also to measure the effective nitrogen-fixing ability, growth, and yield of plants under field conditions.
Materials and methods
Bacteria isolation, PCR amplifications, and cloning of 16S rDNA
Rhizobium (Rhizobium sp. BARIRGm901) was isolated from soybean plants at the research farm of the BARI in the coastal area of Noakhali, Bangladesh. Nodules were collected aseptically and crushed in 100 μL of sterile distilled water using a homogenizer. A loop full of suspension was streaked onto yeast extract mannitol (YEM) agar plates and incubated at 30 °C for 2–3 days. A single colony was isolated and purified by repeated streaking on YEM agar plates. These colonies were preserved on agar slants at 4 °C until further use. Isolated bacteria were cultured in test tubes (Pyrex®, USA) containing 3 mL in YEM liquid broth by shaking in a rotary shaker (KSI-100L Shaking Incubator, Koencon, Hanam, Korea) at 180 rpm, 30 °C, for 48 h, and the cultures were centrifuged at 18,000 g for 5 min at 4 °C. Genomic DNA was extracted from the pellet using the FastDNA SPIN Kit (MP Biomedicals, Santa Ana, CA, USA), and DNA yield was quantified using a spectrophotometer (NanoDrop 2000C, Thermo Scientific, Waltham, MA, USA). The extracted bacterial DNA was used as a template for PCR amplification of 16S rDNA.
Bacterial 16S rDNA was amplified by PCR with the forward primer fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer rD1 (5′-AAGGAGGTGATCCAGC-3′). About 50 ng of template DNA was used in each reaction. The PCR conditions and primer sequences used for sequencing are shown in Table S1 (supplementary online information). PCR products were gel purified using the QIAEX®II Gel Extraction Kit (Qiagen GmbH, Hilden, Germany) and ligated into the pGEM-T Easy Vector (Promega, Madison, USA) via TA cloning. The ligation product was transformed into Escherichia coli DH5α competent cells (Tiangen, Beijing, China), according to the protocol of He et al.Citation22)
Sequencing and phylogenetic analysis
DNA sequencing was performed using an Applied Biosystems 3730 automated sequencer with the M13 primer to obtain nearly full-length bacterial 16S rDNA sequences. The bidirectional gene sequences were compiled using DNAMAN software (DNAMAN version 4.11, Lynnon Biosoft, San, Ramon, CA, USA), and the sequences were analyzed using MEGA 5.2 software. The consensus sequence was used in a BLAST search of the NCBI GenBank database. Phylogenetic analysis was conducted using MEGA version 5.2, and a neighbor-joining tree was constructed using Kimura 2-parameter distances with 1000 replicates to estimate bootstrap support. The compiled sequence of the Rhizobium sp. BARIRGm901 strain was deposited in the GenBank database and assigned accession number was KC570944.
Seedling infectivity test
In order to check the infection ability of Rhizobium sp. BARIRGm901 on soybean seedlings roots, Rhizobium was grown in yeast extract mannitol (YEM) broth culture at 30 °C for 48 h and 0.1 mL of this broth culture (1.2 × 108 CFU mL−1) was added to the YEM agar slants tube (60 mL glass test tube, diameter 3 cm) having 1–2 soybean seedlings and kept for 3–5 weeks at room temperature under aseptic condition and monitored nodulation infectivity. The nitrogen-free nutrient solution was supplied as a nutrient source to YEM agar slant tubes twice in a week (Supplementary document 1).
Experimental site
The experiment was conducted at the central farm of the BARI, Gazipur, Bangladesh over two consecutive years, 2010 and 2011. The physical characteristics and chemical status of initial soil at this location are shown in Table . The experimental site is located at the center of the agro-ecological zone (AEZ) of the Madhupur tract (AEZ-28; latitude 24° 23′ N and longitude 90° 08′ E) and has a mean elevation of 8.4 m above mean sea level. The soil belongs to the Chiata series of the Gray Terrace soils (Aeric Albaquept) under the order Inceptisols in the USDA Soil Taxonomy.Citation23) The textural class was clay loam, with a soil pH 6.02 ± 0.08, and the land type was medium high.
Table 1. Properties of initial soil.
Experimental setup
The experiment was laid out in a randomized complete block design with eight treatments and four replicates of each treatment. The treatments included four soybean genotypes, two soybean varieties (Shohag and BARI Soybean6), and two soybean advanced lines (MTD10 and BGM02026), which were each studied with and without Rhizobium (Rhizobium sp. BARIRGm901) inoculation. The seeds of newly released soybean varieties and advanced lines were obtained from the Pulse Research Centre of BARI, Gazipur, Bangladesh.
The unit plots were 2 × 3 m in size, and seeds were planted at 70 kg ha−1. Rhizobium sp. was grown in YEM liquid medium to a population density of 1.4 × 108 (CFU mL−1, estimated by the serial dilution method) and injected into peat soil (100 g packet). This peat-based rhizobial inoculum was used in field experiments at a rate of 1.5 kg ha−1. Soybean seeds were mixed thoroughly with the peat-based inoculum and a small quantity of water before sowing. Fertilizers were used at the rate of 22/42/40/5 kg PKSZn ha−1, respectively, as a basal dose; triple super phosphate, muriate of potash, gypsum, and zinc sulfate were used as the sources of phosphorus, potassium, sulfur, and zinc, respectively. Weeding, thinning, and other intercultural operations were done when necessary. Nodules were collected by carefully uprooting five sample plants selected randomly from each treatment plot at the 50% flowering stage.
Nodules and biomass collection, and soybean harvesting
During harvesting, the nodules were separated aseptically, and nodule properties were studied without damaging roots and biomass. Fresh weights of root and above-ground biomass were measured for each treatment of plants. For dry weight measurements, samples of nodules, root, and above-ground biomass were dried at 70 °C for 72 h. The dried plant samples were ground and stored for further chemical analysis. Five plants were sampled randomly at maturity from each plot, pods were counted for all the five plants, and the average value was reported as number of pods per plant. The soybean pods, stover (leaves with stem), and seeds were separated from plants and measured the fresh weight. The pods, stover, and seeds were dried at 70 °C for 72 h for the measurements of yield parameters.
Soil and plant analysis
To determine soil chemical properties, soil samples were collected, air-dried, and ground (2 mm sieve) before measurement of pH (soil:water, 1:5 w/v, glass electrode pH meter method), electrical conductivity (conductivity meter), total nitrogen (N) (Kjeldahl digestion method), and organic carbon content (Walkley and Black method).Citation24) Soil samples were digested with a di-acid mixture (HNO3:HClO4 3:1 v/v), and extracts were analyzed using inductively coupled plasma-mass spectroscopy (ICP-MS, Perkin Elmer Optima 3300 DV) at the Central Lab, Gyeongsang National University to determine the concentrations of potassium, calcium, and magnesium. The total nitrogen content in soybean was determined from undigested dry samples using an elemental analyzer (Leco CNS-932, Madrid, Spain). Finally, nitrogen uptake was determined as the product of above-ground biomass yield multiply with the respective nitrogen content in above-ground biomass.
Nitrogenase activity of root nodules
Nitrogenase activity in nodules was measured using the acetylene reduction method.Citation25) Whole root systems of two soybean plants were placed into an incubation bottle (500 mL capacity) with a sealed cap, and 10% of the air was replaced with acetylene gas. The bottle was incubated at 30 °C for 30–40 min with periodic shaking. After incubation, 1 mL of the gas sample was injected into the gas chromatograph (GC). Ethylene produced by the nodules was measured using gas chromatography (Shimadzu, GC-2010, Kyoto, Japan) with Porapak Q Columns (Q 80-100 mesh) and a flame ionization detector. Helium and hydrogen were used as the carrier and burning gases, respectively.
Statistical analysis
Statistical analyses were conducted using standard statistical proceduresCitation26) implemented in SAS.Citation27) The data were examined using analysis of variance (ANOVA). Differences between the treatments were determined using ANOVA. Fisher’s protected least significant difference (LSD) was calculated at the 0.05 probability level for comparisons between treatment means.
Results
Rhizobium characterization and nodulation assay
A preliminary morphological and physiological characterization of isolated Rhizobium sp. BARIRGm901 was performed. Colonies of the selected strain varied in shape and appearance, and their diameters ranged from 0.29 to 0.8 mm. The isolate produced mucilaginous substances after incubation for 5 days. This strain showed normal growth under laboratory conditions (20 ± 0.5 and 30 °C; data not shown). The nodulation efficiency of this isolate was examined in soybean seedlings grown in YEM agar slant in glass tubes before the field experiment and nodules were produced in all soybean genotypes roots (Supplemental document 1). All soybean varieties and advanced lines used in the study were able to form nodules within 2–3 weeks of inoculation.
PCR amplification of 16S rDNA and sequence analysis
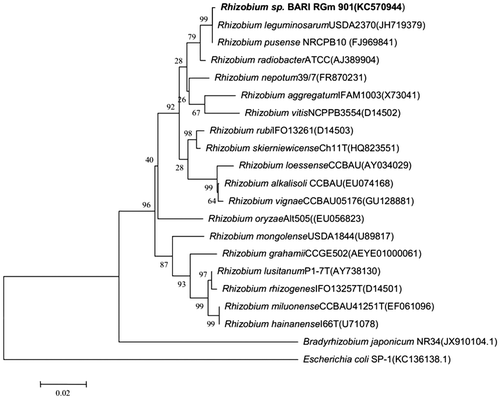
PCR amplification of the 16S rDNA from the Rhizobium sp. BARIRGm901 isolate yielded a 1426 bp product. The nucleotide sequence was deposited in the GenBank database and assigned accession number KC570944. The phylogenetic tree, constructed using the maximum likelihood method, showed both sequence and functional conservation (Fig. ). The separation of the isolate Rhizobium sp. BARIRGm901 from Rhizobium leguminosarum USDA2370 was supported by a bootstrap value of 98%. The isolated rhizobial strain Rhizobium sp. BARIRGm901 in this study belonged to the bacterial order Rhizobiales and the family Rhizobiaceae, and was most similar to Rhizobium leguminosarum.
Fig. 1. Phylogenetic relationship of Rhizobium sp. BARIRGm901 and related organisms were determined by aligning nucleotide sequences derived from a PCR analysis of 16S rDNA recovered from nodules of soybean plants.
Nodulation effect on soybean
Both numbers and weights of nodules were higher in soybean plants inoculated with Rhizobium sp. BARIRGm901 than in non-inoculated plants. Rhizobium-inoculated plants of all genotypes yielded significantly (p ≤ 0.05) higher nodule numbers and weights than non-inoculated plants in both years (2010 and 2011) of the experiment (Table ). Across all treatments, BARI soybean6 + R (“+R” indicates a genotype inoculated with Rhizobium sp. BARIRGm901) produced significantly (p ≤ 0.05) higher nodule numbers and weight than other inoculated and non-inoculated genotypes in both years, with the exception of MTD10 + R and Shohag + R (Table ). In each year, the nodule numbers on all soybean genotypes correlated positively with stover yield (2010: r = 0.691; 2011: r = 0.796) and seed yield (2010: r = 0.743; 2011: r = 0.810) (Table ); however, the dry weight of nodules of all soybean genotypes was positively correlated with stover yield (r = 0.727) and seed yield (r = 0.728) only in 2011 (Table ).
Table 2. Effect of inoculation with Rhizobium sp. BARIRGm901 on nodulation, biomass production, and plant height of soybean genotypes grown in gray terrace soils in 2010 and 2011.
Table 3. Correlation between yield parameters and plant growth characteristics of soybean plants (n = 16) grown in gray terrace soil in 2010 and 2011.
Biomass yield and plant height of soybean
Soybean varieties and advanced lines inoculated with Rhizobium sp. BARIRGm901 produced greater root and shoot weights, and increased plant height, compared with non-inoculated controls in both years of the experiment (Table ). BARI soybean6 + R, BGM02026, and BGM02026 + R produced significantly (p ≤ 0.05) greater shoot biomass in 2010, but BARI soybean6 + R, BARI soybean6, BGM02026, and BGM02026 + R showed significantly (p ≤ 0.05) greater shoot biomass in 2011 compared with other treatments genotypes (Table ). On the other hand, root biomasses were significantly (p ≤ 0.05) higher in BARI soybean6 + R and BGM02026 + R plants in 2010, and in BARI soybean6 + R, BGM02026 + R, MTD10 + R, and MTD10 plants in 2011, compared with other treatments genotypes (Table ). Plant height was significantly (p ≤ 0.05) increased in BARI soybean6 + R and BGM02026 + R plants in 2010, and in BARI soybean6 + R, BARI soybean6, and BGM02026 + R plants in 2011, compared with other treatments genotypes (Table ). Shoot and root biomass, and plant heights, of all soybean genotypes were positively correlated with stover yield (2010: r = 0.983, r = 0.970, and r = 0.987; 2011: r = 0.991, r = 0.719, and r = 0.910) and seed yield (2010: r = 0.771, r = 0.668, and r = 0.902; 2011: r = 0.998, r = 0.709, and r = 0.911) (Table ).
Nitrogenase activity in soybean roots
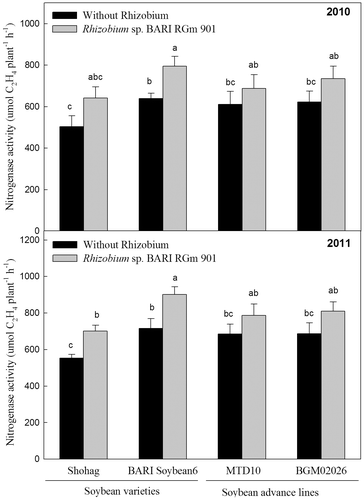
The presence of NA enzyme produced by Rhizobium sp. BARIRGm901 increases nitrogen fixation in root nodules by reducing atmospheric N2 to . Inoculation with Rhizobium influenced NA activity due to the biochemical characteristics of the nodules on soybean roots. Inoculated plants of all genotypes showed significantly higher (p ≤ 0.05) NA activity compared with non-inoculated plants in 2010 (Fig. , upper panel). Similarly, NA activity was significantly higher (p ≤ 0.05) in inoculated soybean varieties and advanced lines compared with non-inoculated plants, except Shohag + R, in 2011 (Fig. , lower panel). In both years, the increase in NA activity due to nodulation by Rhizobium sp. BARIRGm901 on soybean root positively correlated with stover yield (2010: r = 0.988; 2011: r = 0.936) and seed yield (2010: r = 0.986; 2011: r = 0.927) (Table ).
Fig. 2. Effect of Rhizobium sp. BARIRGm901 on nitrogenase activity in soybean genotypes grown in gray terrace soil in 2010 and 2011.
Yield parameters of soybean
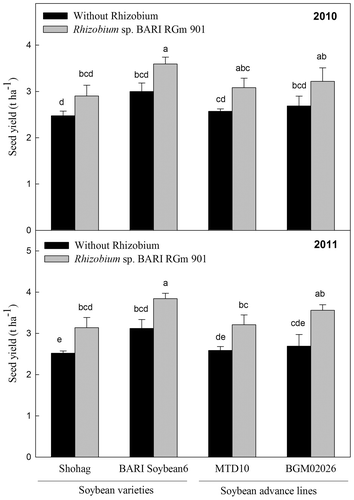
Inoculation of soybean roots with Rhizobium sp. BARIRGm901 resulted in higher pod, stover, and seed yields, compared with yields of non-inoculated plants, in both 2010 and 2011 (Fig. ). BARI Soybean6 + R plants produced significantly (p ≤ 0.05) higher pod yields (2010: 40.94 pods plant−1; 2011: 48.0 pods plant−1) than plants from other treatments and genotypes, other than those of BGM02026 + R in 2011 (Fig. , lower panel). BARI Soybean6 + R plants produced a significantly higher (p ≤ 0.05) stover yield (4.83 t ha−1) than plants from other treatments genotypes in 2010 (Fig. , upper panel). Likewise, in 2011, BARI Soybean6 + R and BGM02026 + R plants showed significantly higher (p ≤ 0.05) stover yield (3.85 t ha and 3.60 t ha−1, respectively) compared with those of other treatments genotypes (Fig. , lower panel). When inoculated with Rhizobium sp. BARIRGm901, three of the four soybean genotypes tested (BARI soybean6 + R, MTD10 + R, and BGM02026 + R) showed significant increases (p ≤ 0.05) in seed yields (3.60, 3.08, and 3.22 t ha−1, respectively) compared with plants of other treatments genotypes in 2010 (Fig. , upper panel). In 2011, plants of BARI soybean6 + R and BGM02026 + R each produced significantly higher (p ≤ 0.05) seed yields (3.84 and 3.56 t ha−1, respectively) compared with other inoculated and non-inoculated soybean varieties and advanced lines (Fig. , lower panel).
Fig. 3. Effect of inoculation with Rhizobium sp. BARIRGm901 on pod yield of soybean genotypes grown in gray terrace soil in 2010 and 2011.
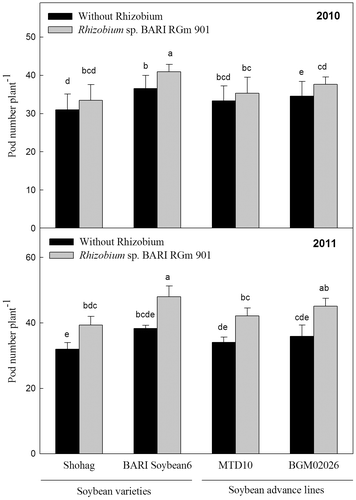
Fig. 4. Effect of inoculation with Rhizobium sp. BARIRGm901 on stover yield of soybean genotypes grown in gray terrace soil in 2010 and 2011.
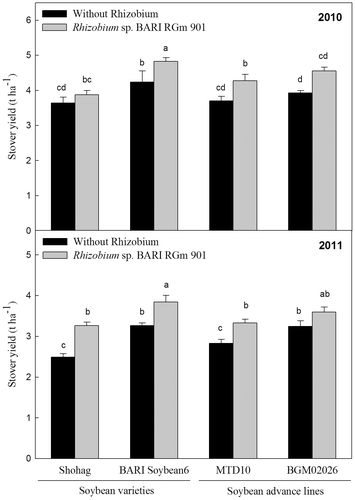
Fig. 5. Effect of inoculation with Rhizobium sp. BARIRGm901 on seed yield of soybean genotypes grown in gray terrace soil in 2010 and 2011.
Nitrogen content and uptake
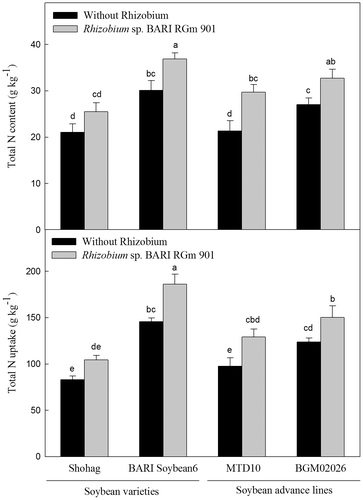
Soybean varieties and advanced lines inoculated with Rhizobium sp. BARIRGm901, grown in 2011, showed higher total nitrogen content and nitrogen uptake than non-inoculated controls. The total nitrogen content of BARI soybean6 + R plants was significantly higher (p ≤ 0.05) than that of plants of other treatments genotypes except for BGM02026 + R plants (Fig. , upper panel). Similarly, BARI soybean6 + R plants showed significantly higher (p ≤ 0.05) levels of total nitrogen uptake compared with other treatments genotypes in 2011 (Fig. , lower panel).
Fig. 6. Total nitrogen (N) content (upper panel) and uptake (lower panel) of above-ground biomass of soybean genotypes grown in gray terrace soil in 2011.
Discussion
The Rhizobium sp. BARIRGm901 strain used in this study was isolated by BARI from a soybean plant grown on a farm in the coastal research area of Bangladesh. Its ability to enter symbiosis with different soybean genotypes was tested. Following inoculation, a huge number of root nodules formed, due to the presence of this effective strain, enabling nitrogen fixation. The relative success of Rhizobium sp. BARIRGm901 probably results from the interaction of many factors affecting rhizobial survival and root colonization, as well as competition for nodule sites. There were significant differences between plant genotypes in their acceptance of Rhizobium sp. BARIRGm901. Soybean genotypes released by BARI showed similar levels of Rhizobium sp. BARIRGm901 in their nodules, and specific interactions among the soybean genotypes were closely related to nodule formation. Pazdernik et al.Citation28) also proposed that soybean genotypes might be used with specific isolates. Plant hosts may, therefore, differ in their capacity to select and uptake Rhizobium sp. BARIRGm901. The number and weight of nodules increased in inoculated soybean genotypes, as an almost twofold increase in nodule number was observed in inoculated plants relative to non-inoculated plants of the same genotype. Sinclair et al.Citation29) and Hungria and BohrerCitation30) showed a twofold increment in nodule number in high nodulation cultivars over those nodulating the least. It is likely, therefore, that the twofold difference in nodule number observed in this study resulted from an increase in NA activity of Rhizobium sp. BARIRGm901 in root nodules. Plants of all genotypes inoculated with Rhizobium sp. BARIRGm901 produced higher nodule dry mass than non-inoculated plants. Taken together, these findings suggest that nodule number and their dry weight might assist breeders in selecting genotypes based on nitrogen fixation.Citation30)
The significant increases in above-ground and root biomass of inoculated soybean genotypes observed in 2010 and 2011 might result from a high association with symbiotic Rhizobium sp. BARIRGm901 that promoted plant growth. JonesCitation31) showed that higher nodulation and biomass yields of inoculated plants could be attributed to high nitrogen fixation incorporated into nitrogen biosynthesis. Specific interactions were also observed between nodule formation in individual genotypes of soybean and Rhizobium sp. BARIRGm901: the BARI soybean6 variety showed a high affinity with this Rhizobium strain, producing high levels of nodulation across the whole root system following inoculation (Table ). Our results are consistent with previous findings of high levels of nodulation in soybean genotypes following inoculation with commercial B. japonicum.Citation30)
Rhizobium is a vital micro-organism in the process of nitrogen fixation because of its effect on the activities of NA.Citation32) Inoculated plants of different genotypes usually displayed significantly higher (p ≤ 0.05) levels of NA activity than non-inoculated controls, with the exception of the Shohag genotype in 2011. Pramanik et al.Citation33) determined that Rhizobium has a strong stimulatory effect on NA enzymes, and enzyme activity increases, in inoculated plants. Nitrogenase activity in inoculated soybeans showed a positive correlation with stover and seed yield in both years (Table ). Experiments with soybean and common bean (Phaseolus vulgaris) also showed that inoculated plants have increases in the rates of NA activity and nodule formation due to an enhanced nitrogen-fixing symbiosis.Citation34)
Inoculation of soybean varieties and advanced lines increased yield parameters, as high levels of nitrogen fixation increased the production of protein molecules that contributed to yield properties. The superior response of BARI Soybean6 to inoculation, and its higher pod, stover, and seed yields compared with other inoculated genotypes or non-inoculated plants, might result from a superior compatibility with Rhizobium sp. BARIRGm901 that enabled high levels of nodulation and NA activity, leading to maximum growth and productivity. RubenDario et al.Citation30) observed that the commercial strain B. japonicum improved nodulation capacity of Argentinean commercial soybean and contributed to a higher yield. The highest nitrogen contents and uptake were recorded in inoculated soybeans might be attributed to the greater ability of these plants to fix and assimilate nitrogen.Citation31,35) Inoculation of newly released soybean varieties and advanced lines with newly isolated Rhizobium sp. BARIRGm901 under experimental conditions, therefore, provided a means to determine the perfect symbiosis, as the plant genotypes that were most compatible with this strain in the test conditions of gray terrace soils would perform best. This compatibility should be considered when introducing Rhizobium sp. BARIRGm901 strain into soybean varieties in new areas.
In conclusion, plants of different soybean varieties and advanced lines inoculated with Rhizobium produced higher nodule numbers, nodule weights, above-ground biomass, root biomass, and plant height than non-inoculated plants in both 2010 and 2011, indicating that Rhizobium sp. BARIRGm901 effectively enhanced nodulation and growth parameters across a range of genotypes. Nitrogenase activity was increased in inoculated plants, and these increases probably contributed to the high levels of nitrogen fixation and nitrogen assimilation found in these plants. Soybean plants inoculated with Rhizobium sp. BARIRGm901 showed higher pod yield, stover yield, and seed yield than non-inoculated plants in both 2010 and 2011, revealing the positive impact of Rhizobium sp. BARIRGm901 on yield parameters of soybean. Therefore, Rhizobium sp. BARIRGm901 established an effective symbiotic association with soybean and was responsible for increased growth and biomass, and improved yield characteristics, of a range of soybean genotypes of diverse genetic background in gray terrace soils.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1044931.
Author contribution
Conceived and designed the experiments: FA, MAHB. Performed the experiments: FA, MAHB. Analyzed the data: FA, MAHB. Contributed bacterial gene sequencing: FA, SSA, PJK, YBL. Contributed reagents/materials/analysis tools: FA, MAHB, SSA, TRW, PJK, YBL. Contributed in writing and improvements the manuscript: FA, MAHB, SSA, TRW, PJK, YBL.
Supplemental Materials
Download MS Word (583.8 KB)Additional information
Funding
Notes
Abbreviations: AEZ, Agro-ecological zone; BARI, Bangladesh Agricultural Research Institute; BNF, biological nitrogen fixation; FW, fresh weight; GC, gas chromatograph; NA, nitrogenase; R: Rhizobium.
References
- Ohyama T, Minagawa R, Ishikawa S, Yamamoto M, Hung NVP, Ohtake N, Sueyoshi K, Sato T, Nagumo Y, Takahashi Y. Soybean seed production and nitrogen nutrition. In: Board JE, editor. A comprehensive survey of international soybean research-genetics, physiology, agronomy and nitrogen relationships. Rijeka: InTech; 2013. p. 115–157.
- Burris RH, Roberts GP. Biological nitrogen fixation. Annu. Rev. Nutr. 1993;13:317–335.10.1146/annurev.nu.13.070193.001533
- Prakamhang J, Tittabutr P, Boonkerd N, Teamtisong K, Uchiumi T, Abe M, Teaumroong N. Proposed some interactions at molecular level of PGPR co-inoculated with Bradyrhizobium diazoefficiens USDA110 and B. japonicum THA6 on soybean symbiosis and its potential of field application. Appl. Soil Ecol. 2015;85:38–49.10.1016/j.apsoil.2014.08.009
- Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey R, Giller K, Alves B, Chalk P. Measuring plant-associated nitrogen fixation in agricultural systems. Canberra, Australia: ACIAR; 2008. http://www.aciar.gov.au/publication/MN136
- Chafi MH, Bensoltane A. Vicia faba (L), a source of organic and biological manures for the Algerian arid regions. World J. Agric. Sci. 2003;5:698–706.
- Lupwayi NZ, Kennedy AC, Chirwa RM. Grain legume impacts on soil biological processes in sub-Saharan Africa. Afr. J. Plant Sci. 2010;5:1–7.
- Brewin JN. Root nodules (Legume-Rhizobium symbiosis). Norwich: Innes Centre; 2010.
- Hubbell DH, Kidder G. Biological nitrogen fixation. University of Florida IFAS Extension Publication, Florida SL16; 1–4 2009.
- Rao NSS. Recent advances in biological nitrogen fixation. New Delhi: Oxford and IBH; 1980.
- Tripathl BR, Psychas PJ. The AFNETA alley farming training manual-Volume 2: source book for alley farming research. Addis Ababa: International livestock centre for Africa; 1992.
- Abi-Ghanem R, Bodah ET, Wood M, Braunwart K. Potential breeding for high nitrogen fixation in Pisum sativum L.: germplasm phenotypic characterization and genetic investigation. Am. J. Plant Sci. 2013;4:1597–1600.10.4236/ajps.2013.48193
- Jordan DC. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int. J. Syst. Bacteriol. 1982;32:136–139.10.1099/00207713-32-1-136
- Saldaña G, Martinez-Alcántara V, Vinardell JM, Bellogín R, Ruíz-Sainz JE, Balatti PA. Genetic diversity of fast-growing rhizobia that nodulate soybean (Glycine max L. Merr). Arch. Microbiol. 2003;180:45–52.10.1007/s00203-003-0559-y
- Keyser HH, Bohlool BB, Hu TS, Weber DF. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982;215:1631–1632.10.1126/science.215.4540.1631
- Peng GX, Tan ZY, Wang ET, Reinhold-Hurek B, Chen WF, Chen WX. Identification of isolates from soybean nodules in Xinjiang region as Sinorhizobium xinjiangense and genetic differentiation of S. xinjiangense from Sinorhizobium fredii. Int. J. Syst. Evol. Microbiol. 2002;52:457–462.
- Chen W, Wang E, Wang S, Li Y, Chen X, Li Y. Characteristics of Rhizobium tianshanense sp. nov., a moderately and slowly growing root nodule bacterium isolated from an arid saline environment in Xinjiang, People’s Republic of China. Int. J. Syst. Bacteriol. 1995;45:153–159.10.1099/00207713-45-1-153
- Van K, Kim MY, Lee SH. Genomic of root nodulation. Genomics assisted crop improvement. 2007;2:435–452.10.1007/978-1-4020-6297-1
- Balatti PA. Competition an old unsolved problem of rhizobia nodulating soybean. The old unsolved problem of competition. Proceedings of the VII world soybean research conference; 2004 Feb 5; Foz do Iguazú.
- Hungria M, Bohrer TRJ. Variability of nodulation and dinitrogen fixation capacity among soybean cultivars. Biol. Fertil. Soils. 2000;31:45–52.10.1007/s003740050622
- Stougaard J. Regulators and regulation of legume root nodule development. Plant Physiol. 2000;124:531–540.10.1104/pp.124.2.531
- Bohrer TRJ, Hungria M. Evaluation of soybean cultivars for biological nitrogen fixation. Pesq. Agropec. Bras. 1998;33:937–953.
- He J, Zhang L, Jin S, Zhu Y, Liu F. Bacterial communities inside and surrounding soil iron-manganese nodules. Geomicrobiol. J. 2008;25:14–24.10.1080/01490450701829014
- Land and Soil Resources use guidelines. Gazipur central sub-district, soil resources development institute. Ministry of Agriculture: Government of the People’s Republic of Bangladesh; 2005.
- Allison LE. Organic carbon. In: Black CA, editor. Methods of soil analysis. Part II. Madison (WI): American Society of Agronomy; 1965. p. 1367–1376.
- Somasegaran P, Hoben HJ. Handbook for Rhizobia: methods in legume Rhizobium technology. New York, NY: Springer-Verlag; 1994.
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2nd ed. New York: John Wiley and Sons; 1984.
- SAS Institute. System for windows release 9.1. Cary (NC): SAS Institute; 2003.
- Pazdernik DL, Vance CP, Sadowsky MJ, Graham PH. A host-controlled, serogroup-specific, ineffective-nodulation system in the bradyrhizobium-soybean (Glycine max) symbiosis. Mol. Plant-Microbe Interact. 1997;10:994–1001.10.1094/MPMI.1997.10.8.994
- Sinclair TR, Soffes AR, Hinson K, Albrecht SL, Pfahler PL. Genotypic variation in soybean nodule number and weight. Crop Sci. 1991;31:301–304.10.2135/cropsci1991.0011183X003100020014x
- RubénDarío S, Monica A, Mariangela H, Pedro Alberto B. Nodulation capacity of Argentinean soybean (Glycine max L. Merr) cultivars inoculated with commercial strains of Bradyrhizobium japonicum. Am. J. Plant Sci. 2011;3:130–140.
- Jones JBJ. Plant nutrition manual. 1st ed. Boca Raton (FL): CRC Press; 1998. p. 149.
- Mendel RR, Hänsch R. Molybdoenzymes and molybdenum cofactor in plants. J. Exp. Bot. 2002;53:1689–1698.10.1093/jxb/erf038
- Pramanik P, Haque MM, Kim PJ. Effect of nodule formation in roots of hairy vetch (Vicia villosa) on methane and nitrous oxide emissions during succeeding rice cultivation. Agric. Ecosyst. Environ. 2013;178:51–56.10.1016/j.agee.2013.06.021
- Vieira RF, Cardoso EJBN, Vieira C, Cassini STA. Foliar application of molybdenum in common beans. I. Nitrogenase and reductase activities in a soil of high fertility. J. Plant Nutr. 1998;21:169–180.10.1080/01904169809365391
- Raven PH, Evert RF, Eichhorn SE. Biology of plants. 6th ed. New York, NY: W.H. Freeman and Company; 1999. p. 686.
