Abstract
Phytases comprise a group of phosphatases that trim inorganic phosphates from phytic acid (IP6). In this study, we aimed to achieve the efficient secretion of phytase by Bacillus subtilis. B. subtilis laboratory standard strain 168 and its derivatives exhibit no phytase activity, whereas a natto starter secretes phytase actively. The natto phytase gene was cloned into strain RIK1285, a protease-defective derivative of 168, to construct a random library of its N-terminal fusions with 173 different signal peptides (SPs) identified in the 168 genome. The library was screened to assess the efficiency of phytase secretion based on clear zones around colonies on plates, which appeared when IP6 was hydrolyzed. The pbp SP enhanced the secretion of the natto phytase most efficiently, i.e. twice that of the original SP. Thus, the secreted natto phytase was purified and found to remove up to 3 phosphates from IP6.
Graphical abstract
The signal peptide of pbp (C) enhanced the secretion of natto phytase up to twofold more efficiently than the original one of the natto starter.
Inositol stands for a group of compounds with a structure of 1,2,3,4,5,6-hexahydroxy-cyclohexane. The epimerization of the 6 hydroxyl groups generates 9 stereoisomers, the most prominent form of which in nature is myo-inositol (MI). MI plays an important role as a moiety for a number of secondary messengers in eukaryotic cells, various inositol phosphates. In addition, MI serves as an important component of the membrane structural phospholipid, phosphatidylinositol. Furthermore, some other isomers are known to have specific biological activities. For example, D-chiro-inositol (DCI) prevents hyperglycemia due to its insulin-like activityCitation1–3) and it is also known to improve polycystic ovary syndrome, which is one of the most common endocrine disorders in women.Citation4) In addition, another isomer, scyllo-inositol (SI), is regarded as a candidate therapeutic agent for treating Alzheimer’s diseaseCitation5,6) because it can inhibit the aggregation of amyloid β protein, which is involved in the development of Alzheimer’s disease. However, these isomers are relatively rare in nature and difficult to produce and thus are insufficiently available to satisfy demand.
Previously, we reported bioconversion methods for producing DCI and SI from MI using metabolically engineered Bacillus subtilis.Citation7,8) To further improve these production methods, we hypothesize that MI hexakisphosphate, which is generally known as phytic acid (IP6), can be used as an alternative starting material for bioconversion because IP6 is abundant in cereals and legumes, as a major storage form of phosphorous.Citation9,10) For example, rice bran is rich in IP6 and it is one of the most abundant agricultural wastes, and thus one of the most inexpensive raw materials as a source of IP6.Citation11) In order to obtain MI, all 6 phosphate groups in IP6 must be removed chemically or enzymatically.
Phytases comprise a group of enzymes that can hydrolyze IP6 into less-phosphorylated MI and inorganic phosphates, which have industrial value as additives in nonruminant animal feed, often for poultry and swine, thereby enhancing the nutritive value of plant materials.Citation10,12,13) It has been shown that phytase is produced by various microorganisms.Citation14–17) B. subtilis is a model gram-positive bacterium and the entire nucleotide sequence of laboratory standard strain 168 genome has been determined,Citation18,19) thereby facilitating its efficient and simple genetic manipulation. A gene that possibly encodes a phytase, phy, has been found in the genome of strain 168, which suggests the potential use of B. subtilis for producing phytase. However, strain 168 exhibits no phytase activity,Citation3) whereas some other strains such as the natto starters used to produce the traditional Japanese food of fermented soybean nattoCitation20) may have phytase activity.
B. subtilis has been used for protein secretion during the industrial production of enzymes, including amylase and cellulase. An enzyme secreted by B. subtilis must possess a specific signal peptide (SP) at its N-terminus as the structural and functional determinant, which is recognized by the secretion machinery and cleaved by signal peptidases during secretion.Citation21,22) A large number of previous studies have demonstrated that an appropriate SP must be selected and added to heterologous proteins to allow them to be secreted efficiently.Citation23–28) Thus, in this study, we aimed to develop a strain of B. subtilis that secretes a phytase derived from a natto starter.
Materials and methods
Bacterial strains, plasmids, and oligonucleotides
The bacterial strains and plasmids used in this study are summarized in Table . A B. subtilis natto starter isolated from a commercial product (Asahimatsu Foods, Osaka, Japan) was selected as the source of the active phytase gene. B. subtilis strain RIK1285 (Takara Bio, Otsu, Japan) is a protease-defective derivative of strain 168, which was used as the host to express the heterogeneous phytase. The pBE-S plasmid vector (Takara Bio) contains the pUB110-derived replication origin and a kanamycin resistance gene that functions in B. subtilis, as well as the pUC-derived replication origin and an ampicillin resistance gene that functions in Escherichia coli. This vector was designed to clone a gene for a secretory enzyme under the control of the aprE promoter and it was fused with the secretory SP in the N-terminus, as well as possessing a His6-tag in the C-terminus. E. coli C600 was used as the cloning host for DNA manipulation. The oligonucleotides used in this study are listed in Table .
Table 1. Bacterial strains and plasmids used in this study.
Table 2. Oligonucleotides used in this study.
Media and growth conditions
In general, the bacterial strains were grown in Luria broth (LB) (Becton Dickinson, NJ) medium at 37 °C in the presence or absence of appropriate antibiotics, i.e. 100 μg/mL ampicillin and 10 μg/mL kanamycin. Phytase secretion was assayed using phytase screening medium (PSM) agar plates [0.1% (w/v) K2HPO4, 0.6% KH2PO4, 0.1% Na3 citrate·2H2O, 0.02% MgSO4·7H2O, 0.2% K2SO4, 0.02% glutamine, 0.5% IP6 sodium salt (pH 6.5) (Sigma-Aldrich, St Louis, MO), 1.5% glucose, 0.3% (NH4)2SO4, 0.05% KCl, 0.01% lysine, 0.005% tryptophan, 0.2% CaCl2·2H2O, 0.05% MgSO4·7H2O, 0.015% MnSO4·5H2O, 0.01% FeSO4·7H2O]. In the liquid cultures used to produce phytase, B. subtilis strains were grown for 24 h at 37 °C with shaking at 200 rpm in various media, including 2 types of commercially available premixed LB media (Becton Dickinson and Nacalai Tesque, Kyoto, Japan), which were also supplemented with 1.5% (NH4)2SO4 and 1.5% Bacto yeast extract (Becton Dickinson) (referred to as modified LB); soytone medium [1% Bacto soytone (Becton Dickinson), 0.5% Bacto yeast extract, and 1% MI (Sigma-Aldrich)]; IP6 medium [1.5% Bacto yeast extract, 1.5% (NH4)2SO4, 2.5% IP6 sodium salt (pH 6.5), 0.025% MgSO4·7H2O, and 0.26% CaCl2]; and rice bran medium [1.5% Bacto yeast extract, 1.5% (NH4)2SO4, 5% rice bran (Boso oil and fat, Chiba, Japan), 0.025% MgSO4·7H2O, and 0.26% CaCl2].
Construction of a plasmid library of phytase fused with various SPs
A DNA fragment that corresponded to a part of the coding region of phy, but without its native SP region in the N-terminus, was amplified by PCR from the genomic DNA of the natto starter strain, using a specific pair of primers (phyM-F-NdeI and phy-R-HindIII) (Table ) to generate the restriction sites at both ends. The fragment was trimmed with the respective restriction enzymes and ligated with the arms of the plasmid pBE-S, which had been previously cleaved with the same enzymes to yield the recombinant plasmid pBE-S-phySPaprE, containing the mature phytase fused with the aprE SP and the His6-tag at the N- and C-termini, respectively, for expression under the control of the aprE promoter. The recombinant plasmid was cleaved with MluI and Eco52I to replace the aprE SP randomly with one of the 173 different SPs identified in the genome of strain 168,Citation23) thereby yielding a mixture of recombinant plasmids, using an In-Fusion HD Cloning Kit (Takara Bio) and a B. subtilis Secretory Protein Expression System Kit (Takara Bio). E. coli C600 was transformed with the plasmid mixture to obtain thousands of independent colonies on LB plates containing ampicillin. All of the colonies on the plates were collected and mixed, and an aliquot of the mixture was used to inoculate liquid LB containing ampicillin to prepare a library of recombinant plasmids.
Another fragment that corresponded to the entire original coding region of phy was amplified from the natto starter using a specific pair of primers (phySPN-F-MluI and phySPN-R-XbaI) (Table ) to generate the restriction sites at both ends. The fragment was trimmed with the respective restriction enzymes and ligated with the arms of the plasmid pBE-S, which had been previously cleaved with the same enzymes, to yield the recombinant plasmid pBE-S-phySPN. This harbored the original phytase fused with the His6-tag at the C-terminus, and it was expressed under the control of the aprE promoter.
Screening for efficient SPs during phytase secretion
Strain RIK1285 was transformed with the plasmid library, which was prepared as described above. A single transformation was performed to obtain thousands of colonies on LB plates containing kanamycin, before hundreds of independent colonies were replicated randomly on the PSM plates. After 4 days of incubation at 37 °C, the clear zones around the colonies corresponded to the active secretion of phytase, where we selected clones with larger clear zones.
Sequencing analysis
Using the genomic DNA extracted from B. subtilis cells, the phy promoter regions were amplified with the primer set Phy-PF and Phy-PR (Table ). The phy coding regions were amplified using the primer set PhyALL-F and phyALL-R (Table ). Sequencing was performed with one of the primers used for PCR and with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA), according to the manufacturer’s protocols. The sequence determined for the natto starter is available in the DDBJ/EMBL/GenBank databases under accession number LC012840. Using the plasmid DNA, the SP region was amplified with the primer set pBE-S-F and pBE-S-R (Table ) and used as a template for sequencing analysis with the primer pBE-S-F (Table ).
Assay and detection of secreted phytase
The B. subtilis strains were cultivated in 100 mL of the rice bran liquid medium in a 500-mL baffled flask at 37 °C for 24 h with shaking at 200 rpm. After centrifuging an aliquot of the culture, 130 µL of the supernatant was mixed with 20 µL of 1 M Tris–HCl (pH 7.0), 40 µL of 500 mM IP6 sodium salt (pH 6.5), and 10 µL of 500 mM CaCl2, which was then incubated at 50 °C for 20 min, before the reaction was stopped by adding 20 µL of 30% trichloroacetic acid. After centrifugation, the supernatant was employed to obtain spectrophotometric measurements of the released inorganic phosphate, as described previously.Citation20) The culture supernatant was subjected to 5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting analysis to probe the phytase fused with the His6-tag, using anti-6×His mouse IgG-к (Nacalai Tesque) and goat anti-mouse IgG-HRP (Nacalai Tesque) as the primary and secondary antibodies, respectively.
Enzyme purification
Strain TSU004 (Table ) was cultivated in 100 mL of the rice bran liquid medium in a 500-mL baffled flask at 37 °C for 24 h with shaking at 200 rpm. After centrifugation, the supernatant was concentrated twice by spinning in an Amicon Ultra-15 (Merck Millipore, Billerica, MA), mixed with the same volume of 2× binding buffer (1 M NaCl, 10 mM imidazole, and 40 mM Tris–HCl; pH 7.4), and applied to a TALON (cobalt) column (Takara Bio). The column was washed twice with 1× buffer (0.5 M NaCl, 5 mM imidazole, and 20 mM Tris–HCl; pH 7.4), and the bound His6-tag fused phytase was then eluted with elution buffer (0.5 M NaCl, 200 mM imidazole, and 20 mM Tris–HCl; pH 7.4).
High-pressure capillary ion chromatography
The purified phytase was incubated with 100 mM IP6 sodium salt (pH 6.5) under the enzyme assay conditions. Aliquots of the reaction mixture were extracted at the indicated time points and frozen immediately at −20 °C to terminate the reaction. After thawing, the sample was diluted 1000 times with pure water and a small aliquot (10 µL) was injected into a high-pressure capillary ion chromatograph using a Dionex ICS-1600 (Thermo Scientific, Sunnyvale, CA) with a Dionex ASRS-300 suppressor and a Dionex IonPac AG11/Dionex IonPac AS11 column, which was kept at 35 °C, and eluted by a constant flow of KOH at 1.2 mL/min with a linear gradient of 8–65 mM for 45 min and then at 65 mM for 5 min.
Results
The phy gene of the natto starter
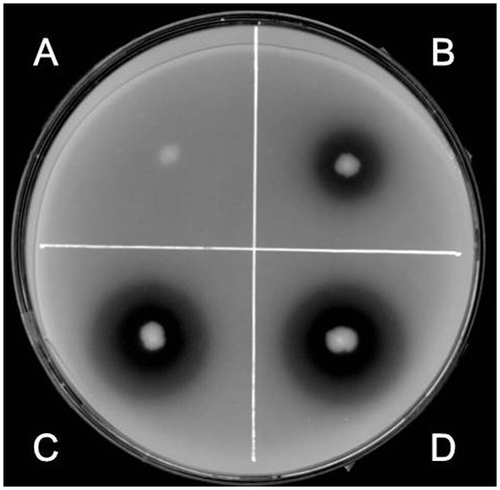
In order to assess phytase secretion, we used PSM plates, upon which the secreted phytases dephosphorylated IP6 to solubilize the IP6-Ca2+ powder present in the agar, thereby forming clear zones around the bacterial colonies (Fig. ). Colonies of B. subtilis strain TSU001 without the active phytase gene (Table ) did not produce clear zones (Fig. A). On the other hand, colonies of the natto starter isolated from a commercial product (Asahimatsu Foods) yielded distinct clear zones, which indicated the active secretion of phytase (data not shown). The natto starter produced viscous slime materials containing polyglutamic acid, which prevented us from characterizing the native natto phytase.
Fig. 1. Clear zones formed on PSM plates.
Notes: After 4 days of incubation on PSM plates, clear zones were observed around colonies and their diameters were measured to compare the secretory activities. The strains used were TSU001 (A), TSU002 (B), TSU004 (C), and TSU005 (D).
In strain 168 and its derivatives (including RIK1285), the phy gene encoding phytase is considered to be cryptic because its promoter was reported to be nonfunctional.Citation3) Thus, the phy locus of the natto starter was sequenced for comparison. As shown in Fig. A, strain 168 and the natto starter shared high similarity in their phy loci, but the promoter regions differed in 8 points: 1 deletion and 7 substitutions, including one within the −10 region, which may damage the promoter activity in 168. In addition, the translated amino acid sequences differed at 3 residues (Fig. B). These substitutions may cause differences in the activity and/or secretion of the enzyme, although they were not examined experimentally.
Fig. 2. Comparison of the nucleotide sequences of the promoter regions (A) and amino acid sequences of the gene products (B) in B. subtilis strain 168 and the natto starter.
Notes: (A) The nucleotide sequences of the phy promoter regions in 168 (embl/genbank/ddbj ID: gb CP010052.1) and the natto starter (natto) were aligned for comparison using CLUSTALW (http://clustalw.ddbj.nig.ac.jp/). Differences between residues are shown as white letters on a black background. The –35 and –10 regions of putative promoter and N-terminal part of the coding region (ORF) are boxed as indicated. (B) The amino acid sequences of the phy gene products were compared in a similar manner.
Selection of SPs to facilitate the efficient secretion of natto phytase
First, using the natto starter, the phy gene was cloned to construct the recombinant plasmid pBE-S-phySPaprE, where the mature phytase was fused with the aprE SP and the His6-tag at the N- and C-termini, respectively, and it was expressed under the control of the aprE promoter. Strain TSU002 harbored pBE-S-phySPaprE and it formed a small clear zone on the PSM plate (Fig. B), thereby indicating its ability to secrete phytase. In pBE-S-phySPaprE, the aprE SP was randomly replaced with 173 different SPs from B. subtilisCitation23) to yield the library of recombinant plasmids, as described above. RIK1285 was transformed with the library to obtain more than 3000 colonies and 700 independent colonies were replicated randomly on the PSM plates to select 38 clones that formed larger clear zones. The sequencing analysis of the SP region of the pBE-S-phySPaprE derivatives from the 38 clones allowed us to identify 13 different additional SPs (Table ), some of which were redundant; thereby, suggesting that the screening process was saturated. Some isolates produced larger clear zones than strains TSU002 and TSU003 (Table ), where that expressing the pbp SP fusion (designated as strain TSU004) produced the largest zone (Fig. C) and its plasmid was designated as pBE-S-phySPpdp. The sizes of the clear zones did not correlate with the D-score, calculated charge, or hydrophobicity of the SP, which implies that none of these parameters was reliable in predicting the efficiency of the SP in phytase secretion (Table ).
Table 3. Efficient SPs during phytase secretion.
Effects of nutritional conditions on phytase secretion
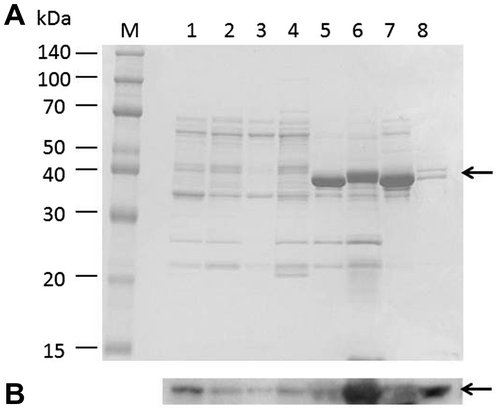
The PSM used for screening contained a large amount of inorganic phosphate, so it was unsuitable for measuring the amounts of phosphate released from IP6 after the hydrolysis catalyzed by the secreted phytase. To select the appropriate medium for further analysis, strain TSU004 was cultivated in various media, including LB (Difco), modified LB (Difco), LB (Nacalai Tesque), modified LB (Nacalai Tesque), IP6 medium, rice bran medium, soytone medium, and PSM. The modified LB media contained (NH4)2SO4 and a larger amount of yeast extract, which have been reported to promote phytase secretion.Citation29) The supernatants obtained from the 24-h cultures were subjected to SDS-PAGE and successive immunoblot analyses to probe the His6-tag fused at the C-terminus of the secreted phytase (Fig. ). The secreted phytase was detected at a size of 40 kDa, which indicates that the N-terminal SP was cleaved, as designed. Intriguingly, the rice bran medium facilitated the most efficient secretion of the enzyme (lane 6), which implies that the efficient secretion of phytase may demand specific nutritional conditions. In IP6 and soytone media (lanes 5 and 7), rather thick protein bands appeared on SDS-PAGE. Since the proteins contained in the bands did not react with anti-6×His mouse IgG-к, we did not examine them further.
Fig. 3. Detection of the secreted phytase.
Notes: Strain TSU004 was cultivated in 7 different types of media (1–7). The culture media were analyzed by SDS-PAGE (A) and successive immunoblot (B) using anti-6×His mouse IgG-к antibody. The secreted phytase was detected as the band at about 40 kDa (indicated by arrowheads). M, marker; 1, LB medium (Nakalai); 2, modified LB medium; 3, LB medium (Difco); 4, modified LB medium (Difco); 5, IP6 medium; 6, rice bran medium; 7, soytone medium; and 8, PSM.
Quantification of the activity of the secreted phytase
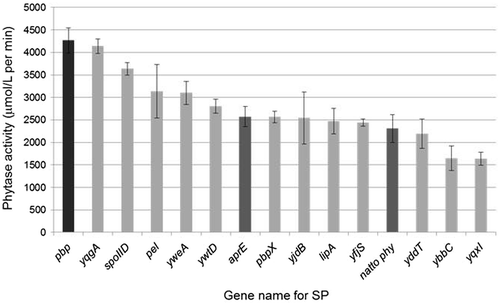
In addition to strains TSU002, TSU003, and TSU004, the other isolates that expressed different SP fusions were cultivated in rice bran medium and we measured the amounts of phytase secreted into the culture medium. The results indicated that isolates with larger clear zones had higher activities (Fig. ). We confirmed that strain TSU004, which expressed the pbp SP fusion, had the highest activity, which was up to twofold higher compared with the activity of strain TSU003, which expressed the original natto phy gene.
Fig. 4. Comparison of the secreted phytase activities with various SP fusions.
Notes: The phytase levels secreted by strains TSU002 (aprE; dark gray bar), TSU003 (natto phy; dark gray bar), TSU004 (pbp; black bar), TSU005 (yqgA; light gray bar), and the other selected clones (their SPs were derived from the gene as indicated; light gray bars) were measured 3 times independently. The values shown represent the mean ± SD.
Analysis of the hydrolysis products from the phytase reaction
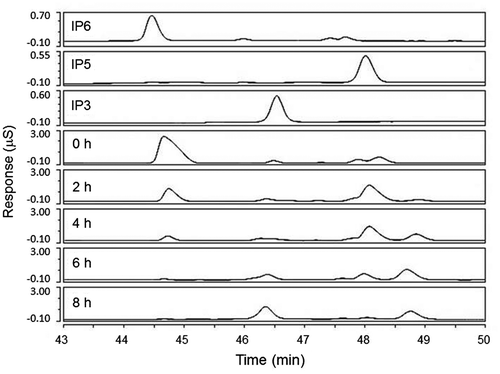
Strain TSU004 was grown in rice bran medium, and the secreted phytase was purified. The purified enzyme was incubated with IP6 and aliquots of the reaction mixture were extracted throughout the time course, before being subjected to high-pressure capillary ion chromatography (Fig. ). As the reaction proceeded, the peak around 44.5 min corresponding to IP6 decreased gradually. The peak around 48 min for MI pentaphosphate (IP5) appeared first, followed by the peak around 49 min for an unidentified compound, and finally, MI triphosphate (IP3) appeared around 46.5 min. After 8 h, IP6 and IP5 disappeared almost completely, and IP3 and the unidentified compound remained, with a greater increase in IP3. The sequential appearance and disappearance of the reaction products implied that the unidentified compound could be MI tetraphosphate, which was not confirmed due to the unavailability of its reference standard compound. Further incubation up to 24 h did not lead to significant changes (data not shown). These results suggest that the secreted natto phytase can remove up to 3 phosphates from 1 IP6 in the conditions employed.
Discussion
B. subtilis strain RIK1285, a derivative of laboratory standard strain 168, exhibited no phytase activity (Fig. A, strain TSU001), whereas the natto starter strain secreted phytase (data not shown). In the sequencing analysis, we compared their phy loci, which detected 1 deletion and 7 substitutions in the promoter region, including a substitution within the −10 region (Fig. A) that could abolish the promoter activity in 168, as reported previously.Citation3) In addition, 3 substitutions were also found in the amino acid residues (Fig. B). The substitution in the N-terminal region is within the SP stretch (the 4th residue was P in 168 but S in natto), which may affect the efficiency of secretion if the enzyme is expressed, since the SP for phy of 168 contained in the tested 173 SPsCitation23) was not selected after the screening. Based on its amino acid sequence, the natto phytase belongs to the β-propeller family. A B. amyloliquefaciens phytase belongs to the same family and its structural analysis identified amino acid residues related to its catalytic activity.Citation31) The 2 substituted residues within the mature enzyme region did not correspond to the residues involved directly in the catalytic activity of the B. amyloliquefaciens enzyme, and thus, the possible significance of the substitution still needs to be examined.
In this study, we expressed the natto phy gene in B subtilis strain RIK1285 to increase the secretion of this enzyme by replacing the SP fused to its N-terminus. We employed screening on PSM plates to detect clear zones that indicated the dephosphorylation of IP6. The 173 different SPs derived from strain 168 were randomly fused to the N-terminus of the phy gene to generate the recombinant plasmid library, which we evaluated. There were apparent significant differences in their capacities for producing clear zones and the isolates that formed larger clear zones (Table ) also secreted higher amounts of phytase into the liquid medium (Fig. ). The pbp SP fusion facilitated the highest secretion of phytase, which was up to twofold higher compared with the original phy gene of the natto starter (Fig. ). In B. subtilis, the secretion of other enzymes, including NprE Citation20) and AprE,Citation23) has been studied intensively and their enhanced secretion was achieved using altered SP fusions,Citation30,32) and thus possible explanations for their efficient secretion have been considered. The D-score is often used to predict the efficiency of a SP in combination with the N-terminal amino acid sequence of the secreted protein.Citation33) D-scores >0.8 may suggest better efficiency, whereas scores <0.5 are regarded as nonfunctional. In addition to the D-score, the calculated charge and hydrophobicity of SPs may also be considered.Citation20) However, for the phytase, the D-scores of the top 3 SPs that we selected were not the highest (0.721 for pbp SP, 0.629 for yqgA, and 0.538 for spoIID), and some of the SPs with higher D-scores (0.835 for yjdB and 0.758 for yddT) did not secrete high levels of phytase (Table ). This also applied to the charge and hydrophobicity of the SPs (Table ). Thus, the efficiency of phytase secretion was not correlated with the D-score, charge, or hydrophobicity of the SP.
In general, a secreted protein is produced in an unfolded state in the cell and the unfolded “preprotein” is recognized by soluble targeting factors to allow its transport to the inside of the cytoplasmic membrane where the protein becomes associated with the translocation machinery. The translocation process is driven by a translocation motor that hydrolyzes ATP, and the protein is transported through a proteinaceous channel in the membrane to the outside. This process results in the release of the transported protein from the translocase, and finally, the protein folds into its native conformation shortly after release from the translocase.Citation34) The SP functions as the transient “zip code,” which is recognized by the cellular sorting and translocation machinery, and thus, it is one of the key determinants of the recognition and translocation steps. After translocation, the SP is finally removed by specialized signal peptidases, which may be required for the efficient maturation of secreted enzymes. The pdp gene encodes a penicillin-binding protein, which shares no significant similarity with natto phytase (data not shown). Thus, we suggest that the pdp SP fusion may streamline all or some of the recognition, translocation, and maturation steps. However, we were not able to elucidate why the pbp SP was so efficient in the secretion of natto phytase.
The purified natto phytase was only able to remove 3 of the 6 phosphate groups from IP6 (Fig. ). Therefore, the natto phytase is not sufficient to produce MI from IP6. Based on the active site geometry and digestive mechanism, phytases are classified into at least 4 families, i.e. histidine acid phosphatase, β-propeller phytase, purple acid phosphatase, and protein tyrosine phosphatase-like phytase families. As mentioned above, the natto phytase is classified into the β-propeller phytase family. Another β-propeller phytase from B. amyloliquefaciens has been structurally characterized and it was shown to possess a property similar to the natto enzyme, where it removed 3 phosphates from IP6.Citation31) It could also remove the additional phosphates but at a very slow rate. Therefore, a limited capacity for removing phosphates from IP6 may be a feature of the β-propeller phytase family, although it might possibly be engineered to extend the capacity by artificial mutagenesis in the future. In addition, an E. coli phytase belonging to the histidine acid phosphatase family was secreted in B. subtilis to dephosphorylate IP6 to MI monophosphate;Citation17) while it was also reported that a Klebsiella pneumoniae enzyme removed all of the phosphates to yield MI.Citation16) These enzymes may be better choices for the production of MI from IP6. However, the problem is that most of the bacterial phytases from the other families require acidic conditions to be active,Citation16,17,35) which are not suitable for B. subtilis. Thus, an alternative strategy must be considered to allow the simultaneous secretion of the natto phytase together with some neutral/alkaline phosphatases with a broader substrate specificity, which could act on IP3 to liberate MI.
Author contribution
STs carried out the molecular genetic studies and participated in drafting the manuscript. TK and STa supervised the high-pressure capillary ion chromatography and enzyme analyses, respectively. KY conceived the study, conducted it, and prepared the final manuscript. All authors read and approved the final manuscript.
Acknowledgments
This study received financial support from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, partly under Special Coordination Funds for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas, the Advanced Low-Carbon Technology Research and Development Program, KAKENHI 26660067, and the NC-CARP project.
Notes
Abbreviations: DCI, D-chiro-inositol; IP3, myo-inositol triphosphate; IP5, myo-inositol pentaphosphate; IP6, phytic acid; LB, Luria broth; MI, myo-inositol; SI, scyllo-inositol; SP, signal peptide.
References
- Bates SH, Jones RB, Bailey CJ. Insulin-like effect of pinitol. Br. J. Pharmacol. 2000;130:1944–1948.10.1038/sj.bjp.0703523
- Brown FD, Rappaport M, McLaurin R. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J. Mol. Med. 2007;85:603–611.
- Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, Richter T, Borriss R. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology. 2002;148:2097–2109.
- Iuorno MJ, Jakubowicz DJ, Baillargeon JP, Dillon P, Gunn RD, Allan G, Nestler JE. Effects of D-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr. Pract. 2002;8:417–423.10.4158/EP.8.6.417
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid β peptide and inhibit Aβ-induced toxicity. J. Biol. Chem. 2000;275:18495–18502.10.1074/jbc.M906994199
- McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, Darabie AA, Cousins JE, French JE, Lan MF, Chen F, Wong SS, Mount HT, Fraser PE, Westaway D, St George-Hyslop P. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006;12:801–808.10.1038/nm1423
- Yamaoka M, Osawa S, Morinaga T, Takenaka S, Yoshida K. A cell factory of Bacillus subtilis engineered for the simple bioconversion of myo-inositol to scyllo-inositol, a potential therapeutic agent for Alzheimer’s disease. Microb. Cell Fact. 2011;10:69.10.1186/1475-2859-10-69
- Yoshida K, Yamaguchi M, Morinaga T, Ikeuchi M, Kinehara M, Ashida H. Genetic modification of Bacillus subtilis for production of D-chiro-inositol, an investigational drug candidate for treatment of type 2 diabetes and polycystic ovary syndrome. Appl. Environ. Microbiol. 2006;72:1310–1315.10.1128/AEM.72.2.1310-1315.2006
- Reddy NR, Pierson MD, Sathe SK, Salunkhe DK. Phytates in cereals and legumes. Boca Raton (FL): CRC Press; 1989. p. 147.
- Cosgrove DJ. Chemistry and biochemistry of inositol polyphosphates. Rev. Pure Appl. Chem. 1966;16:209–214.
- Reddy NR, Sathe SK, Salunkhe DK. Phytates in legumes and cereals. Adv. Food Res. 1982;28:1–92.10.1016/S0065-2628(08)60110-X
- Angel R, Tamim NM, Applegate TJ, Dhandu AS, Ellestad LE. Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poultry Res. 2002;11:471–480.10.1093/japr/11.4.471
- Wyss M, Brugger R, Kronenberger A, Rémy R, Fimbel R, Oesterhelt G, Lehmann M, van Loon AP. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Appl. Environ. Microbiol. 1999;65:367–373.
- Tran TT, Hashim SO, Gaber Y, Mamo G, Mattiasson B, Hatti-Kaul R. Thermostable alkaline phytase from Bacillus sp. MD2: Effect of divalent metals on activity and stability. J. Inorg. Biochem. 2011;105:1000–1007.10.1016/j.jinorgbio.2011.04.005
- Park I, Cho J. The phytase from antarctic bacterial isolate, Pseudomonas sp. JPK1 as a potential tool for animal agriculture to reduce manure phosphorus excretion. Afr. J. Agric. Res. 2011;6:1398–1406.
- Escobin-Mopera L, Ohtani M, Sekiguchi S, Sone T, Abe A, Tanaka M, Meevootisom V, Asano K. Purification and characterization of phytase from Klebsiella pneumoniae 9-3B. J. Biosci. Bioeng. 2012;113:562–567.10.1016/j.jbiosc.2011.12.010
- Gerlach R, Pop O, Müller JP. Tat dependent export of E. coli phytase AppA by using the PhoD-specific transport system of Bacillus subtilis. J. Basic Microbiol. 2004;44:351–359.10.1002/(ISSN)1521-4028
- Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Codani JJ, Connerton IF, Cummings NJ, Daniel RA, Denizot F, Devine KM, Düsterhöft A, Ehrlich SD, Emmerson PT, Entian KD, Errington J, Fabret C, Ferrari E, Foulger D, Fritz C, Fujita M, Fujita Y, Fuma S, Galizzi A, Galleron N, Ghim SY, Glaser P, Goffeau A, Golightly EJ, Grandi G, Guiseppi G, Guy BJ, Haga K, Haiech J, Harwood CR, Hénaut A, Hilbert H, Holsappel S, Hosono S, Hullo MF, Itaya M, Jones L, Joris B, Karamata D, Kasahara Y, Klaerr-Blanchard M, Klein C, Kobayashi Y, Koetter P, Koningstein G, Krogh S, Kumano M, Kurita K, Lapidus A, Lardinois S, Lauber J, Lazarevic V, Lee SM, Levine A, Liu H, Masuda S, Mauël C, Médigue C, Medina N, Mellado RP, Mizuno M, Moestl D, Nakai S, Noback M, Noone D, O’Reilly M, Ogawa K, Ogiwara A, Oudega B, Park SH, Parro V, Pohl TM, Portetelle D, Porwollik S, Prescott AM, Presecan E, Pujic P, Purnelle B, Rapoport G, Rey M, Reynolds S, Rieger M, Rivolta C, Rocha E, Roche B, Rose M, Sadaie Y, Sato T, Scanlan E, Schleich S, Schroeter R, Scoffone F, Sekiguchi J, Sekowska A, Seror SJ, Serror P, Shin BS, Soldo B, Sorokin A, Tacconi E, Takagi T, Takahashi H, Takemaru K, Takeuchi M, Tamakoshi A, Tanaka T, Terpstra P, Tognoni A, Tosato V, Uchiyama S, Vandenbol M, Vannier F, Vassarotti A, Viari A, Wambutt R, Wedler E, Wedler H, Weitzenegger T, Winters P, Wipat A, Yamamoto H, Yamane K, Yasumoto K, Yata K, Yoshida K, Yoshikawa HF, Zumstein E, Yoshikawa H, Danchin A. The complete genome sequence of the Grampositive bacterium Bacillus subtilis. Nature. 1997;390:249–256.10.1038/36786
- Barbe V, Cruveiller S, Kunst F, Lenoble P, Meurice G, Sekowska A, Vallenet D, Wang T, Moszer I, Médigue C, Danchin A. From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology. 2009;155:1758–1775.10.1099/mic.0.027839-0
- Shimizu M. Purification and characterization of phytase from Bacillus subtillis (nato) N-77. Biosci. Biotechnol. Biochem. 1992;56:1266–1269.10.1271/bbb.56.1266
- von Heijne G. The signal peptide. J. Membr. Biol. 1990;115:195–201.10.1007/BF01868635
- Tjalsma H, Antelmann H, Jongbloed JD, Braun PG, Darmon E, Dorenbos R, Dubois JY, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, van Dijl JM. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 2004;68:207–233.10.1128/MMBR.68.2.207-233.2004
- Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in gram-positive bacteria. J. Mol. Biol. 2006;362:393–402.10.1016/j.jmb.2006.07.034
- Borchert TV, Nagarajan V. Effect of signal sequence alterations on export of levansucrase in Bacillus subtilis. J. Bacteriol. 1991;173:276–282.
- Caspers M, Brockmeier U, Degering C, Eggert T, Freudl R. Improvement of Sec-dependent secretion of a heterologous model protein in Bacillus subtilis by saturation mutagenesis of the N-domain of the AmyE signal peptide. Appl. Microbiol. Biotechnol. 2010;86:1877–1885.10.1007/s00253-009-2405-x
- Fu LL, Xu ZR, Shuai JB, Hu CX, Dai W, Li WF. High-level secretion of a chimeric thermostable lichenase from Bacillus subtilis by screening of site-mutated signal peptides with structural alterations. Curr. Microbiol. 2008;56:287–292.10.1007/s00284-007-9077-5
- Mathiesen G, Sveen A, Brurberg MB, Fredriksen L, Axelsson L, Eijsink VG. Genome-wide analysis of signal peptide functionality in Lactobacillus plantarum WCFS1. BMC Genomics. 2009;10:425.10.1186/1471-2164-10-425
- Watanabe K, Tsuchida Y, Okibe N, Teramoto H, Suzuki N, Inui M, Yukawa H. Scanning the Corynebacterium glutamicum R genome for high-efficiency secretion signal sequences. Microbiology. 2009;155:741–750.10.1099/mic.0.024075-0
- Farhat-Khemakhem A, Farhat MB, Boukhris I, Bejar W, Bouchaala K, Kammoun R, Maguin E, Bejar S, Chouayekh H. Heterologous expression and optimization using experimental designs allowed highly efficient production of the PHY US417 phytase in Bacillus subtilis 168. AMB Express. 2012;2:1–11.
- Fu LL, Xu ZR, Li W, Shuai JB, Lu P, Hu CX. Protein secretion pathways in Bacillus subtilis: Implication for optimization of heterologous protein secretion. Biotechnol. Adv. 2007;25:1–12.
- Shin S, Ha NC, Oh BC, Oh TK, Oh BH. Enzyme mechanism and catalytic property of beta propeller phytase. Structure. 2001;9:851–858.10.1016/S0969-2126(01)00637-2
- Nijland R, Kuipers OP. Optimization of protein secretion by Bacillus subtilis. Recent Pat. Biotechnol. 2008;2:79–87.10.2174/187220808784619694
- Dyrløv Bendtsen J, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: signalP 3.0. J. Mol. Biol. 2004;340:783–795.10.1016/j.jmb.2004.05.028
- Tjalsma H, Bolhuis A, Jongbloed JD, Bron S, van Dijl JM. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 2000;64:515–547.10.1128/MMBR.64.3.515-547.2000
- Oh BC, Choi WC, Park S, Kim YO, Oh TK. Biochemical properties and substrate specificities of alkaline and histidine acid phytase. Appl. Microbiol. Biotechnol. 2004;63:362–372.10.1007/s00253-003-1345-0