Abstract
We developed the simple method of soymilk cream production from the high-fat soymilk, which was prepared by papain digestion and heat treatment. As a result of the treatment, high-fat soymilk was aggregated and it became possible to separate soymilk cream as the surface fraction by low-speed centrifugation (6000 × g, 10 min).
Key words:
Soymilk is produced by adding water to soybeans and pulverizing them, then removing the insoluble cell walls and membranes by centrifugation or pressure filtration,Citation1) and heating the resulting emulsion (raw soymilk). Its main constituents are proteins, fats, and sugars, and because of its high nutritional value, soymilk is consumed mainly in the form of drinks and used as the raw material for manufacturing tofu. Recently, soymilk cream (which mainly contains oil bodies) has been produced as a new soymilk-derived ingredientCitation2) and has been commercialized for use by vegans and people with dairy allergies.
Previously reported methods of separating soymilk cream included ultracentrifugation, Na2CO3 washing,Citation3) and the sucrose-alkali method.Citation4) Although these methods can be used at the laboratory level, they are unlikely to be compatible with mass production at the factory level. Methods that are more suitable for comparatively large-scale production include Iwanaga et al.’s methodCitation5) and the technique developed by Fuji Oil Co., Ltd. The former method is based on a combination of aqueous extraction and centrifugation under the optimal conditions, and the latter technique is based on the usage of highly processed soybean (NSI; nitrogen solubility index: 20–70) as a raw material.Citation2)
In this study, we showed an efficient new method of producing a soymilk cream from soymilk as the raw ingredient. The principle of the method is the following. Soymilk supplied by Taishi Food Inc. (Aomori, Japan) was centrifuged to obtain high-fat soymilk (solid content approximately 25–26%, fat approximately 21%), which was then aggregated by inducing with the treatment of papain (Nagase ChemteX, Japan, specific activity was 100,000 PaUN/g) digestion followed by heat treatment. A soymilk cream was separated from the aggregated high-fat soymilk by centrifugation at low speed.
First, we examined the conditions for aggregation of high-fat soymilk by papain digestion. Papain was added to 30 mL of high-fat soymilk (protein concentration 61 mg/mL) to achieve a final concentration of 0.2 mg/mL and reacted for 30 min at 50°C. The papain was then inactivated either by boiling for 10 min at 100°C or by adding leupeptin (final concentration 10 μM). The respective samples were kept at a constant temperature of 25°C, and viscosities of the samples were measured.
There was no change in viscosity as a result of heat treatment (100°C, 10 min) alone. Papain treatment alone increased viscosity by approximately ninefold, although papain treatment and boiling increased the viscosity above the measurable level, revealing that papain digestion followed by heat treatment is required for the significant aggregation of soymilk (Fig. ). Fuke et al. investigated the conditions for soymilk gelation by rennet, pepsin, ficin, bromelain, and papain and reported that gelling by enzyme treatment requires heating to at least 70°C and that papain is a less effective gelling agent than either ficin or bromelain.Citation6,7) Murata et al. investigated the gelation effect of various types of commercially available proteases (Bacillus enzymes, Streptomyces enzymes, fungal enzymes, and plant and animal enzymes) and noted that the most effective were Bacillus enzymes, Aspergillus enzymes, and bromelain, whereas the effectiveness of Endothia enzymes, Rhizopus enzymes, Mucor enzymes, papain, and trypsin were lower.Citation8,9) As shown in Fig. , we observed an increase in viscosity of high-fat soymilk when it was subjected to papain digestion followed by heating. We thought that the increase in viscosity was caused by the formation of aggregation.
Fig. 1. Change in the viscosity of high-fat soymilk by papain digestion and/or boiling.
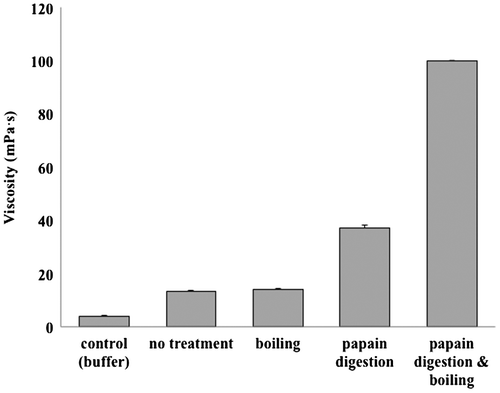
The reason for this difference between our results and those of Fuke and Murata et al. is unclear. In our work, the aggregation of oil bodies might mainly occur in high-fat soymilk.
Then, we examined the condition for separation of soymilk cream by low-speed centrifugation. A total of 0.5 mL high-fat soymilk was placed in 1.5-mL microtubes, and papain (final concentration 0.2 mg/mL) was added and reacted for 30 min at 50°C, after which the samples were boiled for 10 min and cooled to room temperature rapidly. The samples were then centrifuged for 10 min at a range of different gravitational forces, and their contents observed. As shown in Fig. , the high-fat soymilk samples that underwent papain treatment and heat treatment exhibited a clear boundary between an upper cream layer and a lower layer (whey) after low-speed centrifugation (6000 × g), indicating that the separation of soymilk cream is feasible. In contrast, those that did not undergo papain treatment (heat treatment only) did not exhibit any such clear separation even at the highest speed used in this study (13,400 × g).
Fig. 2. Determination of optimal centrifugation condition for soymilk cream separation.
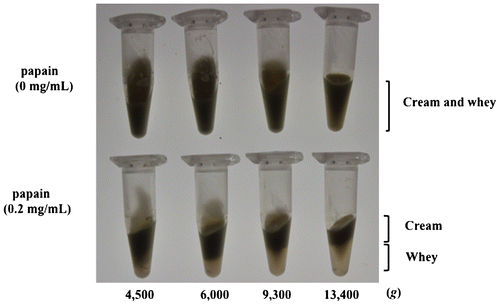
In this way, we have succeeded in developing a highly efficient method of producing soymilk cream from high-fat soymilk by means of papain digestion, heat treatment, and low-speed centrifugation. The mechanism underlying this method may be explained in terms of papain digestion of not only protein particles (mainly 7S and 11S globulins)Citation10,11) but also oleosins and heat treatment to aggregate and coalesce oil bodies, and hence the aggregates of oil bodies with modified surface float to the top as cream with low specific gravity, enabling the separation of soymilk cream by means of low-speed centrifugation. De Moura et al. reportedCitation12) an enzyme-associated aqueous extraction processing of oil and protein from soybeans with the endoprotease Protex 6L, which is a bacterial alkaline protease from Bacillus licheniformis. This process is seemed to be using a similar principle to our method except for the difference of protease type and ability to separate both cream and free oil.
The method developed by us does not require ultracentrifugation but can be carried out using the existing centrifuges in production plants, making it a highly practicable new method.
Author contribution
N.A., T.F., and K.A. designed the study. N.A., C.W., and Y.K. performed the experiments and analyzed the results. N.A., T.F., and K.A. prepared the manuscript. All authors discussed the results.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
This project was supported by a Scheme to Revitalize Agriculture and Fisheries in Disaster Area through Deploying Highly Advanced Technology, Ministry of Agriculture, Forestry and Fisheries of Japan. The authors also would like to thank Mr. Yasushi Iwamoto for supply of soymilk.
References
- Idogawa S, Ito K, Fujii T. Filtration behavior during soymilk separation process. Food Sci. Technol. Res. 2013;19:1071–1075.10.3136/fstr.19.1071
- Japanese Patent No. 5077461.
- Fujiki Y, Hubbard AL, Fowler S, et al. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol. 1982;93:97–102.10.1083/jcb.93.1.97
- Gustav W, Birgitta S, Thomas AV, et al. Soy bean oleosomes behavior at the air–water interface. J. Phys. Chem. B. 2012;116:10832–10841.
- Iwanaga D, Gray DA, Fisk ID, et al. Extraction and characterization of oil bodies from soy beans: a natural source of pre-emulsified soybean oil. J. Agric. Food. Chem. 2007;55:8711–8716.10.1021/jf071008w
- Fuke Y, Matsuoka H. Coagulation of soymilk by proteolytic enzyme treatment. Nippon Shokuhin Kogyo Gakkaishi. 1980;27:275–280. Japanese.10.3136/nskkk1962.27.6_275
- Fuke Y, Matsuoka H. Preparation of fermented soybean curd using stem bromelain. J. Food Sci. 1984;49:312–313.10.1111/jfds.1984.49.issue-1
- Murata K, Kusakabe I, Kobayashi H, et al. Selection of commercial enzymes suitable for making soymilk-curd. Agric. Biol. Chem. 1987;51:2929–2933.10.1271/bbb1961.51.2929
- Murata K, Kobayashi H, Kusakabe I, et al. Preparation of fermented soymilk curd with commercial proteinases. Nippon Shokuhin Kogyo Gakkaishi. 1989;36:417–423.10.3136/nskkk1962.36.5_417
- Ono T, Takeda M, Guo ST. Interaction between protein particles with lipids in soybean milk. Biosci. Biotechnol. Biochem. 1996;60:1165–1169.
- Guo ST, Ono T, Mikami M. Interaction between protein and lipid in soybean milk at elevated temperature. J. Agric. Food. Chem. 1997;45:4601–4605.
- de Moura JMLN, Johnson LA. Two-stage countercurrent enzyme-associated aqueous extraction processing of oil and protein from soybeans. J. Am. Oil Chem. Soc. 2009;86:283–289.10.1007/s11746-008-1341-8
